Significance
Sensitive periods in human functional brain development were tested in humans who had been blind from birth and whose sight was restored as long as 14 y later. In investigating this rare population, our data demonstrate a general principle of brain development: rather than being born with highly specialized neural systems (e.g., for specific object categories such as faces), the functional differentiation of neural circuits seems to depend on early (visual) experience involving a decrease in responsiveness to certain events during sensitive periods. The functional tuning of neural systems seems necessary to achieve high processing proficiency.
Abstract
The aim of the study was to identify possible sensitive phases in the development of the processing system for human faces. We tested the neural processing of faces in 11 humans who had been blind from birth and had undergone cataract surgery between 2 mo and 14 y of age. Pictures of faces and houses, scrambled versions of these pictures, and pictures of butterflies were presented while event-related potentials were recorded. Participants had to respond to the pictures of butterflies (targets) only. All participants, even those who had been blind from birth for several years, were able to categorize the pictures and to detect the targets. In healthy controls and in a group of visually impaired individuals with a history of developmental or incomplete congenital cataracts, the well-known enhancement of the N170 (negative peak around 170 ms) event-related potential to faces emerged, but a face-sensitive response was not observed in humans with a history of congenital dense cataracts. By contrast, this group showed a similar N170 response to all visual stimuli, which was indistinguishable from the N170 response to faces in the controls. The face-sensitive N170 response has been associated with the structural encoding of faces. Therefore, these data provide evidence for the hypothesis that the functional differentiation of category-specific neural representations in humans, presumably involving the elaboration of inhibitory circuits, is dependent on experience and linked to a sensitive period. Such functional specialization of neural systems seems necessary to archive high processing proficiency.
Brain development is characterized by transient phases of enhanced neuroplasticity during which learning is particularly efficient. These epochs are called “sensitive phases.” They are named “critical periods” if neural systems are irreversibly shaped during this time (1, 2). The mechanisms of sensitive periods have often been investigated in animal research using a visual deprivation approach (see pioneering work in ref. 3). However, their timing and their neural mechanisms in humans are yet largely unknown (e.g., ref. 4). This is because only a few human models exist that allow for a systematic investigation of the existence, time course, and neural correlates of sensitive periods in functional development.
Analogous to studies in animals, the visual deprivation model has been used to investigate sensitive periods in humans (5). In particular, individuals born with dense bilateral cataracts, i.e., opaque lenses preventing patterned light from reaching the retina, have been investigated after the cataractous lenses were surgically removed. The behavioral restoration of both basic (2) and more complex visual functions (6–8), as well as multisensory processes (7, 9, 10), have been studied in this population. For example, face detection (2) and the processing of biological motion (11) have been found to be largely unaffected by periods of early visual deprivation lasting for a few months. By contrast, visual functions that rely on an automatic binding of visual features seem to be permanently impaired by short periods of visual deprivation, including configural face processing (6), the ability to recognize faces from different perspectives (7, 12), and the detection of illusory contours (8).
Because only a few months of complete visual deprivation after birth seem to be sufficient to cause permanent deficits in higher visual processes typically characterized by a protracted developmental time course, it has been argued that these impairments originate in compromised early developing basic visual functions (13). However, it might also be argued that what is linked to sensitive phases is not predominantly the setting up of basic neural circuits but rather their fine tuning, known as functional specialization, often accompanied by “perceptual narrowing” (14) at the behavioral level. Functional specialization seems to be crucial to achieve a high processing efficiency and automaticity (15, 16). This hypothesis is compatible with the finding that individuals with cataracts were able to detect faces, but the configural processing of faces, a hallmark of face processing, was compromised (13). Similarly, individuals with a history of congenital cataracts were able to identify illusory contours only by engaging an effortful, slow search strategy, rather than an automatic parallel processing mode (8). Finally, a recent brain imaging study has demonstrated that individuals with a history of congenital cataracts are able to read lips, although this ability was reduced compared with healthy controls, possibly because of a reduced specificity in the neural systems involved in lip-reading (17).
