Significance
Stem cells and the ovarian steroids estrogen and progesterone are essential for leiomyoma tissue growth. The underlying mechanisms are unknown, particularly because leiomyoma stem cells are deficient in estrogen and progesterone receptors. Expression of these receptors is much higher in surrounding mature myometrial or leiomyoma smooth muscle cells. Here, we demonstrate that wingless-type (WNT) acts as a paracrine signal from estrogen/progesterone receptor-rich mature cells to activate the canonical β-catenin pathway in leiomyoma stem cells. Our findings suggest a paracrine role for the canonical WNT pathway in the growth of leiomyoma tumor.
Keywords: WNT/β-catenin signaling, paracrine signaling, tumor biology
Abstract
Uterine leiomyomas are extremely common estrogen and progesterone-dependent tumors of the myometrium and cause irregular uterine bleeding, severe anemia, and recurrent pregnancy loss in 15–30% of reproductive-age women. Each leiomyoma is thought to arise from a single mutated myometrial smooth muscle stem cell. Leiomyoma side-population (LMSP) cells comprising 1% of all tumor cells and displaying tumor-initiating stem cell characteristics are essential for estrogen- and progesterone-dependent in vivo growth of tumors, although they have remarkably lower estrogen/progesterone receptor levels than mature myometrial or leiomyoma cells. However, how estrogen/progesterone regulates the growth of LMSP cells via mature neighboring cells is unknown. Here, we demonstrate a critical paracrine role of the wingless-type (WNT)/β-catenin pathway in estrogen/progesterone-dependent tumorigenesis, involving LMSP and differentiated myometrial or leiomyoma cells. Estrogen/progesterone treatment of mature myometrial cells induced expression of WNT11 and WNT16, which remained constitutively elevated in leiomyoma tissues. In LMSP cells cocultured with mature myometrial cells, estrogen-progesterone selectively induced nuclear translocation of β-catenin and induced transcriptional activity of its heterodimeric partner T-cell factor and their target gene AXIN2, leading to the proliferation of LMSP cells. This effect could be blocked by a WNT antagonist. Ectopic expression of inhibitor of β-catenin and T-cell factor 4 in LMSP cells, but not in mature leiomyoma cells, blocked the estrogen/progesterone-dependent growth of human tumors in vivo. We uncovered a paracrine role of the WNT/β-catenin pathway that enables mature myometrial or leiomyoma cells to send mitogenic signals to neighboring tissue stem cells in response to estrogen and progesterone, leading to the growth of uterine leiomyomas.
Uterine leiomyomas (LM), the most common pelvic tumor in women, are benign smooth muscle tumors originating from the myometrium (1). Uterine LMs occur in ∼70% of white women and more than 80% of African-American women by age 50 y and cause excessive uterine bleeding, anemia, recurrent pregnancy loss, preterm labor, pelvic discomfort, and urinary incontinence in ∼15–30% of cases (2, 3). They are the most common indication for hysterectomies (2, 4). Despite their high prevalence, the cellular and molecular origins of uterine LM remain poorly understood.
Somatic stem cells are a subset of cells residing in normal adult tissues that, through asymmetric division, retain their ability to self-renew while producing daughter cells that go on to differentiate and play a role in tissue regeneration and repair (5, 6). Likewise, tumor-initiating cells are a subset of cells within a tumor cell population, which also through asymmetric division retain the ability to sustain tumors (7, 8). The side population (SP) cell phenotype was described first in bone marrow, where a somatic stem cell population was identified based on its ability to extrude the DNA-binding dye Hoechst 33342, a phenomenon that is associated with the expression of ATP-binding cassette transporter G2 (8). The SP phenotype is thought to be a universal marker of somatic stem cells and has been used to isolate them from many adult tissues, such as the myometrium, endometrium, and mammary gland (9–12). We and another group have reported that SP cells from human LM exhibit key features of the tumor-initiating cells (13, 14).
It has been proposed that each LM originates from a single transformed somatic stem cell of the myometrium in an ovarian steroid-dependent manner (15); however, this suggestion has not been proven definitively. Human and mouse myometrial (MM) tissues contain multipotent somatic stem cells, a subset of tissue responsible for regeneration in an estrogen-and-progesterone (E+P)-dependent fashion (5, 10, 16). E+P-dependent in vivo growth of human LM tissue requires the presence of these multipotent somatic stem cells (13, 14). Compared with the main, mature LM cell population or normal MM cells, however, LM stem cells express remarkably lower levels of estrogen and progesterone receptors (13, 14), and their growth requires the presence of mature MM or LM cells with higher levels of the steroid receptors and their ligands. This requirement suggests that steroid hormone action on LM stem cells is mediated via mature MM cells (tumor initiation) or mature LM cells (growth maintenance) in a paracrine fashion. It is likely that this paracrine interaction with the surrounding cells supports the self-renewal of LM stem cells (10, 14).
