Significance
Bacillus anthracis produces protein toxins that present a major biodefense challenge. Host genes exploited for toxin entry by pathogens are potential targets for therapies that circumvent resistance-inducing pathogen mutations. We report that (i) anthrax lethal toxin enters macrophages by associating with higher-order actin filaments that provide the dynamic force for membrane invagination during endocytic trafficking of integrin-containing focal adhesion complexes (FACs) and (ii) calpain-mediated cleavage of talin-1, which anchors FACs at the cell surface, facilitates such exploitation. Our findings elucidate steps of anthrax toxin endocytosis and identify a potential target for mitigation of toxicity.
Keywords: lethal factor, calpastatin, MDL28170
Abstract
The protective antigen component of Bacillus anthracis toxins can interact with at least three distinct proteins on the host cell surface, capillary morphogenesis gene 2 (CMG2), tumor endothelial marker 8, and β1-integrin, and, with the assistance of other host proteins, enters targeted cells by receptor-mediated endocytosis. Using an antisense-based phenotypic screen, we discovered the role of calpains in this process. We show that functions of a ubiquitous Ca2+-dependent cysteine protease, calpain-2, and of the calpain substrate talin-1 are exploited for association of anthrax toxin and its principal receptor, CMG2, with higher-order actin filaments and consequently for toxin entry into host cells. Down-regulated expression of calpain-2 or talin-1, or pharmacological interference with calpain action, did not affect toxin binding but reduced endocytosis and increased the survival of cells exposed to anthrax lethal toxin. Adventitious expression of wild-type talin-1 promoted toxin endocytosis and lethality, whereas expression of a talin-1 mutant (L432G) that is insensitive to calpain cleavage did not. Disruption of talin-1, which links integrin-containing focal adhesion complexes to the actin cytoskeleton, facilitated association of toxin bound to its principal cell-surface receptor, CMG2, with higher-order actin filaments undergoing dynamic disassembly and reassembly during endocytosis. Our results reveal a mechanism by which a bacterial toxin uses constitutively occurring calpain-mediated cytoskeletal rearrangement for internalization.
The ability of pathogenic bacteria and viruses to exploit normal functions of host cells during pathogenesis is well recognized (1–3). Host gene functions are exploited also for interaction of toxins with and entry of toxins into targeted cells, as well as for later steps along the pathway to pathogenicity (4–7). Among such toxins are two produced by Bacillus anthracis. Both toxins include a pore-forming component, named “protective antigen” (PA), which employs lipid rafts to carry an injurious toxin moiety—either lethal factor (LF) or edema factor (EF)—into cells by clathrin-mediated endocytosis (CME) (8–10). The host cell capillary morphogenesis gene product, CMG2, a type I membrane protein whose primary function in cells remains poorly understood, has a dominant role in PA binding to the cell surface and in anthrax toxin lethality; multiple other host proteins, including the tumor endothelial marker protein TEM8, the low-density lipoprotein family member LRP6, and ARAP3, a multidomain protein having GTPase activity, as well as integrin complexes which mediate cell–cell attachments, participate in and promote toxin internalization (11–16). Integrins, CMG2, and TEM8 are transmembrane proteins containing a highly conserved extracellular von Willebrand factor type A domain that can function as a PA docking site (17) and can enter cells along with the toxin (14, 16). Apoptosis and caspase-1–dependent necrosis have been implicated as mechanisms underlying the cell death that occurs following exposure to anthrax lethal toxin (PA-LF) (18–20).
Calpains, a ubiquitous family of Ca2+-dependent cysteine proteases that regulate functions of substrates by carrying out limited proteolysis (21), are encoded in mammals by at least 15 distinct genes (22, 23). Calpain substrates include cytoskeletal proteins, kinases/phosphatases, membrane-associated proteins, and transcription factors (21), and calpain function has been implicated in diverse cellular events ranging from the remodeling of cytoskeletal anchorage complexes to cell-cycle control (24, 25). Additionally, calpains are exploited by fungal and viral pathogens to facilitate cell invasion (26, 27) and have been implicated in pathogen-mediated or toxin-mediated apoptosis or necrosis occurring after infection by Streptococcus pneumonia, Shigella dysenteriae, Neisseria spp., and Clostridium septicum (28–31).
Using an antisense-RNA–based phenotypic screen for host proteins that modulate macrophage killing by anthrax lethal toxin, we found that B. anthracis toxins exploit the homeostatic actions of calpains to promote toxin entry into targeted cells. Here, we report these findings and establish the mechanism underlying this event. We show that internalization of anthrax toxin complexes is promoted by exploitation of the calpain-dependent disruption of talin, a calpain substrate that links integrins to the actin cytoskeleton (32), and further show that interference with calpain function impedes the association of CMG2-bound PA with dynamically reassembling actin filaments during endocytosis of PA. Because chemical interference with calpain function can mitigate the effects of exposure to anthrax lethal toxin, we suggest that calpain inhibitors, which have been developed as potential treatments for a variety of human diseases (33–35), may be useful in treatment of or prophylaxis for anthrax toxicity.
Results
Reduction in Anthrax Toxin Lethality by Adventitious Expression of Calpastatin.
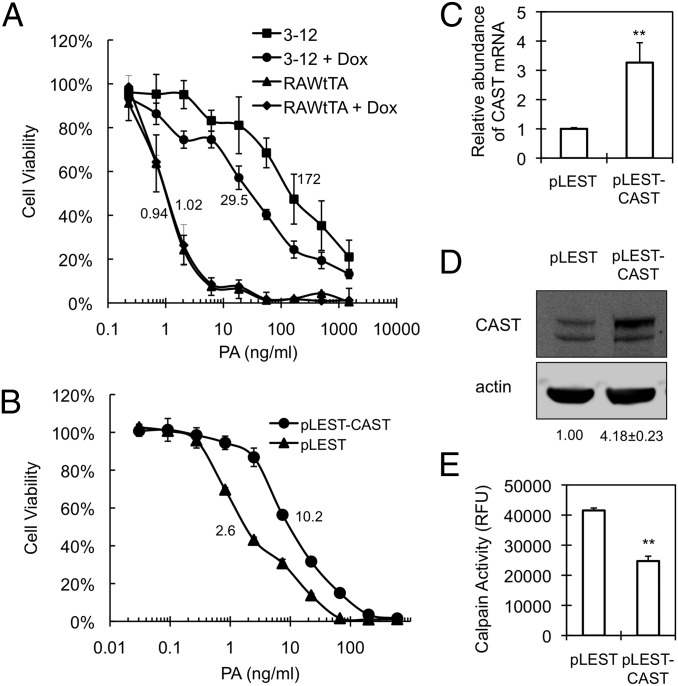
We used regulated transcription from a lentivirus-based human EST library to perturb host gene expression globally and randomly (14, 15, 36, 37) in murine RAW267.4 macrophages and isolated 96 macrophage colonies that survived exposure to the PA-LF complex under conditions that normally are lethal. Doxycycline, which represses a modified CMV promoter controlling expression of ESTs in the library we used (15), partially reversed the reduced toxin sensitivity observed in several of these clones, implying a role for the corresponding ESTs in the phenotype. One clone (clone 3-12; Fig. 1A) showed a sixfold higher LD50 in the absence of doxycycline than in its presence and was selected for further study. PCR amplification and sequencing indicated that 3-12 cells contain a chromosomally inserted EST derived from calpastatin (CAST), a cellular down-regulator of calpain activity (21). The CAST gene sequence, which, notwithstanding its presence in an EST library, is annotated in GenBank as intronic (IMAGE clone no. 71286), was inserted in the sense direction relative to the pLEST lentivirus promoter. The de novo introduction of the 3-12 CAST EST into parental RAW264.7tTA cells behind the pLEST promoter (15) reproduced the anthrax toxin-resistance phenotype and increased the abundance of CAST mRNA and protein (Fig. 1 B–D). Consistent with the known biological role of calpastatin as a highly specific calpain inhibitor (21), cells expressing the CAST EST showed decreased cellular calpain activity (Fig. 1E). Collectively, these findings argue that expression of the 3-12 CAST EST can down-regulate calpain activity and that such down-regulation is associated with decreased cellular lethality of anthrax toxin.