However, direct neurophysiological evidence demonstrating that the functional specialization of neural systems, such as the face processing system, depends on adequate input during early development is missing. Testing this hypothesis with a neural marker is crucial, because the same overt behavior might arise from different neural circuits, e.g., as a result of functional reorganization or strategy change (1). Here, we made use of the high temporal resolution of noninvasive event-related potentials (ERPs) in humans to test whether the development of the human face-specific neural processing system depends on visual input during the first years of life (7, 18). In particular, the N170 response (negative peak around 170 ms), known to indicate the structural encoding of faces by integrating configural cues and facial features (19, 20), was assessed. Moreover, because it is unknown whether a complete visual deprivation from birth for more than 1 y might result in larger impairments, including deficits in face detection, we included cataract individuals who underwent surgery as late as 14 y of age. A total of 11 participants with a history of congenital bilateral dense cataracts [congenital cataract (cc) group], who had undergone cataract surgery between the age of 2 mo and 14 y of life, were tested. Age- and sex-matched healthy individuals (mcc group) and five individuals with developmental or incomplete cataracts [developmental cataract (dc) group] with a separate age-matched control group (mdc group) served as controls (Table 1).
Table 1.
Description of participants
| Participant | Age, y | Cataract onset timing | Age at surgery, mo | Postsurgical visual acuity in better eye | Mean accuracy in EEG task, % |
| cc-a | 22 | Congenital | 48 | 0.15 | 99.3 |
| cc-b | 11 | Congenital | 84 | 0.03 | 95.75 |
| cc-c | 8 | Congenital | 72 | 0.08 | 90.15 |
| cc-d | 17 | Congenital | 36 | 0.60 | 95.75 |
| cc-e | 33 | Congenital | 24 | 0.31 | 100 |
| cc-f | 7 | Congenital | 9 | 0.25 | 95.9 |
| cc-g | 9 | Congenital | 4 | 0.25 | 99.3 |
| cc-h | 11 | Congenital | 5 | 0.30 | 99.85 |
| cc-i | 9 | Congenital | 2 | 0.50 | 91.5 |
| cc-j | 15 | Congenital | 168 | 0.12 | 92.15 |
| cc-k | 7 | Congenital | 60 | 0.50 | 83.9 |
| cc mean (n = 4 female) | 13.4 | — | 47 | 0.28† | 94.87‡ |
| dc-l | 9 | Congenital incomplete, better eye before surgery 0.12 | 60 | 0.20 | 96.48 |
| dc-m | 11 | Accident 2 y before surgery with rapid decline before surgery | 96 | 0.80 | 92.35 |
| dc-n | 11 | Unknown onset; able to read with one eye | 120 | 0.60 | 96.9 |
| dc-o | 11 | Congenital incomplete, decline in light perception before surgery | 72 | 0.60 | 98.95 |
| dc-p | 10 | Congenital incomplete, better eye before surgery 0.1 | 84 | 0.40 | 97.7 |
| dc mean (n = 2 female) | 10.4 | — | 86 | 0.52† | 96.48¶ |
Bold represents group means.
Age at surgery and maximal archived visual acuity marginally correlated negatively at r = −0.47 (P = 0.072). The N170 effect correlated with neither highest archived visual acuity nor age at surgery (see text; P > 0.3).
Mean accuracy of matched controls for cc, 98.06%.
Mean accuracy of matched controls for dc, 97.98%.
Results
The EEG was recorded while black-and-white images of faces and houses, intermixed with their scrambled versions, preserving global low-level properties of the original image (21), were presented. Additional pictures of butterflies served as behavioral targets and had to be detected.
Behavioral Results.
Before the experiment, all participants, even those who had been blind for several years, were able to name the pictures as faces, houses, and butterflies. They were all able to discriminate between the intact faces and houses as well as the intact faces and houses from their scrambled versions. [Moreover, a short screening procedure revealed that participants of all groups were able to discriminate different faces (SI Text and Table S1).]