β-Catenin exists in multiple cellular pools and serves diverse functions. In its basal state, β-catenin is localized mainly to the intercellular (adherens) junctions, where it is bound to the cytoplasmic domain of E-cadherin and plays a role in the regulation of cell–cell adhesion (17). In the presence of canonical wingless-type (WNT) MMTV integration site family signaling or as a consequence of the destabilization of the cadherin–catenin complex, activated β-catenin translocates to the nucleus and binds to the T-cell factor (TCF)/lymphocyte enhancer factor (LEF) family of transcription factors, resulting in the expression of specific target genes (17, 18). Because many β-catenin target genes, such as c-Myc, WISP1, and cyclin D1, are involved in cell proliferation, β-catenin signaling plays an important role in development and neoplasia (17). Selective overexpression of constitutively activated β-catenin in uterine mesenchyme during embryonic development and in adults gives rise to LM-like tumors in the uterus of all female mice (19), suggesting that signaling by WNT/β-catenin may play a role in somatic stem cell function in the myometrium and in uterine LM tissue. Selective deletion of β-catenin in uterine mesenchyme during the embryonic development significantly reduces uterine size and replaces it with adipocytes, thus completely disrupting the normal MM smooth muscle differentiation and regeneration (16). This effect suggests that β-catenin plays a key role in stem cell renewal and differentiation to the smooth muscle phenotype observed in MM and LM tissues (5). The size and number of β-catenin–driven LM-like tumors increases with parity in mice, suggesting that steroid hormones may interact with activated β-catenin to accelerate tumorigenesis (19). Moreover, activated β-catenin induced the expression of TGF-β3, which was shown to induce proliferation and ECM formation in human LM tissue (19).
These observations indicate that unique interactions exist between β-catenin activation, estrogen and progesterone action, and growth via stem cell renewal; we hypothesize that these interactions ultimately give rise to clonal expansion of uterine LM. Given that WNT signaling is known to control cell-fate decisions throughout development and in adult stem cells, we further posit that the relevant target of β-catenin–mediated tumorigenesis is a progenitor cell type that gives rise to LM cells rather than a mature smooth muscle cell. Here, we uncover a paracrine role of WNT ligand, originating from mature MM or LM smooth muscle cells in response to E+P, which then acts on adjacent LM stem cell-like tumor-initiating cells to stimulate self-renewal and proliferation, eventually leading to tumor growth.
Results
Inhibitor of β-Catenin and T-Cell Factor 4 Inhibits the Generation of Human LM-Like Tumors in Immunodeficient Mice.
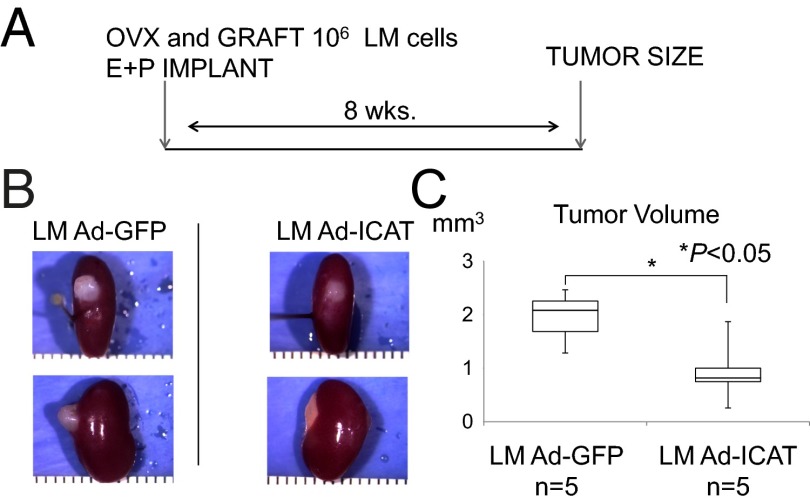
We assessed whether the β-catenin pathway is required for E+P-dependent tissue growth in human LM xenografts. Primary cultures were prepared from human whole LM tissues and were transfected with adenoviral vectors expressing inhibitor of β-catenin and TCF4 (Ad-ICAT) or GFP (Ad-GFP), as control (Fig. 1A). One million LM cells were transplanted under the kidney capsule of ovariectomized immunosuppressed mice, which then were treated with E+P for 8 wk (SI Materials and Methods). Each xenografted cell pellet was analyzed for tumor formation (Fig. 1B) and tumor volume (Fig. 1C). Although cells expressing Ad-ICAT were capable of generating human LM tumors, these tumors were significantly smaller (0.94 ± 0.59 mm3) than those derived from LM cells infected with Ad-GFP (1.95 ± 0.21 mm3; P < 0.05; n = 5 paired patient samples; Fig. 1 A–C).
Fig. 1.
Blocking WNT activity inhibits the growth of LM tumors in NOD-SCID (IL2Rγnull) mice. (A) Experimental design and hormone treatment. Mice were ovariectomized and xenotransplanted with LM cells infected by Ad-GFP or Ad-ICAT and then were treated with s.c. implantation of E2 and P4 pellets. The mice underwent nephrectomy 8 wk after xenotransplantation. (B) Macroscopic visualization of the transplanted site 8 wk after xenotransplantation. (C) Xenografts were analyzed in terms of tumor volume. Error bars indicate SD; *P < 0.05; n = 5 paired patient samples.
The β-Catenin Pathway Is Intact in MM and LM Cells.
Prior studies using embryological models indicated that LiCl stimulates β-catenin activation by inhibiting glycogen synthase kinase-3β (GSK-3β) activity directly (20, 21). To demonstrate the integrity and function of the β-catenin pathway in MM and LM cells, we investigated the effects of LiCl on intracellular β-catenin levels and signaling. Members of the TCF/LEF transcription factor family and β-catenin mediate WNT target gene transcription in the nucleus; the basal activity of this pathway in MM and LM cells is unknown. To assess the activity of the β-catenin signaling pathway, we used a reporter gene construct containing a β-catenin/TCF/LEF response element and performed dual-luciferase reporter assays.