Fig. 1.
Characteristics of expression of CAST EST. (A) Effect of doxycycline (Dox) on the susceptibility of clone 3-12. Cells were preincubated in the presence or absence of 1 µg/mL Dox for 2 d and then were seeded at a concentration of 2 × 104 cells/mL in a 96-well plate containing or lacking 1 µg/mL of Dox. Cells were treated with a mixture containing the indicated concentrations of PA plus 250 ng/mL LF for 2 d, and an MTT assay was performed as described in Materials and Methods. Cell viability is shown as the percent survival relative to treatment without toxin and represented as mean ± SD. Data are from one representative experiment (n = 3) performed in quadruplicate. LD50 values of PA for clone 3-12 in the absence and presence of Dox are 172 ± 13.8 ng/mL and 29.5 ± 2.2 ng/mL, respectively. P < 0.01. (B–D) The CAST EST identified in clone 3-12 was reintroduced into the pLEST lentiviral vector, yielding pLEST-CAST, which then was used to infect RAW264.7tTA cells. Selective growth of cells containing pLEST-CAST or the empty lentiviral vector (pLEST) was accomplished by treating cultures with 800 µg/mL G418. (B) Effect of CAST EST expression on the cytotoxicity of PA-LF. Pooled pLEST-CAST–infected cells were treated with a mixture of PA plus LF, as in A. An MTT assay was performed 1 d after toxin treatment. The percent of cell viability is represented as mean ± SD. Data are from one representative experiment (n = 3) carried out in triplicate. LD50 values of PA in pLEST-CAST and pLEST are 10.2 ± 0.8 ng/mL and 2.6 ± 0.1 ng/mL, respectively. P < 0.01. (C) Effect of CAST EST on CAST mRNA expression. Relative mRNA abundance was quantified by real-time RT-PCR using primers corresponding to a segment of exons 1–3, as indicated in Materials and Methods and normalized to β-actin. Data represent mean ± SD of three independent experiments. **P < 0.01. (D) Abundance of CAST protein. Cell lysates were analyzed by Western blotting using anti-CAST antibody. The numbers below the Western blots indicate mean ± SD of relative CAST level (upper band) normalized to actin from two independent experiments. (E) Calpain activity in cells infected by pLEST-CAST or pLEST was determined in total cell lysates using synthetic fluorogenic substrates as described in Materials and Methods. Data represent mean ± SD of three independent experiments. **P < 0.01. RFU, relative fluorescence units.
Effect of Pharmacological Inhibition of Calpain on Anthrax Toxin Lethality.
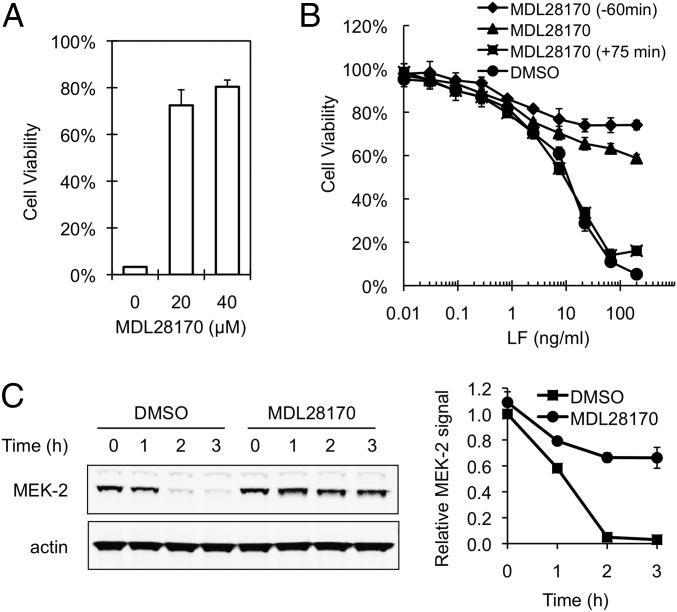
Treatment of cells with a cell-permeable synthetic agent MDL28170, which broadly inhibits the activity of calpains (38) (Fig. S1), confirmed that calpain function affects anthrax toxin lethality. RAW264.7 cells exposed to a dose of anthrax lethal toxin that normally results in <3% cell survival after 4 h of exposure showed up to 80% survival when treated with MDL28170 either before (Fig. 2A) or concurrently with (Fig. 2B) toxin exposure. However, MD28170 failed to protect against toxin lethality when added 75 min after the cells were exposed to toxin (Fig. 2B), suggesting that the calpain function exploited for the normal lethality of anthrax toxin is provided soon after toxin exposure. Consistent with this notion, calpain inhibition also specifically protected against cleavage of the LF substrate MEK-2 (Fig. 2C), which normally occurs 1–2 h after exposure of cells to PA-LF (39). Control experiments showed that MDL28170 had no direct effect on the enzymatic activity of LF (Fig. S2).
Fig. 2.
Effects of calpain inhibition on cellular susceptibility to PA-LF. (A) Effect of MDL28170 on killing of RAW264.7 cells by PA-LF. MDL28170 (20 or 40 µM) dissolved in DMSO or DMSO alone as a control was added to cells 1 h before toxin treatment. After cells were incubated with 0.5 µg/mL PA-LF for 4 h, cell viability was assessed by MTT assay, as indicated in Fig. 1. The percent survival relative to cells cultured in the absence of toxin is represented as mean ± SD of triplicate experiments. Data are from one representative experiment (n = 3). (B) Effect of the time of addition of MDL28170 on PA-LF–induced cytotoxicity. RAW264.7 cells were treated with 40 µM MDL28170 for 60 min before (♦), concurrently with (▲), or 75 min after (■) exposure of the cells to toxin. DMSO was added to a final concentration of 0.1% to cells in wells lacking MDL28170 (●). After cells were incubated for 4 h with serially diluted LF in the presence of 200 ng/mL PA, an MTT assay was performed. Values shown are the mean ± SD of experiments performed in triplicate. (C) Effect of MDL21870 on PA-LF-mediated cleavage of MEK-2. RAW264.7 cells were incubated in the absence or the presence of 80 µM MDL28170 for 1 h and then were exposed to 0.5 µg/mL PA-LF for the indicated time. (Left) Cell extracts were analyzed by Western blotting using anti–N-terminal MEK-2 antibody to detect intact MEK-2. (Right) Mean ± SD of the relative level of intact MEK-2 normalized to actin from three independent experiments.
Calpain Dependence of Anthrax Toxin Internalization.