Mean detection performance for targets (i.e., pictures of butterflies) during the EEG session did not differ between groups (mean percentage correct, 97%; range, 84–100%; Table 1).
ERP Results.
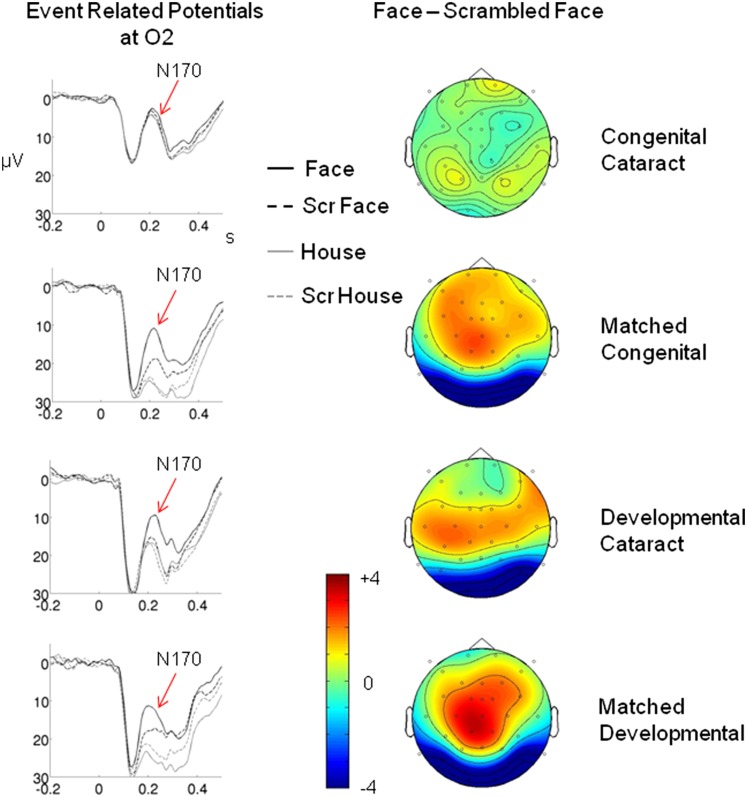
A face-specific enhancement of the N170 was observed in the mcc, dc, and mdc groups, but such a significant N170 enhancement was not observed in the cc group (Figs. 1 and 2).
Fig. 1.
ERP results. (Left) ERPs for each of the four groups—cc, dc, mcc, and mdc—and for each of the four stimulus conditions (faces, houses, and scrambled version of faces and houses) at the right occipital electrode O2. (Right) Potential maps of the difference wave, ERP (faces) minus ERP (scrambled faces), for each group. N170 was defined as the mean amplitude of a 30-ms epoch around the N170 peak. All groups except the cc individuals show a significantly enhanced N170 response to faces (arrows).
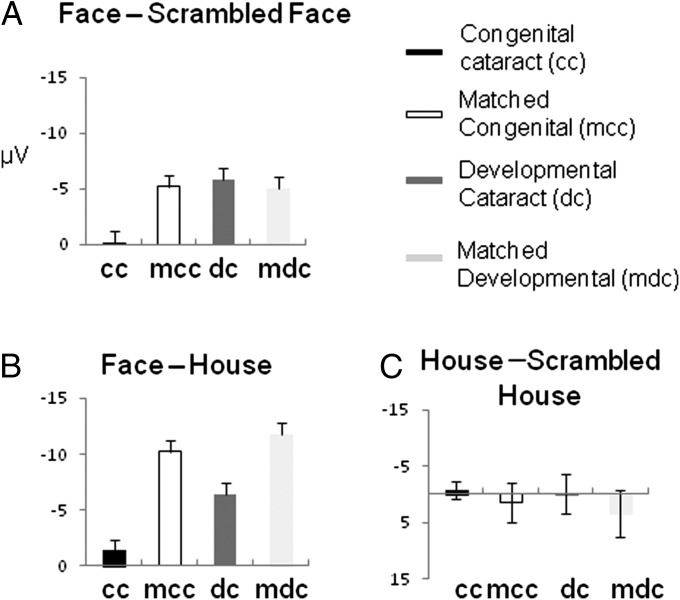
Fig. 2.