Cells were incubated with LiCl (30 mM) for 24 h. ICAT pull-down assays revealed that active β-catenin levels were increased significantly in LiCl-treated MM and LM cells (Fig. S1A). LiCl-induced β-catenin activation also led to increased transcription from the TCF/LEF reporter gene with an average twofold increase in luciferase activity in MM and LM cells compared with NaCl-treated controls (n = 6, P < 0.05) (Fig. S1B). Curiously, LM and MM primary cultures appear to manifest a substantial baseline of GST-ICAT–precipitable β-catenin. Activation of β-catenin signaling has been observed spontaneously in a number of primary cell cultures (22, 23), suggesting that it may be a common feature of adapting primary cells to in vitro cultures.
We next tested whether E+P treatment stimulates β-catenin–dependent TCF/LEF reporter activity in MM and LM cells. E+P treatment increased TCF/LEF reporter activity by 1.5-fold in MM cells, whereas no E+P induction was observed in LM cells (n = 6 in each group; P < 0.05) (Fig. S1C). These results indicate that the β-catenin signaling pathway leading to target gene transcription is intact in both MM and LM cells, but that β-catenin signaling is further activated by E+P treatment only in MM cells. This result suggests that the β-catenin pathway is constitutively active in the majority of LM cells and may play an important role in LM. In support of these findings, adenoviral expression of an HA-tagged nondegradable form of β-catenin (Ad-S37A–β-catenin–HA) (Fig. S2 A and B) activated β-catenin signaling and target reporter gene expression in both MM and LM cells (Fig. S2) in the TCF/LEF dual-luciferase reporter assay system (Fig. S2C).
E+P Stimulates WNT Signaling Activity in Cocultures of MM and LMSP Cells.
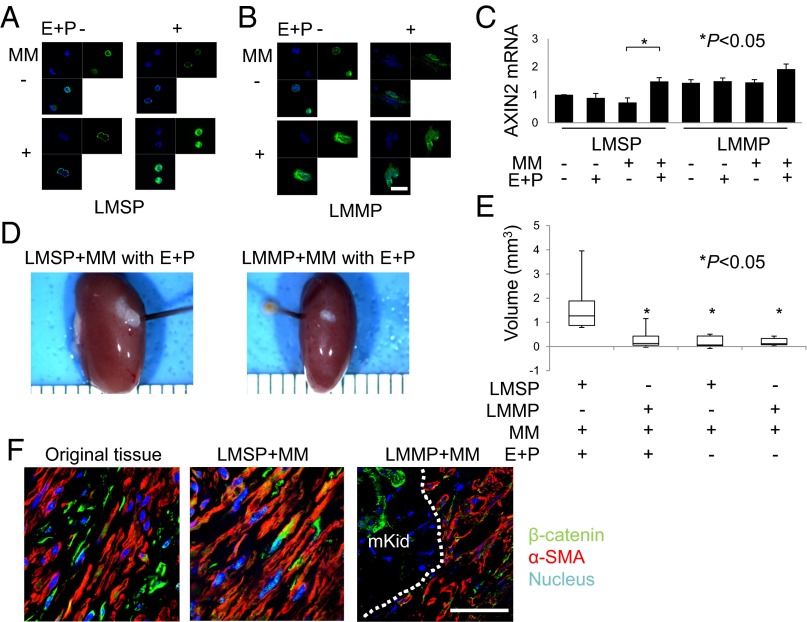
Activation of the WNT signaling pathway results in nuclear translocation of β-catenin and heterodimer formation with TCF to activate target gene transcription (17, 24). We examined the paracrine effects of E+P on β-catenin activation in Transwell cocultures of MM cells with LMSP or LM mature population (LMMP) cells (Fig. 2 A and B). In LMMP cells, β-catenin was localized to the nucleus constitutively regardless of E+P treatment or coculture with MM cells in Transwells (Fig. 2B). In contrast, E+P treatment alone did not support β-catenin nuclear translocation in LMSP cells; in these cells, E+P treatment and coculture with MM cells were necessary for nuclear localization of β-catenin (Fig. 2A). In Transwell cocultures both E+P treatment and MM cells also were required for the induction of the β-catenin target gene AXIN2 in LMSP cells, whereas AXIN2 expression was constitutively high under all culture conditions in LMMP cells (Fig. 2C). These results suggest the presence of paracrine signaling from MM cells to LMSP cells upon E+P stimulation.
Fig. 2.
E+P indirectly increases WNT signaling activity in LMSP cells. (A and B) Subcellular localization of β-catenin in LMSP or LMMP cells cultured in Transwells with or without MM coculture and in the presence or absence of E+P treatment. Indirect immunofluorescence was performed on LMSP and LMMP cells using anti–β-catenin antibody (1:100; green), followed by Hoechst nuclear counterstaining (blue). The overlay of β-catenin (green) and Hoechst (blue) is shown also. (Scale bar, 20 µm.) (C) Total RNA was extracted from LMSP or LMMP cells cultured in Transwells with or without MM to quantify the expression of AXIN2 by real-time quantitative PCR. Results are expressed as the mean ± SD of AXIN2 relative to GAPDH from four independent experiments. *P < 0.05. (D) Freshly isolated LMSP cells mixed with freshly isolated MM cells are able to generate larger LM tumors than mixes of LMMP-MM cells, with tumor sizes dependent on E+P treatment. Macroscopic visualization of the transplanted site 8 wk after xenotransplantation is shown. (E) Tumor volume of xenografts after 8 wk. Data are shown as mean ± SD. *P < 0.05. (F) Original tissue and generated tumor were analyzed by immunofluorescence. Immunostaining was performed to confirm the presence of β-catenin and α-SMA. Nuclei were stained with DAPI (blue). mKid, mouse kidney. Dotted line indicates the border between mouse kidney and tumor. (Scale bar, 30 µm.)