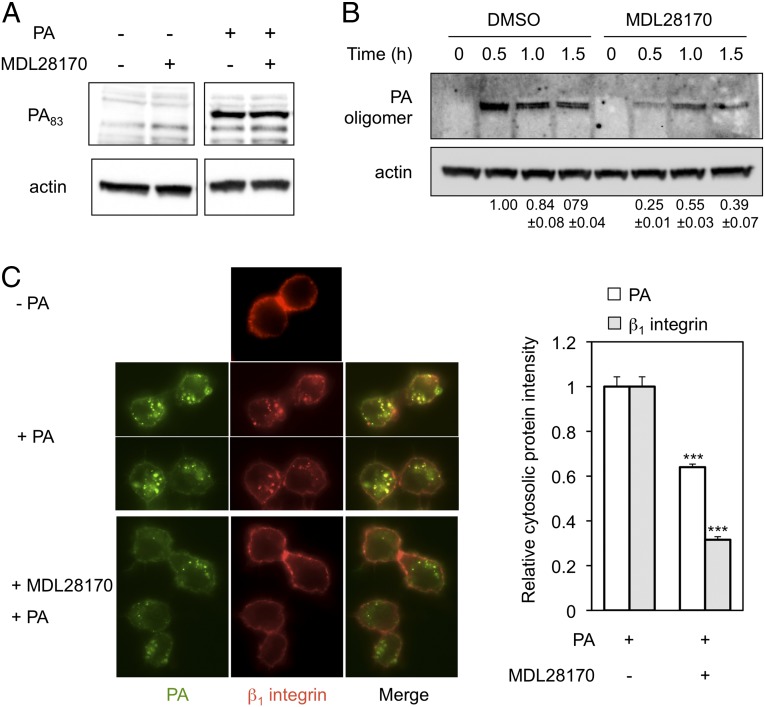
The early steps toward anthrax toxin lethality are binding of the 83-kDa PA protein (PA83) to receptors on the cell surface, furin-mediated cleavage of PA83 to generate the 63-kDa PA subunit (PA63), formation of a heptameric oligomer of PA63, interaction of heptameric PA63 with EF or LF, and entry of the toxin and receptor complex into cells by CME (10, 40, 41). A conformational change in PA structure caused by the acidic pH of endosomes renders the tertiary structure of the PA63 heptamer resistant to disruption by the detergent SDS (40, 42). Internalized (SDS-resistant) PA thus can be distinguished from surface-bound (SDS-sensitive) PA during electrophoresis on SDS/PAGE gels, enabling us to determine whether calpain inhibition affects events that occur before or after the conformational change in the PA63 heptamer that takes place in endosomes. Western blot analysis of cell lysates from RAW264.7 macrophages that were exposed to PA and maintained at 4 °C, which allows binding of the toxin but prevents endocytosis (40), showed that treatment with MDL28170 did not detectably alter the amount of SDS-sensitive (i.e., surface-bound) PA (Fig. 3A). In contrast, calpain inhibition was associated with a 75% decrease in SDS-resistant heptameric PA, as assayed after the shift of toxin-exposed cells for 0.5 h at 37 °C to enable endocytosis (Fig. 3B), arguing that the actions of calpain that affect anthrax toxicity occur subsequent to binding but before the entry of PA into acidic endosomes. Calpain inhibition also delayed endocytosis: the peak for SDS-resistant PA was seen at 1 h instead of 0.5 h (Fig. 3B). Consistent with these effects, fluorescence microscopy indicated that the addition of MDL28170 sharply reduced the internalization of PA and also of integrin β1 (Fig. 3C), which previously has been shown accompany the toxin during its entry into cells (16).
Fig. 3.
Effect of calpain inhibition on PA internalization. (A and B) RAW264.7 cells were incubated with 80 µM MDL28170 or DMSO control for 1 h. The cells then were exposed to 0.5 µg/mL PA and maintained at 4 °C for 1 h for binding assays (A) or at 37 °C for the indicated time for internalization assays (B). Cell lysates were analyzed by Western blotting using anti-PA antibody as described in Materials and Methods. The SDS-resistant PA oligomer signal was quantified by normalization against actin, and the resulting value was compared with the signal obtained from DMSO-treated cells at time point 0.5 h (which was assigned a value of 1.0). The mean ± SD for values obtained in three independent experiments are shown below the lanes in the Western blot in B. (C) Immunofluorescence analysis of cellular localization of PA and β1 integrin during PA internalization. RAW264.7 cells were preincubated with or without 80 µM MDL28170 for 1 h, followed by exposure to Alexa-Fluor 488–labeled PA in the presence of 0.5 µg/mL anti–β1 integrin-APC (HMβ1–1) antibody at 37 °C for 20 min. (Left) The cellular localization of PA (green fluorescence) and β1 integrin (red fluorescence) was monitored microscopically. (Right) The intensities of cytoplasmic PA and β1-integrin signal were quantified by ImageJ analysis as described in Materials and Methods. Data represent mean ± SEM values (n > 30). ***P < 0.001.
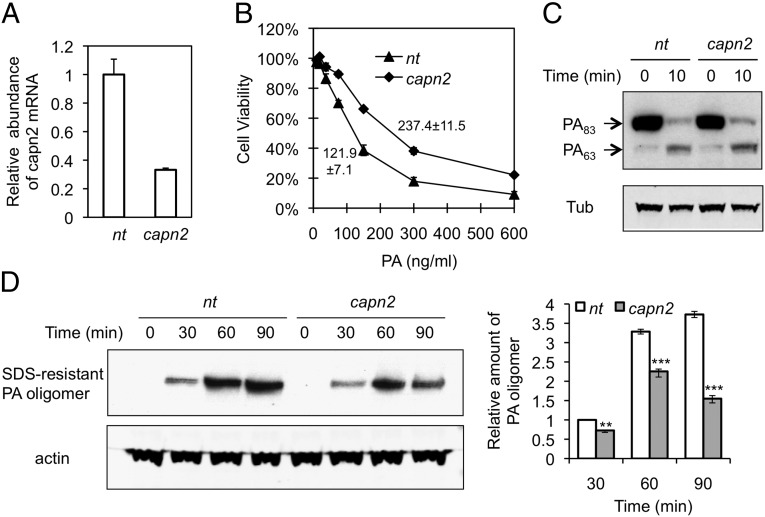
Calpain-2 (capn2) is required for proteolysis of cytoskeletal and focal adhesion proteins, including talin, paxillin, spectrin, and FAK (43, 44), and colocalizes with lipid rafts (45, 46) in which both integrin β1 and the PA–receptor complex are redistributed during CME (8, 47). Using lentivirus that produces shRNA directed specifically against capn2, we investigated a possible role for capn2 in PA internalization. Knockdown of capn2 to ∼40% of normal by this shRNA (Fig. 4A) resulted in an approximate doubling of the LD50 for PA, from 121.9 ± 7.1 ng/mL to 237.4 ± 11.5 ng/mL PA (Fig. 4B). shRNA directed against capn2 had no detectable effect on PA binding or processing as determined by Western blotting (Fig. 4C). However, like the overall pharmacological inhibition of calpains by MD21870, shRNA directed specifically against the expression of capn2 resulted in a decrease in the amount of internalized PA (Fig. 4D), indicating that a function provided specifically by capn2 is exploited for endocytosis of PA. In contrast, overexpression of capn2 had no effect on the endocytosis of PA (Fig. S3), suggesting that endogenous expression of capn2 is not rate-limiting.
Fig. 4.
Effect of capn2 deficiency on PA internalization. RAW264.7 cells were infected with lentivirus expressing capn2 shRNA or nontargeting shRNA (nt). (A) The capn2 mRNA expression determined by quantitative RT-PCR was normalized to the expression of β-actin. Data represent mean ± SD from three independent experiments. (B) Cellular susceptibility to PA-LF. The cells were exposed for 4 h to a mixture containing the indicated concentration of PA and 200 ng/mL LF; then cell survival was determined by MTT assay. The percent of cell viability is represented as mean ± SD. Data are from one representative experiment (n = 3) carried out in triplicate. LD50 values of PA in cells infected with lentivirus expressing nt or capn2 shRNA are 121.9 ± 7.1 ng/mL and 237.4 ± 11.5 ng/mL, respectively. P < 0.01. (C) Binding and processing of PA. The cells were exposed to 1 µg/mL PA at 4 °C for 1 h and then were shifted to 37 °C for 10 min. Cell lysates were analyzed by Western blotting using anti-PA antibody. Tub, tubulin. (D) PA-internalization assay. Cells were exposed to 1 µg/mL PA at 37 °C for the indicated time, and cell lysates were analyzed by Western blotting using anti-PA antibody as a probe. (Left) A representative blot. (Right) Relative abundance of SDS-resistant PA oligomers was quantified by normalization to actin in three independent experiments; data are shown as mean ± SD. **P < 0.01; ***P < 0.001 compared with nt.
Calpain-Cleavable Talin-1 Promotes Anthrax Toxin Internalization.