N170 amplitude effects (at O2) are shown as bar plots with SE bars for each of the four groups. (A) Face-specific effect: ERP (faces) minus ERP (scrambled faces). (B) ERP (faces) minus ERP (houses). (C) ERP (houses) minus ERP (scrambled houses).
The N170 amplitude was submitted to an ANOVA with the between-participant factor group (cc vs. mcc) and the repeated-measurement factors object (faces vs. houses) and scrambling (intact vs. scrambled). The same ANOVA was calculated for the dc and mdc groups.
The relevant three-way interaction group * object * scrambling was significant for the cc group vs. mcc group, but not for the dc group vs. mdc group, indicating that the N170 face-sensitive response differed for the cc group vs. mcc group only (Table 2). Next, an ANOVA with the repeated-measurement factors object and scrambling was calculated for each group. The relevant two-way interaction of factors object and scrambling (indicating a face-specific response) was significant in each group except for the cc group (Table 2). In a next step, the face-specific response of the N170 was further assessed with an ANOVA for intact face stimuli and scrambled face stimuli only: a group (cc vs. mcc) * face (intact vs. scrambled) ANOVA, as well as a corresponding ANOVA for the dc group vs. the mdc group, was performed. The relevant two-way interaction was significant only for the cc group vs. mcc group (Table 2), indicating that the face-specific N170 effect differed only between these two groups. The follow-up analysis for each group revealed a significant face-specific effect for the mcc group and marginally significant effects for the dc group and mdc group, whereas the difference for the cc group was far from being significant (Table 2 and Fig. 2A).
Table 2.
Relevant higher-order interactions of ANOVAs for N170 amplitude
| Statistic | df1/df2 | F | P Value | F | P Value |
| Between group | Group * object† * scrambling‡ | Group * scrambling (intact vs. scrambled face) | |||
| cc vs. mcc | 1/20 | 11.388 | 0.003 | 9.361 | 0.006 |
| dc vs. mdc | 1/8 | 0.599 | 0.476 | 0.004 | 0.952 |
| Within group | Object† * scrambling‡ | Scrambling (intact vs. scrambled face) | |||
| cc | 1/10 | 0.037 | 0.851 | 0.372 | 0.556 |
| mcc | 1/10 | 16.909 | 0.002 | 10.880 | 0.008 |
| dc | 1/4 | 7.830 | 0.049 | 4.336 | 0.106 |
| mdc | 1/4 | 19.967 | 0.011 | 6.272 | 0.066 |
Two levels: faces vs. houses.
Two levels: intact vs. scrambled.
The corresponding ANOVA for houses vs. scrambled houses did not reveal any significant effect, neither a two-way interaction of group * scrambling (intact vs. scrambled houses; P > 0.5 and P > 0.4 for the cc vs. mcc and dc vs. mdc analysis, respectively) nor for any group (cc group, P > 0.6; mcd group, P > 0.2; dc, P > 0.9; mdc, P > 0.07; Fig. 2C).
A cluster-based permutation test (22) was used to analyze the N170 face-specific effect (intact vs. scrambled faces) for each single individual across all recoding sites (Fig. 1 displays potential maps). Single time points of the ERP elicited by faces and the ERP elicited by scrambled faces were contrasted within 60-ms time windows centered on the N170 peak of each participant. Significant clusters were the result of a time series of significant differences taking into account the number and the location of the electrodes involved (22).
Significant N170 effects (intact faces vs. scrambled faces) were found in 72% of individuals in the mcc group (8 of 11), 80% of individuals in the dc group (4 of 5), and 80% of individuals in the mdc group (4 of 5), but in only 18% (2 of 11) of the cc group (the face-specific N170 for each participant is shown in Fig. S1).