To confirm the paracrine effect of MM cells on LMSP cells in vivo, we conducted xenotransplantation under the mouse kidney capsule. Mice inoculated with a freshly isolated single-cell suspension (100,000 cells) of whole myometrium (MM cells only) were used as negative controls, because these cells never have produced tumors in our laboratory (25). The largest tumors, which also had a robust growth pattern, were observed only with transplantation of 100,000 freshly isolated LMSP cells and 100,000 MM cells in E+P-treated mice (Fig. 2 D and E). Replacing LMSP cells with LMMP cells or withholding E+P treatment did not result in significant tumor growth (P < 0.05, Fig. 2E). The tumors were characterized by the presence of α-smooth muscle actin (αSMA)-positive cells under the renal capsule (Fig. 2F). We confirmed that the expression of β-catenin is similar to that in the original tissue from which LMSP or LMMP were harvested (Fig. 2F).
Disruption of WNT Signaling Suppresses Proliferative Effects of E+P on LMSP Cells in Coculture with MM Cells.
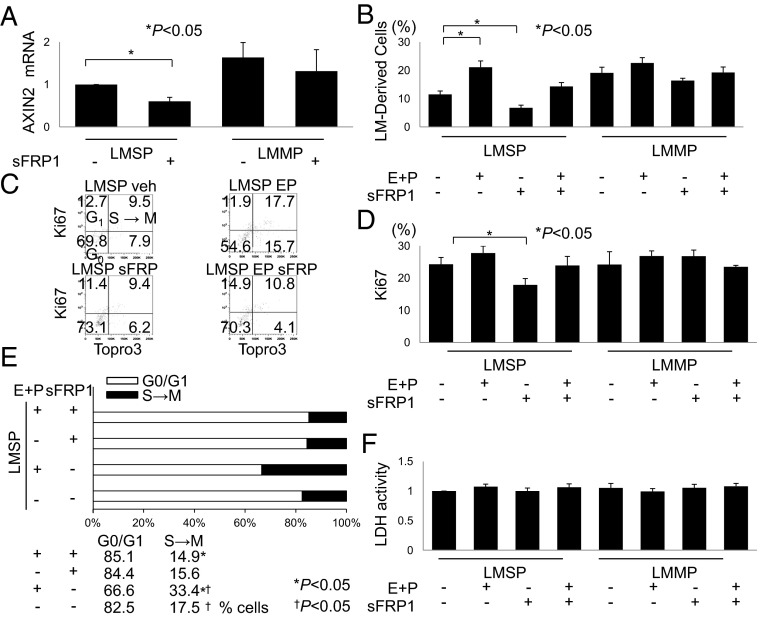
To determine whether the observed E+P enhancement of β-catenin activation and tumor growth in the mixed MM-LMSP coculture microenvironment was mediated by WNT secretion, we determined the effects of secreted frizzled-related protein 1 (sFRP1), a natural inhibitor of WNT, on LMSP cell growth. As shown in Fig. 3A, sFRP1 inhibited basal expression of the β-catenin target gene AXIN2 in LMSP cells but not in LMMP cells cocultured with MM cells. In the presence or absence of E+P, sFRP1 had little effect on the growth of LMMP cells in mixed coculture with MM cells. In contrast, sFRP1 disrupted E+P-dependent and independent growth of LMSP cells in coculture, implying that the effects of MM coculture on LMSP were WNT dependent (Fig. 3B). These findings also indicated that E+P-induced growth of LMSP cells in coculture with MM cells is mediated by WNT signaling. The lack of inhibitory effects of sFRP1 on AXIN2 expression and cell growth in LMMP cells in coculture with MM cells suggested that WNT secretion is not a crucial mechanism for β-catenin activation in this cell type (Fig. 3 A and B).
Fig. 3.
Inhibition of WNT signaling suppresses and E+P enhances the growth of LMSP cells cocultured with MM cells. (A) Effect of WNT signaling inhibition on LMSP-MM cocultured cells was determined by quantitative PCR of the WNT target gene AXIN2. Cells were seeded for mixed coculture as described previously and were treated with vehicle or sFRP1 (10 μg/mL) for 7 d. (B) Percentages of LMSP and LMMP cells among total cells were counted using PKH-26 dye and flow cytometry. Cells were seeded for mixed coculture and subjected to treatment with vehicle, 10−7 mol/L E+P, or sFRP1 (10 μg/mL) either alone or in combination with E+P (10−7 mol/L) for 7 d. Data are reported as the means ± SD. *P < 0.05. (C–E) Cell-cycle analyses of LMSP and LMMP cells were performed using flow cytometry with the Ki-67 proliferation marker and DNA-specific labeling with the TO-PRO III fluorochrome. The percentages of positive or negative cells for both markers are indicated in each quadrant of the dot plots. The percentage of cells positive for the Ki-67 antigen was decreased after incubation with the WNT blocker sFRP1. Incubation with E+P increased the percentage of cells at the S→M phase compared with vehicle treatment, whereas incubation with E+P and sFRP did not increase the percentage of S→M phase cells. n = 3. *P < 0.05; †P < 0.05. (F) LDH cell-viability assay. LMSP and LMMP cells were cultured with or without E+P and/or sFRP1 for 72 h, and media were assessed for LDH activity as described in SI Materials and Methods. Neither E+P treatment nor inhibition of WNT/β-catenin signaling promoted cell death. Results are expressed as levels of LDH activity (mean ± SD) from five independent experiments.