The proteolytic actions of calpains have been shown to regulate dynamically the functions of cell-adhesion complexes containing integrin and cytoskeletal actin (48–50). The cytoskeletal protein talin-1 (TLN1), which is highly sensitive to cleavage by capn2, links integrins to the actin cytoskeleton directly or indirectly by recruiting other actin-binding proteins and regulating focal adhesion complex dynamics (43, 51, 52). Earlier work has shown that cleavage of talin by capn2 is critical for the disassembly of focal adhesions and that a tln1−/− cell line is defective in the turnover of integrins and other protein components of focal adhesion complexes (32, 43). Although adventitious expression of wild-type TLN1 in such cells reversed this defect, expression of a TLN1 variant, TLN-L432G, that is insusceptible to capn2 cleavage did not (32). Our finding that interference with capn2 function decreased endocytosis of both PA and integrins raised the possibility that altered cleavage of TLN1 may be the mechanism underlying the effects of capn2 on the toxin.
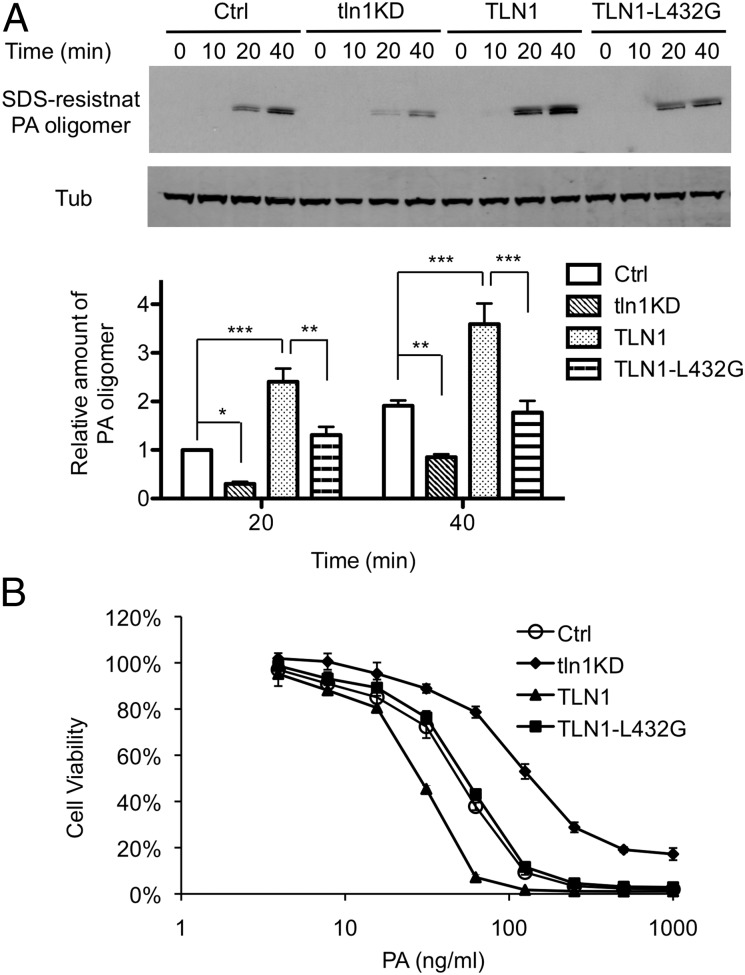
Adventitious expression of TLN1, which had no effect on the binding of PA to the cell surface or the processing of PA (Fig. S4), increased the time-dependent appearance of SDS-resistant PA oligomers (Fig. 5A) and enhanced the lethality of the PA-LF complex (Fig. 5B) in macrophages, indicating a specific role for TLN1 in toxin internalization. Neither increased internalization of PA nor increased killing by lethal toxin was observed in cells that express a TLN1 mutant, TLN-L432G (32), that is insusceptible to calpain cleavage, indicating that disruption of TLN1 integrity, which has been shown to be mediated by capn2 (43), facilitates these TLN1-dependent effects. Moreover, the ability of shRNA directed against endogenous TLN1 (Fig. S5) to reduce toxin internalization and lethality (tln1KD; Fig. 5 A and B) argues that dynamic turnover of focal adhesion complexes, which, as Franco et al. (32) have shown, requires both the presence and cleavage of TLN1, has a positive role in endocytosis of the toxin.
Fig. 5.
Effect of TLN1 on anthrax toxin internalization and lethality. RAW264.7 cells (Ctrl) were infected with lentivirus expressing TLN1 shRNA (tln1KD) to reduce the expression of TLN1. pEGFP-C1–derived plasmids expressing wild-type TLN1 or mutant TLN1-L432G were introduced by transfection. (A) Kinetics of PA internalization during knockdown or adventitious expression of TLN1. Cells were treated with 1 μg/mL of PA at 4 °C for 1 h and then were shifted to 37 °C for the indicated time. Cell extracts were analyzed by Western blotting using anti-PA and anti-tubulin antibodies. (Upper) A representative Western blot. (Lower) The bar graph shows mean ± SD values of relative amounts of SDS-resistant PA oligomer normalized to tubulin in four independent experiments. Statistical significance was determined by two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001. (B) Cellular susceptibility to PA-LF. Cells were exposed for 4 h to a mixture containing the indicated concentrations of PA and 500 ng/mL LF, and then an MTT assay was performed. The percent of cell viability is presented as mean ± SD. Data are from one representative experiment (n = 3) carried out in triplicate. LD50 values of PA in Ctrl, tln1KD, TLN1, and TLN1-L432G cells are 50.1 ± 3.6, 137.7 ± 1.6, 28.0 ± 0.7, and 54.6 ± 2.1 ng/mL, respectively. P < 0.01 between Ctrl and tln1KD or TLN1 and between TLN1 and TLN1-L432G.
Calpain Cleavage of TLN1 Leads to Association of CMG2 and PA with Triton X-100–Insoluble Actin Filaments.
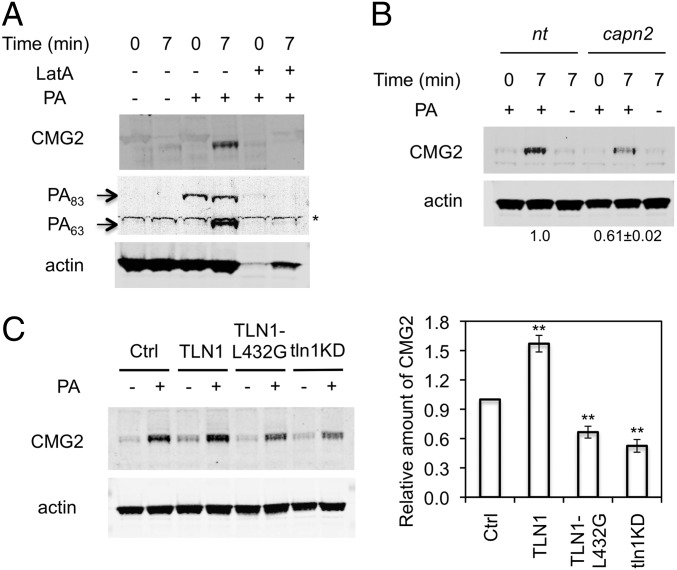
Published experiments by others aimed at detecting interaction between highly overexpressed CMG2 and actin have yielded disparate results (10, 53, 54). In cells expressing a native amount of CMG2, we found that the addition of PA promotes the association of the toxin/receptor complex with actin cytoskeleton fractions that remain insoluble and sediment rapidly in preparations treated with the nonionic detergent Triton X-100 (54–56), facilitating endocytosis of both PA and CMG2 (Fig. 6A). Such Triton X-100–insoluble factions are thought to represent higher-order actin filaments assembled during the progressive invagination of membranes that occurs during the formation of endocytic vesicles (57–59). The Triton X-100–resistant association of CMG2 with the actin cytoskeleton was minimal in macrophages that had not been exposed to PA and in cells that were maintained at 4 °C after such exposure (Fig. 6A). However, shifting PA-treated cells from 4 °C to 37 °C to initiate endocytosis dramatically increased the association of CMG2 and PA63 with the detergent-resistant actin cytoskeleton (Fig. 6A). Together, these findings suggest that CMG2 interaction with PA, which was shown earlier to result in the entry of CMG2 into cells (14, 16), enables CMG2 to exploit mechanisms that have been reported to disassemble and reassemble actin filaments dynamically during CME (60, 61). Treatment of cells with latrunculin A (LatA), which inhibits polymerization of filamentous actin (62), prior to initiation of PA endocytosis eliminated CMG2 from the detergent-insoluble fractions (Fig. 6A), supporting the notion that CMG2 internalization is dependent on dynamic rearrangement of the actin cytoskeleton.