The lack of an N170 face-specific response in the cc group depended on neither age at surgery (r = 0.131, P > 0.3) nor achieved visual acuity (r = 0.06, P > 0.4). [The lack of a face-specific N170 response was seen for individuals in the cc group with relatively high visual acuity (>0.3) as well (Fig. S2).]
Discussion
The goal of the present study was to identify sensitive phases in the development of the processing system for human faces. Humans with a history of congenital bilateral dense cataracts watched pictures of faces and houses and the scrambled versions of these pictures. They had to detect randomly intermixed pictures of butterflies. Individuals with a history of developmental or congenital but not dense cataracts, as well as healthy, age- and sex-matched individuals served as controls. ERPs were recorded, and the N170, which is known to indicate the structural encoding of faces based on the integration of features and configural cues (20), was analyzed.
Here, we show that the lack of any visual input at birth may render the neural face-processing system functionally ill-specified (23), but, importantly, responsive overall.
In the context of ocular-dominance plasticity, it has been observed that, shortly after eye opening, “all prerequisites for critical-period plasticity are in place, except for the appropriate level or type of inhibitory input” (ref. 23, p. 312). The initiation of GABA-mediated inhibition seems to be linked to the availability of neurotrophic factors such as BDNF or insulin-like growth factor 1. There is evidence that visual experience-dependent epigenetic mechanisms regulate the availability of neurotrophins such as BDNF, and, as a consequence, the elaboration of inhibitory circuits important for functional specialization. Moreover, the retina seems to produce factors such as the transcription factor OTX2, which is transmitted to the visual cortex and modulates perineural nets that interact with inhibitory interneurons and seem to play a role in the closure of the critical period in functional development (23). Hence, the lack of visual experience seems to prevent a change in the excitatory/inhibitory balance of neural systems, which has been proposed as a key factor of critical-period plasticity (23, 24).
These findings from animal studies link to findings from brain imaging research in children: electrophysiological and brain imaging studies in children have shown that the functional differentiation and specialization of brain systems associated with face processing are characterized by a protracted developmental time course lasting into adolescence (25–27). Importantly, a recent brain imaging study has suggested that the functional specialization of neural systems for faces and objects is caused by a decrease of responsiveness of the associated neural systems to the nonpreferred category (16). The functional differentiation therefore seems to be linked to the establishment of inhibitory circuits. Moreover, the functional specialization as assessed with brain imaging techniques positively correlated with face processing skills (16). In the individuals with cc tested in the present study, no significant face-specific response emerged in the N170 epoch. Rather, the N170 amplitude was of the same size for all intact and scrambled objects (faces and houses). Indeed, the P1 (positive peak between 70 and 150 ms)–N170 peak-to-peak amplitudes of the cc and mcc groups were indistinguishable for faces (P > 0.5) but were larger for the cc group than the mcc group for houses (P > 0.028; Fig. 1). This pattern of results might suggest that inhibitory mechanisms established during typical development that mediate the functional tuning of neural systems had not been established in the cc group. By contrast, the overall responsiveness of the underlying brain tissue did not seem to be impaired in this group. One could speculate that these areas might prevail in a precritical period-like state during the deprivation epoch. A delay of critical periods after complete sensory deprivation has repeatedly been observed in animal research (23, 28), and the suppression of inhibitory circuit elaboration, as typically trigged by experience, is known to extend the critical period (23). Here, we provide evidence of the maintenance of neural circuits in humans throughout a deprivation period lasting as long as 14 y. Hence, our data suggest that the functional specialization, rather than the general setting-up of these neural systems, depends on early vision.
Our data for the human face processing system seem to be incompatible with the proposal (1) that a lack of appropriate input to a neural system for an extended period after birth results in a random organization of these neural circuits (1). Moreover, our data argue against the assumption that degraded basic visual functions predominantly cause face processing deficits in cc individuals (13).