To assess cell-cycle stage and proliferation, we incubated mixed cocultures of LMSP and MM cells in the presence or absence of E+P or sFRP1 and then evaluated Ki-67 expression and DNA content (TO-PRO-3 iodide; Fig. 3C). We found that LMSP cells treated with E+P gave rise to the most abundant population that progressed to S, G2, and M phase (Fig. 3C). E+P did not stimulate Ki-67 in LMSP cells; however, sFRP1 decreased Ki-67 levels in LMSP but not in LMMP cocultures with MM (Fig. 3D). E+P did decrease the percentage of cells in G0/G1 and increase the percentage of S-phase cells, suggesting an acceleration of progression through the G1/S interphase (Fig. 3E). sFRP1 inhibited this E+P-dependent effect on cell-cycle progression, indicating involvement of WNT secretion (Fig. 3E). None of these interventions altered cellular lactose dehydrogenase (LDH) levels, ruling out a general toxic effect of sFRP1 (Fig. 3F). These experiments demonstrate a key role of WNT secretion in E+P-dependent β-catenin activation in and proliferation of LMSP cells cocultured with MM cells.
Wnt Expression of Frizzled and Low-Density Lipoprotein Receptor-Related Protein 5/6 Genes in LMSP and LMMP Cells.
Frizzled (FZD) is a family of G protein-coupled receptor proteins that serve as receptors in the signaling pathway. We systemically assessed the expression of all known members of the FZD gene family using RNA extracted from LMSP and LMMP cells. Real-time quantitative PCR analysis revealed expression of all known FZD genes in both cell types (Fig. S3 A–J). FZD1 and FZD7 mRNA levels were significantly higher in LMSP cells than in total LM cells or LMMP cells (Fig. S3 A and G). Low-density lipoprotein-related receptor protein 5 (LRP5) and LRP6 function as cell-surface receptors or, with FZD, as coreceptors for WNT that transduce the canonical WNT/β-catenin signaling cascade. Both LRP5 and LRP6 are expressed in LMSP and LMMP cells (Fig. S3 K and L). LRP5 expression is significantly lower in LMSP cells (P < 0.05) than in LMMP cells. Thus, both LMSP and LMMP cells express receptors for WNT, indicating that WNT secretion from surrounding cells can be received as a paracrine factor by LMSP and LMMP cells.
Estrogen and Progestin Activate WNT11 and WNT16 in MM Cells.
Because E+P have important roles in LM growth (25), we investigated the effects of E+P on a wider range of WNT signaling pathway genes. mRNA from E+P-treated and untreated MM cells was analyzed using Human WNT Signaling Pathway RT2 Profiler PCR Arrays. These PCR expression arrays focus on a selected panel of 84 genes related to WNT-mediated signal transduction. Compared with untreated control cells, E+P treatment induced expression of various members of the WNT pathway (Fig. S4).
Estrogen Plus Progestin Treatment Induces WNT Expression Selectively in MM Cells.
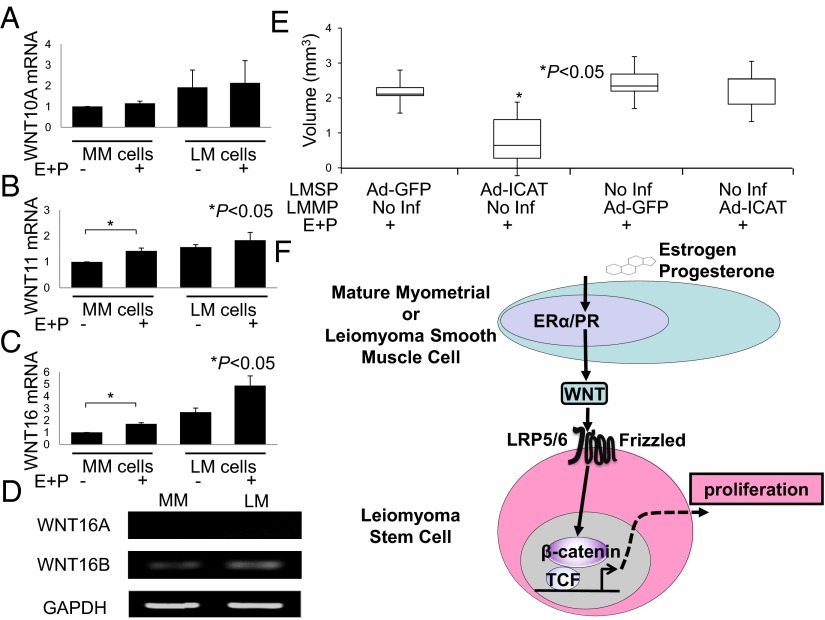
We used real-time quantitative PCR to verify the mRNA levels of the genes that showed a greater than twofold induction in MM cells after E+P treatment (Fig. 4 A–C and Fig. S5). Overall, WNT expression was higher in LM than in MM cells. E+P treatment induced WNT11 and WNT16 mRNA levels in MM cells but not in LM cells. In contrast, WNT10A induction was not verified in MM cells after E+P treatment by real-time PCR (Fig. 4A). Activation (Ad-S37A-β-cat) or inhibition (Ad-ICAT) of β-catenin signaling did not affect the expression of WNT genes in MM cells treated with E+P (Fig. S6). These results suggest that WNT expression in MM and LM cells is independent of β-catenin activity. Finally, RT-PCR showed that the WNT16 isoform WNT16B is expressed predominantly in both MM and LM cells (Fig. 4D).