Fig. 6.
Effect of TLN1 cleavage by calpain on association of CMG2 with the actin cytoskeleton. (A) Dependency of CMG2 association with actin cytoskeleton on PA endocytosis. RAW264.7 cells were preincubated with or without 1 μM of LatA for 40 min at 37 °C. After being chilled on ice for 15 min, cells were treated for 1 h with 1 μg/mL PA at 4 °C for 1 h and were lysed in CSK buffer containing 1% Triton X-100 at 4 °C for 30 min or were lysed similarly after being shifted to 37 °C for 7 min. Lysates were centrifuged at 16,000 × g for 5 min to pellet the actin cytoskeleton. The detergent-insoluble fractions were analyzed by Western blotting using anti-CMG2, anti-PA, and anti–β-actin antibodies as probes. The asterisk indicates a band that we conclude is nonspecific because of its presence in extracts of cells that have not been exposed to PA. The Western blot shown is representative of three independent experiments. (B) Effect of capn2 knockdown on CMG2 association with actin cytoskeleton. The detergent-insoluble actin cytoskeleton was extracted from capn2 knockdown (capn2) and control (nt) cells as described in A and was analyzed by Western blotting using anti-CMG2 and anti–β-actin antibodies as probes. The Western blot shown is from one representative experiment (n = 3). (C) Effect of knockdown or adventitious expression of TLN1 on CMG2 association with actin cytoskeleton. The detergent-insoluble actin cytoskeleton was extracted from RAW264.7 parental cells (Ctrl), TLN1 cells, TLN1-L432G cells, and TLN1 knockdown (tln1KD) cells as described in A. CMG2 and actin were analyzed by Western blotting. (Left) A representative blot. (Right) Mean ± SD of relative CMG2 levels normalized to actin in three independent experiments. **P < 0.01 compared with Ctrl.
The above findings, together with the previously known role of calpains and TLN1 in actin cytoskeletal rearrangements (24, 48), suggested that the association of CMG2 with the Triton X-100–resistant actin cytoskeleton would be sensitive to calpain inhibition, and this hypothesis was confirmed by evidence that shRNA directed against capn2 reduced such association (Fig. 6B). Additionally, although adventitious overexpression of TLN1 increased CMG2 linkage to actin, a decrease in linkage suggestive of dominant-negative effects was observed (Fig. 6C) upon similar overexpression in naive RAW264.7 cells of the TLN1 mutant TLN1-L432G, which is insensitive to cleavage by capn2 (32). shRNA-induced knockdown of TLN1 also reduced CMG2 association with the actin cytoskeleton (Fig. 6C), further implying that endocytosis of the toxin–receptor complex is mediated by the turnover of focal adhesion complexes linked by TLN1 to the actin cytoskeleton. Collectively, these findings and those described above argue that the normal biological roles of both capn2 and TLN1 are exploited to promote the association of the PA/CMG2 complex to the actin cytoskeleton and endocytosis of the toxin.
Discussion
Calpains are known to affect a wide variety of cellular events, including migration, differentiation, and apoptosis (21, 48, 63, 64). These ubiquitous proteases also have been shown to enable rearrangement of the actin cytoskeleton by cleaving multiple cytoskeletal components, including TLN1, vinculin, and FAK (24, 48). Microbial pathogens can trigger actin cytoskeleton rearrangement and/or CME by recruiting adhesion adaptors and downstream signaling molecules (65–67). Recently, it has been recognized that microbial pathogens also have evolved more direct mechanisms to benefit from the actions of calpains, for example by activating calpains to destabilize intercellular interactions that impede invasion of cells (68, 69). Our findings now demonstrate that calpain protease actions are exploited for anthrax toxin internalization and show that the calpain target for this event is the cytoskeletal protein TLN1. Unlike the exploitation of calpain actions by bacterial cells, exploitation of calpain by B. anthracis toxins is passive: we found no evidence that exposure of cells to anthrax toxin increases calpain production or activity.
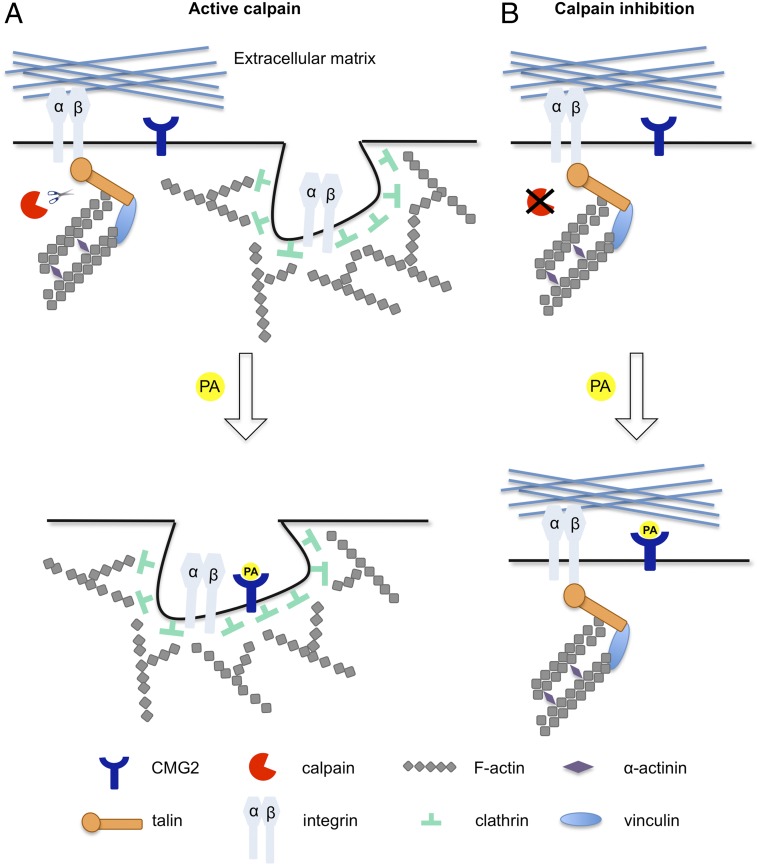
CME in mammalian cells is thought to involve the disassembly—with the assistance a variety of adaptor proteins—of actin-based focal adhesion complexes and subsequent cycles of reassembly and disassembly of the components of these complexes to impart increasing curvature to developing endocytic vesicles (for recent reviews, see refs. 61, 70, and 71). During these events, β1 integrins and other cell-receptor proteins—and the ligands bound to them—can be internalized (60, 72, 73); integrin-mediated signaling is regulated by adaptor proteins that include TLN1 (74). Previous work (14) has shown that PA promotes the internalization of the integrin-like CMG2 anthrax receptor protein [also known as ANTXR2 (11)], and the experiments reported here argue that this event depends on the disassembly and reassembly of actin complexes. Our findings suggest a model (Fig. 7) in which these repetitive events are promoted by both TLN1 linkage of focal adhesion complexes to the actin cytoskeleton and by cleavage of TLN1 by capn2.
Fig. 7.