An astonishing finding of the present study is that, despite the well known crossmodal plasticity emerging as a consequence of total visual loss—i.e., nonvisual activation of visual brain areas (29), including the fusiform face area (30)—“visual” brain areas are recruited for visual processing even after a congenital blindness lasting for several years. It might be argued that crossmodal plasticity following congenital visual deprivation is partially reversible. Indeed, it has been speculated that crossmodal plasticity in congenital blindness might contribute to the maintenance of neural circuits typically associated with the visual system (31). This line of arguments would suggest that, rather than prevailing in a precritical period-like state, visual brain areas are reorganized in congenitally blind individuals to engage in nonvisual processing; this process might be partially reversible such that these areas regain visual responsiveness after visual restoration but would remain unable to acquire a functional specialization within the visual processing system.
The present data do not allow dissociating between these alternatives. Moreover, whether longer-lasting visual deprivation or visual deprivation during other epochs in life than those covered by the individuals of the dc group might result in similar effects as observed in the cc group remains unknown.
Finally, it might be argued that the genetic variation that caused the cc in individuals with familial cataracts also prevented their neural face processing system to functionally differentiate. However, this account seems rather unlikely: first, we included only individuals with isolated cataracts, i.e., individuals who were healthy apart from their history of cataracts. Second, it is known that isolated cases of cc emerge from different gene mutations (more than 20 are known), and different genes are often affected in different families (e.g., refs. 32, 33). It seems highly implausible that each of these different mutations would cause, in addition to isolated cataracts, alterations of the neural face processing system.
In conclusion, our data might demonstrate a general principle of human brain development compatible with the perceptual narrowing (14) or an “interactive specialization” (34) account: the functional specialization involving a decrease of responsiveness to certain stimuli, possibly mediated by the establishment of inhibitory circuits, rather than the initial setting up of a neural system, seems to be linked to early sensitive or even critical periods. Sensory experience, such as visual input, must be available during this time to functionally tune neural circuits of highly specialized systems such as the face system in humans.
Materials and Methods
Participants (Table 1).
Eleven individuals treated for cc were recruited at the L V Prasad Eye Institute in Hyderabad, India (mean age, 13.4 y; range, 7–33 y). Cataract history was based on their medical records. Individuals in the cc group were born with dense, complete, bilateral cataracts. Age at surgery in Table 1 refers to the surgery of the first eye. Surgery of the second eye took place within 1 mo. In five individuals with dc, incomplete cataracts existed at birth (n = 3), or cataracts developed later in life (n = 2; mean age of dc group, 10.4 y; range, 9–11 y). All dc-group individuals had fair vision from birth that had partially declined before surgery (Table 1) All individuals with cataracts had isolated cataracts and, apart from their history of cataracts, were healthy (e.g., no metabolic diseases, no mental retardation, no central diseases).
Eleven healthy age- and sex-matched control participants (n = 4 female; mean age, 12.8 y; range, 8–33 y) served as controls for the cc group (i.e., mcc group), and five healthy age- and sex-matched controls (n = 2 female; mean age, 10.4 y; range, 9–11 y) served as controls for the dc group (i.e., mdc group). All healthy controls had normal or corrected-to-normal vision.
All participants were right-handed and neurologically healthy according to self-report and medical records (for the individuals with cataracts). Participants (or, for minors, legal guardians) gave informed consent. The study was approved by the ethics committee of the German Society of Psychology and the ethics committee of the Hyderabad Eye Research Foundation.
Procedure.
Black-and-white images of faces (with covered outer facial features; n = 110), houses (n = 110), and butterflies (n = 60; all extending a visual angle of 8.6 × 12.3°) were presented in a random sequence with a presentation time of 180 ms each, separated by an interstimulus interval of 1,800 ± 200 ms. In three participants with vision below 0.15, the screen was moved closer to the participant (subjects cc-b, cc-c, and and cc-j). The scrambled versions of the faces (n = 110) and houses (n = 110) were randomly intermixed. Scrambled images had a randomized phase spectrum of the original images while preserving the original amplitude spectrum so that low-level physical properties (luminance, contrast, spatial frequency) were equivalent to those of the original images. Participants had to lift their right hand whenever they had detected the image of a butterfly (P = 0.15). Participants were instructed in their native language. The task was practiced before the start of the EEG session.