Fig. 4.
E+P treatment alters the expression of WNT genes in MM cells. (A–C) MM and LM cells were treated with E+P or vehicle before isolation of RNA and evaluation of WNT expression by real-time quantitative PCR as described in SI Materials and Methods. Results represent the mean of a minimum of four independent experiments. Error bars indicate ± SD. *P < 0.05. (D) RT-PCR of WNT16B mRNA expression in MM and LM cells. GAPDH was used as a housekeeping gene control. (E) Ectopic ICAT expression in LMSP cells severely inhibited E+P-dependent tumor growth. *P < 0.05. (F) Interactions between ovarian steroids and WNT/β-catenin in LM tissue. Because estrogen receptor α (ERα) and progesterone receptor (PR) levels are remarkably higher in mature MM or LM cells than in stem cells, estrogen and progesterone likely affect LM stem cells in a paracrine fashion via estrogen receptor α and progesterone receptor expressed on adjacent mature MM or LM cells. In this model, estrogen and progesterone increase the secretion of WNT ligands from mature smooth muscle cells surrounding the stem cells. WNT via its FZD receptor then activates the β-catenin/TCF pathway in LM stem cells, inducing β-catenin target gene expression, cell proliferation, and tumorigenesis.
Selective β-Catenin Inhibition in LMSP Cells Blocks Tumor Growth.
As indicated above, ICAT inhibits β-catenin activity. To determine whether β-catenin activity in LMSP or LMMP cells is necessary for tumor growth in vivo, mixtures of freshly isolated LMSP and LMMP cells with or without adenoviral expression of ICAT were engrafted under the kidney capsule and assessed for E+P-dependent growth (Fig. 4E). Only ICAT expression in LMSP cells severely inhibited E+P-dependent tumor growth (P < 0.05). This in vivo experiment clearly demonstrates the critical role of β-catenin activity in LMSP cells in tumor growth.
Discussion
We demonstrated that WNT/β-catenin signaling plays a crucial role in mediating the paracrine effects of E+P on LMSP cells with stem/progenitor cell properties. We found that a redundant system involving a number of WNT ligands (e.g., WNT11 and WNT16B) and their cell-surface FZD family receptors (FZD1 and FZD7) comprises a paracrine pathway between mature MM cells or LMMP cells and LMSP cells (i.e., LM stem cells). In our model, stimulation of mature MM or LM cells with E+P leads to secretion of WNT, which in adjacent LM stem cells activates the nuclear translocation of β-catenin and its interaction with TCF family proteins in chromatin to induce the expression of genes supporting the proliferation and growth (Fig. 4F). Together with the strikingly lower expression of estrogen receptor α and progesterone receptor observed in LMSP cells compared with the mature cell populations (MM and LMMP) (13, 14), our data suggest that E+P primarily induces secretion of WNT ligands such as WNT11 and WNT16 from MM or LMMP cells, which have abundant expression of estrogen receptor α and progesterone receptor (Fig. 4F).
The WNT/β-catenin signaling pathway plays a critical role in both development and adult tissue homeostasis (26). Our model is consistent with previously described classical functions of the WNT/β-catenin pathway (26) in that it supports regeneration, proliferation, and in vivo tumor growth in LM stem cells. Recent studies also have implicated WNT/β-catenin signaling in LM and fibrogenesis (27, 28). One group used selective deletion of β-catenin in uterine mesenchyme during the embryonic development to show that β-catenin plays a key role in stem cell renewal and differentiation to the smooth muscle phenotype observed in MM and LM tissues (5). Our observations also are in agreement with previous reports describing that the size and number of β-catenin–driven LM-like tumors are associated with increases in steroid hormone production, TGF-β activation, and ECM formation (19, 29).
We found that the tumorigenic capacity of LMSP cells is enhanced with E+P stimulation, despite our previous work showing that LMSP cells are deficient in estrogen and progesterone receptors (13). In the mammary structures in humans and mice, the proliferation and repopulation of mammary stem cells are regulated by steroid hormones, particularly progesterone, despite the absence of estrogen and progesterone receptors (30–33). Thus, our current observations in LM stem cells may underlie a similar progesterone-dependent paracrine interaction between mature and progenitor cells of the breast that is crucial for breast development and perhaps carcinogenesis.
Uterine LM are monoclonal tumors, with growth of the neoplasm occurring via clonal expansion from a single progenitor cell; this characteristic raises the possibility for development of new, targeted therapeutic interventions (34). Most LM contain specific genetic mutations, such as MED12, suggesting that transformation of normal myocytes into abnormal myocytes is required at some point during the genesis of an LM (35). MED12 encodes a subunit of the Mediator complex, which consists of at least 26 subunits and regulates transcription initiation and elongation by bridging regulatory elements in gene promoters to the RNA polymerase II initiation complex (35). It was determined that MED12 is altered in 70% of LM tumors (35). All mutations resided in exon 2, suggesting that aberrant function of this region of MED12 contributes to tumorigenesis. MED12 binds directly to β-catenin and regulates canonical WNT signaling (36). Because MED12 limits β-catenin–dependent tissue growth during embryonic development, a critical question is whether absent or defective MED12 in uterine LM stem cells or LMMP cells causes β-catenin pathway-dependent tumor growth (29, 37). Interestingly, expression of WNT4 is markedly higher in LM with MED12 mutations than in those without mutations (38). These observations point to a mechanism involving MED12 mutations and WNT/β-catenin activation that supports stem cell renewal, proliferation, and fibrosis in uterine LM tissue (36, 39).