Proposed model for calpain-mediated PA endocytosis. (A) Integrin complexes such as α5β1 are dynamically internalized and recycled to the plasma membrane during the assembly and disassembly of focal adhesion complexes at the cell surface (60). Calpain-mediated cleavage of TLN1 disrupts the linkage between integrins and the actin cytoskeleton, enabling integrin endocytosis by clathrin-dependent and clathrin-independent mechanisms (70, 82, 83). The PA/CMG2 complex at the cell surface is internalized by exploitation of mechanisms for clathrin-dependent endocytosis of integrins. During such endocytosis, the PA/CMG2 complex becomes associated with Triton X-100–insoluble higher-order actin filaments that undergo repeated cycles of disassembly and reassembly as the developing endocytic vesicles acquire increasing curvature and eventually are excised to form nascent endosomes. (B) Inhibition of capn2 function interferes with the disassembly of integrin-containing focal adhesion complexes and reduces PA-induced internalization of both integrins and the PA/CMG2 complex.
Consistent with the proposed role of calpain cleavage in endocytosis of the PA/CMG2 complex, calpain inhibition subsequent to endocytosis of toxin did not affect toxin lethality. This finding contrasts with the effects of the caspase-1 inhibitor Boc-d-CMK and the proteosome inhibitor MG132, which interfere with caspase-1 activation and can rescue cells even when added at later times (75, 76). Although CMG2 is required for rapid killing of cells in culture by PA-LF (16) and has a dominant role in the death of animals exposed to anthrax toxin (12, 77), integrins can assist CMG2 function and enhance cell killing by low doses of toxin (16). We hypothesize that trafficking of integrin-containing complexes facilitates association of the PA/CMG2 complex with the actin cytoskeleton during endocytosis. TEM8, one of three cell-surface receptors for PA identified in mammalian cells, also is an integrin-like protein (13) that interacts with actin as well as with cytoskeletal proteins that include TLN1, vinculin, and/or myosin II (10, 54, 78). However, the actions of calpain and talin in the experiments reported here are necessarily unrelated to TEM8, which is not expressed in RAW264.7 cells (16, 79).
FP59, a fusion of the highly toxic ADP ribosylation domain of Pseudomonas aeruginosa exotoxin A to the PA-binding domain of LF (80), has been used extensively as an anthrax toxin surrogate effector protein to identify PA receptors and in studies of PA-mediated toxin internalization using cell death as an end point (12, 14, 15, 77). However, during our investigations we observed that, although uptake of PA bound to FP59 was reduced by calpain inhibition, as was uptake of PA bound to LF (Fig. S6), calpain inhibition by MD28170 did not affect the survival of cells exposed to the PA-FP59 complex. Moreover, inhibition by MD28170 increased cellular susceptibility to Pseudomonas aeruginosa exotoxin A (i.e., PE toxin) (Fig. S6B), raising the prospect that calpain inhibition may increase the activity of the Pseudomonas aeruginosa ADP ribosyltransferase and concurrently, as shown by our results, reduce the entry of PA. Consistent with this hypothesis, the bovine poly-ADP ribosyltransferase protein has been shown to be a target for calpain proteolysis (81). Thus, disparate actions of calpains on PA-mediated entry of FP59 and the lethality of the FP59 moiety may have prevented the effects of calpains on PA entry from being detected during earlier screens for host genes in assays that tested the lethality of PA-FP59 (15). In any case, the divergent effects of calpain inhibition on PA-LF vs. PA-FP59 emphasize the perils of inferences (77) made about host gene effects on anthrax toxicity using hybrid toxins that may enter cells similarly but which kill cells by different mechanisms.
Calpain inhibitors such as MD28170 have been tested in preclinical animal studies, where they have been shown to prevent motor disturbances in rats subjected to spinal cord injury (34) and also to prevent amyloid β-induced neuronal death in mice (33). We suggest that such inhibitors may be of value in reducing the cellular lethality of exposure to anthrax toxin and perhaps of exposure of other microbial toxins that also may depend on the mechanisms we have identified.
Materials and Methods
Cell Culture, Transfection, and Infection.
RAW264.7 mouse macrophage cells were maintained in DMEM (Invitrogen) supplemented with FBS (HyClone) at 10% (vol/vol) and 100 units/mL penicillin and 100 μg/mL streptomycin. Plasmid DNA transfection was performed using the FuGENE6 transfection reagent (Roche). Viral Infection was performed using lentiviral-based methods (15). The lentiviral constructs pGIPZ targeting capn2 and pLKO.1 targeting TLN1 were purchased from Open Biosystems and Sigma, respectively. RAW264.7 cells were infected with shRNA-expressing lentivirus, and the infected cells were selected by 4 µg/mL puromycin. EGFP-expression vectors for TLN1 (wild type and L432G) were purchased from Addgene Inc. (Addgene plasmid 26724 and 26725, respectively) (32), and the transfected cells were selected in medium containing 1 mg/mL G418 (Geneticin; Life Technologies).
Chemicals and Reagents.
PA and LF were purchased from List Biological Laboratories. Calpain inhibitors III (MDL28170) and LatA were purchased from Calbiochem and Sigma, respectively. Batches of PA and LF had different potency, accounting for the different amounts used in different experiments, each of which included control data for the same toxin batch. The fluorescently labeled monoclonal antibody anti–β1 integrin-APC (HMβ1–1) was purchased from BioLegend. Anti–N-terminal MEK-2 and anti-PA antibodies were purchased from Santa Cruz Biotechnology, and anti-calpastatin (CAST) and anti-CMG2 antibodies were purchased from Cell Signaling Technology and R&D Systems, respectively.
EST Library Screening.
Screening of the EST library was performed as described by Lu et al. (15) with a slight modification. Briefly, RAW264.7tTA cells established by introducing a gene encoding the tetracycline-repressed transactivator (tTA) were infected with the pLEST-based human EST library reported in Lu et al. (15). A pool of cells expressing ESTs was treated with 500 ng/mL PA-LF for 2 d and was grown in toxin-free medium for 10 d. Surviving clones were picked and expanded. Genomic DNA was extracted from each clone using the DNeasy kit (Qiagen), and the EST in each clone was identified by PCR amplification and sequencing.
Expression of the CAST EST in Naive Cells.
pLEST-CAST lentivirus was constructed by PCR amplification of the CAST EST insert identified in clone 3-12 using the primers ESTF_NheI and ESTR_NheI (15), and the amplified DNA was cloned using the pLEST vector (15). Naive RAW264.7tTA cells were infected with lentivirus containing pLEST-CAST or the empty pLEST vector, and the infected cells were cultured in the presence of 800 μg/mL G418 for 2 wk to prevent the growth of cells lacking the pLEST constructs prior to testing the cell pool for sensitivity to anthrax toxin.
Calpain Activity Assay.
Calpain activity in total cell lysates was determined using a calpain activity assay kit (Biovision) according to the manufacturer’s instructions. Briefly, RAW264.7 cells were collected and lysed in Extraction buffer (Biovision). Equal amounts of protein were added to the calpain substrate Ac-LLY-AFC (Biovision). Fluorescence intensity indicating calpain activity was measured at 400 nm excitation and 505 nm emission wavelengths using a microplate reader (Infinite 200;TECAN).
Quantitative Real-Time PCR.
Total RNA was isolated using the RNeasy kit (Qiagen) and then was reverse-transcribed using M-MLV reverse transcriptase (Invitrogen) and random primers. Real-time PCR was performed for EGFP using the Bio-Rad iCycler iQ system (Bio-Rad) with SYBR green detection and the following primers: CAST: 5′-TCGCAAGTTGGTGGTACAAG for CAST1 and 5′-CTCCCCAAACTTGCTGCTT for CAST2; capn2: 5′-TTGACAATTTTGTGCGGTGT for capn2-1 and 5′-GAAAAACTCAGCCACGAAGC for capn2-2; TLN1: 5′-AATGGCTCCCATCCTGTCTCCTTT for tln1-1 and 5′-TCTGCTTCACATACTCCTTGGGCA for tln1-2; β-actin: 5′-CTAAGGCCAACCGTGAAAAG for actin1 and 5′-ACCAGAGGCATACAGGGACA for actin2; EGFP: 5′-GCAGAAGAACGGCATCAAGGT for EGFP1 and 5′-ACGAACTCCAGCAGGACCATG for EGFP2. Fluorescence threshold values were calculated using iCycler iQ system software.