The EEG was recorded with 32 passive Ag/AgCl scalp electrodes mounted in elastic caps (EasyCap) against a right ear lobe reference. The EEG signal was sampled at 500 Hz (bandpass, 0.01–200 Hz) using BrainAmps (Brainproducts). Offline, the recordings were rereferenced to an average reference. Lateral and vertical eye movements were monitored with two electrodes placed at the outer canthi and frontopolar electrodes, respectively.
Data Analyses.
Artifacts caused by blinks, eye movements, and heartbeat were eliminated by independent component analysis (35) [runica version, implemented in EEGLAB (36)]. The continuous EEG was segmented into epochs starting 500 ms before and ending 1000 ms after the onset of a visual stimulus. Any segment with signal variation of ±80 µV was eliminated. The remaining epochs were low-pass filtered (upper cutoff at 40 Hz) and averaged for each participant and condition (intact faces, intact houses, scrambled faces, and scrambled houses).
The mean amplitude of a 30-ms interval centered on the N170 peak (i.e., negative peak between 170 and 230 ms) was extracted individually for each participant at the right occipital electrode O2 and submitted to an ANOVA with factor group (cc vs. mcc or dc vs. mdc) and the repeated-measurement factors object (faces vs. houses) and scrambling (intact vs. scrambled). In addition, for each group, an ANOVA with the two repeated-measurement factors was conducted (Table 2, left). These ANOVAs were repeated for ERPs to intact faces vs. scrambled faces (i.e., with one repeated-measurement factor; Table 2, right).
Moreover, individual P1 amplitudes were defined as 30 ms around the positive peak between 70 and 150 ms. The P1-to-N170 peak to peak amplitudes were calculated at electrode site O2 for each participant and each of the four conditions and compared between the cc group and mcc group with t tests for independent samples.
For single-participant cluster-based statistics, the ERPs to intact faces and scrambled faces were compared (t test) at each ERP sample point within a 60-ms time window centered on the N170 peak of each participant. Sample points with significant t tests were selected and clustered in sets on the basis of temporal (no gaps) and spatial adjacency. Cluster-level statistics are calculated by taking the sum of the t-values within each cluster; the highest of the cluster-level statistics was taken. The significance of this cluster level statistic was tested by using a Monte Carlo method with 1,000 iterations: trials of the two experimental conditions were randomly assigned to two subsets, creating a random partition of the data. The same test statistic was calculated as for the observed data. The proportion of random partitions that resulted in a larger test statistic than the test statistic of the observed data were calculated. This proportion is the Monte Carlo significance probability, i.e., the P value. If the P value was lower than 0.05, we concluded that the data in the two experimental conditions were significantly different.
Supplementary Material
Acknowledgments
We thank D. Balasubramanian for supporting the study at the Hyderabad Eye Research Foundation and Ralf Sürig for programming and help in testing the paradigm. This work was supported by European Research Council Grant ERC-2009-AdG 249425-CriticalBrainChanges (to B.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 16702.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309963110/-/DCSupplemental.
References
- 1.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 2.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46(3):163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 3.Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 4. Stevens C, Neville H, Specificity of experiential effects in neurocognitive development. The Cognitive Neurosciences V, ed Gazzaniga M (The MIT Press, Cambridge, MA), in press.