It has been reported that WNT16B expression is regulated by NF-κB after DNA damage and subsequently signals in a paracrine manner to activate the canonical WNT pathway in tumor cells (40). It also has been shown that WNT16B is activated in fibroblasts through NF-κB and promotes an epithelial-to-mesenchymal transition through paracrine signaling in prostate cancer. Exploring a potential link between WNT and NF-κB in mature and stem cells of LM is an exciting future direction of our research. Our work has relied on Hoechst 33342-based isolation of SP cells to study stem/reservoir cell biology in uterine LM (10, 13, 14, 41). In the future it will be necessary to develop alternative independent methodologies, such as cell sorting via a surface antigen, to isolate an LM cell population enriched in stem cells. The development of in vivo tumors from originally labeled LMSP cells would have demonstrated their stem cell nature conclusively. Despite the lack of this experiment, we have demonstrated that LMSP cells are essential for ovarian steroid-dependent in vivo tumor development, which is mediated via estrogen and progesterone receptors in a different cell population sending paracrine signals via the WNT/β-catenin pathway.
Because LM cell proliferation is thought to be a critical step in LM tumorigenesis, we addressed the contribution of WNT/β-catenin signaling to these phenotypes using cultures of human LM cells and adenoviruses (Ad-ICAT) that can inhibit WNT/β-catenin signaling. We found that inhibition of WNT/β-catenin signaling inhibits tumor growth in vivo (Fig. 1). Recently, Vuga and et al. (42) found that WNT5A can increase fibroblast proliferation through a noncanonical or β-catenin/TCF-independent signaling mechanism, indicating that both canonical and noncanonical WNTs may contribute to tumorigenesis. WNT10A overexpression has been observed in some tumor cells (43, 44); however, we did not detect a significant difference between normal myometrium and LM. Moreover, WNT10A induction was not verified by real-time PCR in MM cells after E+P treatment, perhaps because various WNT members may be overexpressed in different tumors in a cell type- and context-dependent fashion (40, 44, 45). Given that WNT/β-catenin target genes are cell type and cell context dependent, defining the WNT/β-catenin–regulated target genes in human LM requires similar unbiased approaches.
The role of aberrant WNT signaling in the development and progression of tumors such as colorectal cancer has been studied extensively (46–48). Thus, components of the WNT pathway, particularly the accessible plasma membrane FZD receptors, are prime targets for drug development. There are, however, no drugs currently approved by the Food and Drug Administration that regulate WNT signaling at the level of the FZD receptors. It has been reported previously that niclosamide promotes FZD1 internalization and inhibits WNT/FZD function, suggesting that it may have antitumor effects (49). It was also reported that XAV939, which inhibits the stabilization and nuclear accumulation of β-catenin by targeting tankyrase 1 and 2, which down-regulates AXIN levels, blocks the growth of colorectal and lung cancer cells (50, 51). These and other related compounds may serve as future LM therapeutics.
Taken together, our results demonstrate that sustained activation of WNT/β-catenin signaling specifically in LM stem cells enhance their regeneration and proliferation leading to in vivo tumor growth. Given that WNT/β-catenin signaling controls cell-fate decisions throughout development, often controlling the balance between progenitor cells and their descendants, selective WNT/β-catenin inhibition in LM stem cells is a long-term therapeutic objective and would represent a paradigm-shifting strategy for controlling the size and associated symptoms of LM (52).
Materials and Methods
Only cell preparation and adenoviral infection procedures are described here. Detailed protocols can be found in SI Materials and Methods. Antibodies used in this study are listed in Table S1. Primer sets used in this study are listed in Table S2.
Preparation of Human LM and MM Cells.
LM and MM tissues were obtained from women (age range, 33–53 y) undergoing hysterectomy or myomectomy. Basic endocrine information (e.g., day of menstrual cycle, parity, whether oral contraception is being taken) was collected also. Written informed consent was obtained from each patient, and the Institutional Review Board of Northwestern University approved the use of human tissue specimens for human research. None of the women had a previous history of uterine cancer, and all samples were confirmed by histopathological examination to be free of malignancy. The tissues were prepared as previously described (10, 13).
Cell Culture and Adenoviral Constructs.
LM cells were cultured in DMEM/F12 1:1 (GIBCO/BRL) containing 10% (vol/vol) FBS and were grown in a humidified atmosphere in 5% (vol/vol) CO2 at 37 °C. LiCl and NaCl were purchased from Sigma. We treated freshly cultured normal MM and LM cells with 30 mmol/L of LiCl, which is known to inhibit GSK-3β activity, thereby activating β-catenin (53). Cells were treated in the presence or absence of 17β-estradiol (E2; 10−7 M; Sigma-Aldrich) and R5020 (P; 10−7 M; PerkinElmer) in phenol red-free DMEM/F12 with 0.2% charcoal-stripped FBS. Coculturing in two separate compartments or with indirect contact with MM cells was performed using Transwell Permeable Supports (0.4-μm pore; Corning Inc.). Side or main cell populations in mixed cocultures with MM at a 1:1 ratio were identified after initially dye-labeling each cell type. We labeled the cytosolic membranes of LMSP or LMMP cells using PKH-26 (red; Sigma-Aldrich). Cells originating from LMSP or LMMP cells in mixed cocultures were identified using long-lasting status PKH-26. The Ad-S37A-β-cat-HA construct was kindly provided by Jan Kitajewski (Columbia University, New York). The myc-ICAT cDNA was provided by Tetsu Akiyama (The University of Tokyo, Tokyo) and was reengineered by Vector Biolabs to encode a bicistronic mRNA that translates myc-ICAT and GFP proteins [Ad-ICAT (Myc)-Internal Ribosome Entry Site-GFP]. Ad-GFP viruses were supplied by Tong Chuan He (University of Chicago, Chicago).