Toxin Treatment and Cell-Viability Assay.
Cells were seeded in a 96-well plate at a density of 2 × 105 cells/mL 1 d before toxin treatment. Various concentrations of PA combined with a fixed concentration of LF (200 ng/mL) were added to the wells, and cells were incubated for 4 h at 37 °C. Cell viability was measured by adding 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma) to a final concentration of 1 mg/mL for 2 h at 37 °C. The supernatant was removed carefully before the addition of 50 µL of lysis solution [10% (wt/vol) SDS in 0.01 N HCl]. After cell lysates were incubated for 30 min at 37 °C, 100 µL of PBS was added. Spectrophotometer readings at 570 nm were determined using a microplate reader (Infinite 200;TECAN). Cell viability was normalized to wells lacking toxin. Each data point shown for MTT assays indicates the mean ± SD value obtained in triplicate assays done in a representative experiment. At least three such experiments were routinely carried out.
Immunofluorescence Microscopy.
PA protein was labeled with Alexa-Fluor 488 using the protein-labeling kit (A10235; Molecular Probes, Inc.). The potency of PA after labeling was fully retained as indicated by the MTT assay. Immunostaining was performed as follows. RAW264.7 cells were preincubated with or without 80 µM MDL28170 for 1 h in serum-free Iscove’s modified Dulbecco medium (Invitrogen) and then were treated with 1 µg/mL PA–Alexa-Fluor 488 in the presence of 0.5 µg/mL anti–β1 integrin-APC (HMβ1–1) antibody for 20 min at 37 °C. Cells were washed thee times in PBS, fixed in 4% (wt/vol) paraformaldehyde, and examined under a fluorescence microscope (DM5500 B; Leica). The intensities of cytoplasmic PA and β1-integrin signals were quantified by ImageJ analysis (US National Institutes of Health); pixel-intensity values were analyzed in at least 30 cells per condition. The intensities of background determinations from identically sized areas of cytoplasm of untreated control cells were subtracted from experimental values. The numbers shown are the arithmetic means of the deltas and indicate the SEM.
Biochemical Assay of PA Binding and Internalization.
Cells were exposed to 1 µg/mL of PA at 4 °C for 1 h for the binding assay and then were shifted to 37 °C for the indicated time for the internalization kinetics assay. After being washed three times with cold PBS and lysed in RIPA buffer containing a protease inhibitor mixture (Roche), cell lysates were quantified using a BCA protein assay kit (Pierce) and were loaded onto 4–12% denaturing gels (Criterion XT Precast Gel; Bio-Rad). After electrophoresis, and then transfer overnight to nitrocellulose membranes, membranes were probed with anti-PA or anti–β-actin antibodies. Quantitative Western blot analysis of the bands was done using the VersaDoc 1000 instrument (Bio-Rad) or Odyssey infrared imaging system (LI-COR Biosciences).
Cytoskeleton Isolation.
RAW264.7 cells were lysed with cytoskeleton (CSK) buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2, and 1% Triton X-100) containing protease and phosphatase inhibitors (Roche) on ice for 30 min. Triton X-100–insoluble fractions were pelleted by centrifugation at 16,000 × g for 5 min, washed with CSK buffer, and resuspended in Laemmli buffer.
Statistical Analysis.
P values were calculated using a two-tailed Student t test assuming equal variance or two-way ANOVA for comparisons between multiple cell-line groups. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Anna Huttenlocher for providing pEGFP-C1-talin-1 plasmids. This work received support from Award HDTRA1-06-C-0039 from the Department of Defense Chemical and Biological Defense Program through the Defense Threat Reduction Agency.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316852110/-/DCSupplemental.
References
- 1.Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195(7):1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartlova A, Cerveny L, Hubalek M, Krocova Z, Stulik J. Membrane rafts: A potential gateway for bacterial entry into host cells. Microbiol Immunol. 2010;54(4):237–245. doi: 10.1111/j.1348-0421.2010.00198.x. [DOI] [PubMed] [Google Scholar]
- 3.Mañes S, del Real G, Martínez-A C. Pathogens: Raft hijackers. Nat Rev Immunol. 2003;3(7):557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 4.Lin AE, Guttman JA. Hijacking the endocytic machinery by microbial pathogens. Protoplasma. 2010;244(1-4):75–90. doi: 10.1007/s00709-010-0164-2. [DOI] [PubMed] [Google Scholar]
- 5.Gupta VR, et al. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 2008;4(5):e1000073. doi: 10.1371/journal.ppat.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kounnas MZ, et al. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992;267(18):12420–12423. [PubMed] [Google Scholar]
- 7.Spooner RA, Lord JM. How ricin and Shiga toxin reach the cytosol of target cells: Retrotranslocation from the endoplasmic reticulum. Curr Top Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 8.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160(3):321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Goot G, Young JA. Receptors of anthrax toxin and cell entry. Mol Aspects Med. 2009;30(6):406–412. doi: 10.1016/j.mam.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrami L, Bischofberger M, Kunz B, Groux R, van der Goot FG. Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 2010;6(3):e1000792. doi: 10.1371/journal.ppat.1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100(9):5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA. 2009;106(30):12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414(6860):225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124(6):1141–1154. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 15.Lu Q, Wei W, Kowalski PE, Chang AC, Cohen SN. EST-based genome-wide gene inactivation identifies ARAP3 as a host protein affecting cellular susceptibility to anthrax toxin. Proc Natl Acad Sci USA. 2004;101(49):17246–17251. doi: 10.1073/pnas.0407794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martchenko M, Jeong SY, Cohen SN. Heterodimeric integrin complexes containing beta1-integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci USA. 2010;107(35):15583–15588. doi: 10.1073/pnas.1010145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: An anthrax toxin receptor. Proc Natl Acad Sci USA. 2004;101(17):6367–6372. doi: 10.1073/pnas.0401506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muehlbauer SM, et al. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007;6(6):758–766. doi: 10.4161/cc.6.6.3991. [DOI] [PubMed] [Google Scholar]
- 19.Reig N, et al. Maturation modulates caspase-1-independent responses of dendritic cells to Anthrax lethal toxin. Cell Microbiol. 2008;10(5):1190–1207. doi: 10.1111/j.1462-5822.2008.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30(6):439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59(5):549–566. doi: 10.1538/expanim.59.549. [DOI] [PubMed] [Google Scholar]
- 23.Croall DE, Ersfeld K. The calpains: Modular designs and functional diversity. Genome Biol. 2007;8(6):218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebart MC, Benyamin Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006;273(15):3415–3426. doi: 10.1111/j.1742-4658.2006.05350.x. [DOI] [PubMed] [Google Scholar]
- 25.Jánossy J, et al. Calpain as a multi-site regulator of cell cycle. Biochem Pharmacol. 2004;67(8):1513–1521. doi: 10.1016/j.bcp.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Cordon G, Nie W, Schmidt D, Tzipori S, Feng H. Involvement of host calpain in the invasion of Cryptosporidium parvum. Microbes Infect. 2011;13(1):103–107. doi: 10.1016/j.micinf.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozym RA, Morosky SA, Kim KS, Cherry S, Coyne CB. Release of intracellular calcium stores facilitates coxsackievirus entry into polarized endothelial cells. PLoS Pathog. 2010;6(10):e1001135. doi: 10.1371/journal.ppat.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmeck B, et al. Streptococcus pneumoniae-induced caspase 6-dependent apoptosis in lung epithelium. Infect Immun. 2004;72(9):4940–4947. doi: 10.1128/IAI.72.9.4940-4947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SY, Lee MS, Cherla RP, Tesh VL. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008;10(3):770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 30.Müller A, et al. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999;18(2):339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy CL, Smith DJ, Lyras D, Chakravorty A, Rood JI. Programmed cellular necrosis mediated by the pore-forming alpha-toxin from Clostridium septicum. PLoS Pathog. 2009;5(7):e1000516. doi: 10.1371/journal.ppat.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6(10):977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 33.Lopes JP, Oliveira CR, Agostinho P. Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: The role of Cdk5. Aging Cell. 2010;9(1):64–77. doi: 10.1111/j.1474-9726.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- 34.Arataki S, et al. Calpain inhibitors prevent neuronal cell death and ameliorate motor disturbances after compression-induced spinal cord injury in rats. J Neurotrauma. 2005;22(3):398–406. doi: 10.1089/neu.2005.22.398. [DOI] [PubMed] [Google Scholar]
- 35.Wang CH, et al. Protective effect of MDL28170 against thioacetamide-induced acute liver failure in mice. J Biomed Sci. 2004;11(5):571–578. doi: 10.1007/BF02256121. [DOI] [PubMed] [Google Scholar]
- 36.Chang AC, et al. Phenotype-based identification of host genes required for replication of African swine fever virus. J Virol. 2006;80(17):8705–8717. doi: 10.1128/JVI.00475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccone ME, et al. Identification of cellular genes affecting the infectivity of foot-and-mouth disease virus. J Virol. 2009;83(13):6681–6688. doi: 10.1128/JVI.01729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991;16(4):150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 39.Vitale G, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKS and induces tyrosine/threonine phosphorylation of MAPKS in cultured macrophages. J Appl Microbiol. 1999;87(2):288. doi: 10.1046/j.1365-2672.1999.00893.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278(7):5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 41.Deuquet J, Lausch E, Superti-Furga A, van der Goot FG. The dark sides of capillary morphogenesis gene 2. EMBO J. 2012;31(1):3–13. doi: 10.1038/emboj.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milne JC, Furlong D, Hanna PC, Wall JS, Collier RJ. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem. 1994;269(32):20607–20612. [PubMed] [Google Scholar]
- 43.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299(1):179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Azam M, et al. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol Cell Biol. 2001;21(6):2213–2220. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morford LA, et al. Calpain II colocalizes with detergent-insoluble rafts on human and Jurkat T-cells. Biochem Biophys Res Commun. 2002;295(2):540–546. doi: 10.1016/s0006-291x(02)00676-9. [DOI] [PubMed] [Google Scholar]
- 46.Nuzzi PA, Senetar MA, Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell. 2007;18(3):795–805. doi: 10.1091/mbc.E06-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassilieva EV, Gerner-Smidt K, Ivanov AI, Nusrat A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G965–G976. doi: 10.1152/ajpgi.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco SJ, Huttenlocher A. Regulating cell migration: Calpains make the cut. J Cell Sci. 2005;118(Pt 17):3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 49.Cortesio CL, Boateng LR, Piazza TM, Bennin DA, Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J Biol Chem. 2011;286(12):9998–10006. doi: 10.1074/jbc.M110.187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, et al. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med. 2010;207(11):2421–2437. doi: 10.1084/jem.20100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayal A, Webb DJ, Horwitz AF. Talin: An emerging focal point of adhesion dynamics. Curr Opin Cell Biol. 2004;16(1):94–98. doi: 10.1016/j.ceb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Goult BT, et al. RIAM and vinculin binding to talin are mutually exclusive and regulate adhesion assembly and turnover. J Biol Chem. 2013;288(12):8238–8249. doi: 10.1074/jbc.M112.438119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castanon I, et al. Anthrax toxin receptor 2a controls mitotic spindle positioning. Nat Cell Biol. 2013;15(1):28–39. doi: 10.1038/ncb2632. [DOI] [PubMed] [Google Scholar]
- 54.Go MY, Chow EM, Mogridge J. The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun. 2009;77(1):52–59. doi: 10.1128/IAI.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woronowicz K, et al. The platelet actin cytoskeleton associates with SNAREs and participates in alpha-granule secretion. Biochemistry. 2010;49(21):4533–4542. doi: 10.1021/bi100541t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown S, Levinson W, Spudich JA. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- 57.Taylor MJ, Lampe M, Merrifield CJ. A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis. PLoS Biol. 2012;10(4):e1001302. doi: 10.1371/journal.pbio.1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13(9):1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu C, et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 2010;29(21):3593–3606. doi: 10.1038/emboj.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bridgewater RE, Norman JC, Caswell PT. Integrin trafficking at a glance. J Cell Sci. 2012;125(Pt 16):3695–3701. doi: 10.1242/jcs.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 62.Coué M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213(2):316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 63.Moyen C, et al. Involvement of micro-calpain (CAPN 1) in muscle cell differentiation. Int J Biochem Cell Biol. 2004;36(4):728–743. doi: 10.1016/S1357-2725(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 64.Dean P, Mühlen S, Quitard S, Kenny B. The bacterial effectors EspG and EspG2 induce a destructive calpain activity that is kept in check by the co-delivered Tir effector. Cell Microbiol. 2010;12(9):1308–1321. doi: 10.1111/j.1462-5822.2010.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young VB, Falkow S, Schoolnik GK. The invasin protein of Yersinia enterocolitica: Internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;116(1):197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7(9):894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 67.Humphreys D, Davidson A, Hume PJ, Koronakis V. Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe. 2012;11(2):129–139. doi: 10.1016/j.chom.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumitomo T, et al. Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier. J Biol Chem. 2011;286(4):2750–2761. doi: 10.1074/jbc.M110.171504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergounioux J, et al. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium’s epithelial niche. Cell Host Microbe. 2012;11(3):240–252. doi: 10.1016/j.chom.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Briñas L, Vassilopoulos S, Bonne G, Guicheney P, Bitoun M. Role of dynamin 2 in the disassembly of focal adhesions. J Mol Med (Berl) 2013;91(7):803–809. doi: 10.1007/s00109-013-1040-2. [DOI] [PubMed] [Google Scholar]
- 71.Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol. 2012;24(5):569–581. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Alexander RA, et al. VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc Res. 2012;94(1):125–135. doi: 10.1093/cvr/cvs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du J, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA. 2011;108(23):9466–9471. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122(Pt 2):187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 75.Squires RC, Muehlbauer SM, Brojatsch J. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J Biol Chem. 2007;282(47):34260–34267. doi: 10.1074/jbc.M705687200. [DOI] [PubMed] [Google Scholar]
- 76.Alileche A, Squires RC, Muehlbauer SM, Lisanti MP, Brojatsch J. Mitochondrial impairment is a critical event in anthrax lethal toxin-induced cytolysis of murine macrophages. Cell Cycle. 2006;5(1):100–106. doi: 10.4161/cc.5.1.2283. [DOI] [PubMed] [Google Scholar]
- 77.Liu S, Zhang Y, Hoover B, Leppla SH. The receptors that mediate the direct lethality of anthrax toxin. Toxins (Basel) 2013;5(1):1–8. doi: 10.3390/toxins5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garlick KM, Mogridge J. Direct interaction between anthrax toxin receptor 1 and the actin cytoskeleton. Biochemistry. 2009;48(44):10577–10581. doi: 10.1021/bi9015296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dal Molin F, et al. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006;25(22):5405–5413. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arora N, Klimpel KR, Singh Y, Leppla SH. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J Biol Chem. 1992;267(22):15542–15548. [PubMed] [Google Scholar]
- 81.Buki KG, Bauer PI, Kun E. Isolation and identification of a proteinase from calf thymus that cleaves poly(ADP-ribose) polymerase and histone H1. Biochim Biophys Acta. 1997;1338(1):100–106. doi: 10.1016/s0167-4838(96)00189-6. [DOI] [PubMed] [Google Scholar]
- 82.Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583(8):1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187(5):733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.