Supplementary Material
Acknowledgments
We thank Gidon Berger, David J. Escobar, and Annette S. Flozak for providing reagents. This work was supported by the Northwestern University Flow Cytometry Facility and the Northwestern University Cell Imaging Facility; by National Cancer Institute Cancer Center Support Grant CA060553 and National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P01-HD057877; and, in part, by the Kanae Foundation for the Promotion of Medical Science, the Kanzawa Medical Research Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Uehara Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313650110/-/DCSupplemental.
References
- 1.Walker CL, Stewart EA. Uterine fibroids: The elephant in the room. Science. 2005;308(5728):1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 2.Wallach EE, Vlahos NF. Uterine myomas: An overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 3.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 5.Szotek PP, et al. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem Cells. 2007;25(5):1317–1325. doi: 10.1634/stemcells.2006-0204. [DOI] [PubMed] [Google Scholar]
- 6.Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 7.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2):7–25. [PubMed] [Google Scholar]
- 8.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 9.Smalley MJ, Clarke RB. The mammary gland “side population”: A putative stem/progenitor cell marker? J Mammary Gland Biol Neoplasia. 2005;10(1):37–47. doi: 10.1007/s10911-005-2539-0. [DOI] [PubMed] [Google Scholar]
- 10.Ono M, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA. 2007;104(47):18700–18705. doi: 10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda H, et al. Stem cell-like properties of the endometrial side population: Implication in endometrial regeneration. PLoS ONE. 2010;5(4):e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervelló I, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS ONE. 2011;6(6):e21221. doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono M, et al. Role of stem cells in human uterine leiomyoma growth. PLoS ONE. 2012;7(5):e36935. doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mas A, et al. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98(3):741–751.e746. doi: 10.1016/j.fertnstert.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: Utilization as a cell marker in the study of leiomyomas. Science. 1965;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- 16.Arango NA, et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288(1):276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanwar PS, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedgepeth CM, et al. Activation of the Wnt signaling pathway: A molecular mechanism for lithium action. Dev Biol. 1997;185(1):82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235(11):3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 23.Flozak AS, et al. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285(5):3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willert K, Jones KA. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006;20(11):1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa H, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: Regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10(4):276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 27.Lam AP, Gottardi CJ. β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23(6):562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottardi CJ, Königshoff M. Considerations for targeting β-catenin signaling in fibrosis. Am J Respir Crit Care Med. 2013;187(6):566–568. doi: 10.1164/rccm.201301-0144ED. [DOI] [PubMed] [Google Scholar]
- 29.Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development. 2010;137(16):2723–2731. doi: 10.1242/dev.053660. [DOI] [PubMed] [Google Scholar]
- 30.Lydon JP. Stem cells: Cues from steroid hormones. Nature. 2010;465(7299):695–696. doi: 10.1038/465695a. [DOI] [PubMed] [Google Scholar]
- 31.Asselin-Labat ML, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 32.Joshi PA, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Valdivia R, Lydon JP. From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol Cell Endocrinol. 2012;357(1-2):91–100. doi: 10.1016/j.mce.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto K, et al. Clonal determination of uterine leiomyomas by analyzing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynecol Obstet Invest. 1995;40(3):204–208. doi: 10.1159/000292336. [DOI] [PubMed] [Google Scholar]
- 35.Mäkinen N, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/beta-catenin signaling. J Biol Chem. 2006;281(20):14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Rinaldo L, Fazly AF, Xu X. Depletion of Med10 enhances Wnt and suppresses Nodal signaling during zebrafish embryogenesis. Dev Biol. 2007;303(2):536–548. doi: 10.1016/j.ydbio.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 38.Markowski DN, et al. MED12 mutations in uterine fibroids—their relationship to cytogenetic subgroups. Int J Cancer. 2012;131(7):1528–1536. doi: 10.1002/ijc.27424. [DOI] [PubMed] [Google Scholar]
- 39.Catherino WH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40(3):204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18(9):1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang HL, et al. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci. 2010;17(2):158–167. doi: 10.1177/1933719109348924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuga LJ, et al. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol. 2009;41(5):583–589. doi: 10.1165/rcmb.2008-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu D, et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2004;101(9):3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu RJ, et al. WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway. PLoS ONE. 2012;7(10):e47649. doi: 10.1371/journal.pone.0047649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You L, et al. Wnt-1 signal as a potential cancer therapeutic target. Drug News Perspect. 2006;19(1):27–31. doi: 10.1358/dnp.2005.19.1.965871. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 47.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 48.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25(57):7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 49.Osada T, et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71(12):4172–4182. doi: 10.1158/0008-5472.CAN-10-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulsamer A, et al. Axin pathway activity regulates in vivo pY654-β-catenin accumulation and pulmonary fibrosis. J Biol Chem. 2012;287(7):5164–5172. doi: 10.1074/jbc.M111.322123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casás-Selves M, et al. Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition. Cancer Res. 2012;72(16):4154–4164. doi: 10.1158/0008-5472.CAN-11-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 53.Derksen PW, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101(16):6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.