- 5.Röder B. Sensory deprivaton and the development of multisensory integration. In: Bremner A, Lewkowicz DJ, Spence D, editors. Multisensory Development. Oxford: Oxford Univ Press; 2012. pp. 301–324. [Google Scholar]
- 6.Robbins RA, Nishimura M, Mondloch CJ, Lewis TL, Maurer D. Deficits in sensitivity to spacing after early visual deprivation in humans: A comparison of human faces, monkey faces, and houses. Dev Psychobiol. 2010;52(8):775–781. doi: 10.1002/dev.20473. [DOI] [PubMed] [Google Scholar]
- 7.Putzar L, Hötting K, Röder B. Early visual deprivation affects the development of face recognition and of audio-visual speech perception. Restor Neurol Neurosci. 2010;28(2):251–257. doi: 10.3233/RNN-2010-0526. [DOI] [PubMed] [Google Scholar]
- 8.Putzar L, Hötting K, Rösler F, Röder B. The development of visual feature binding processes after visual deprivation in early infancy. Vision Res. 2007;47(20):2616–2626. doi: 10.1016/j.visres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Putzar L, Goerendt I, Lange K, Rösler F, Röder B. Early visual deprivation impairs multisensory interactions in humans. Nat Neurosci. 2007;10(10):1243–1245. doi: 10.1038/nn1978. [DOI] [PubMed] [Google Scholar]
- 10.Putzar L, Gondan M, Röder B. Basic multisensory functions can be acquired after congenital visual pattern deprivation in humans. Dev Neuropsychol. 2012;37(8):697–711. doi: 10.1080/87565641.2012.696756. [DOI] [PubMed] [Google Scholar]
- 11.Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Dev Sci. 2011;14(6):1330–1339. doi: 10.1111/j.1467-7687.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 12.Geldart S, Mondloch CJ, Maurer D, de Schonen S, Brent HP. The effect of early visual deprivation on the development of face processing. Dev Sci. 2002;5(4):490–501. [Google Scholar]
- 13.Maurer D, Mondloch CJ. Sensitive periods in face perceptioin. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford Univ Press; 2011. pp. 779–796. [Google Scholar]
- 14.Werker JC, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behav Dev. 1984;7:49–63. [Google Scholar]
- 15.Lewkowicz DJ, Ghazanfar AA. The emergence of multisensory systems through perceptual narrowing. Trends Cogn Sci. 2009;13(11):470–478. doi: 10.1016/j.tics.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 2011;21(1):191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putzar L, et al. The neural basis of lip-reading capabilities is altered by early visual deprivation. Neuropsychologia. 2010;48(7):2158–2166. doi: 10.1016/j.neuropsychologia.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Le Grand R, Mondloch CJ, Maurer D, Brent HP. Neuroperception. Early visual experience and face processing. Nature. 2001;410(6831):890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- 19.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eimer M. The face-sensitivity of the n170 component. Front Hum Neurosci. 2011;5:119. doi: 10.3389/fnhum.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuefner D, de Heering A, Jacques C, Palmero-Soler E, Rossion B. Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Front Hum Neurosci. 2010;3:67. doi: 10.3389/neuro.09.067.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 24.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 25.Scherf KS, Luna B, Avidan G, Behrmann M. “What” precedes “which”: Developmental neural tuning in face- and place-related cortex. Cereb Cortex. 2011;21(9):1963–1980. doi: 10.1093/cercor/bhq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev Sci. 2007;10(4):F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 27.de Haan M. The neurodevelopment of face perception. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford Univ Press; 2011. pp. 731–751. [Google Scholar]
- 28.Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43(4):1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- 29.Pavani F, Röder B. Crossmodal plasticity as a consequence of sensory loss: Insights from blindness and deafness. In: Stein BE, editor. The New Handbook of Multisensory Processes. Cambridge, MA: MIT Press; 2012. pp. 737–760. [Google Scholar]
- 30.Pietrini P, et al. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci USA. 2004;101(15):5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Röder B, Neville H. Developmental functional plasticity. In: Grafman J, Robertson I, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 2003. pp. 27–49. [Google Scholar]
- 32.Ponnam SP, et al. Mutational screening of Indian families with hereditary congenital cataract. Mol Vis. 2013;19:1141–1148. [PMC free article] [PubMed] [Google Scholar]
- 33.Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19(2):134–149. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson MH. Interactive specialization: A domain-general framework for human functional brain development? Dev Cogn Neurosci. 2011;1(1):7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mennes M, Wouters H, Vanrumste B, Lagae L, Stiers P. Validation of ICA as a tool to remove eye movement artifacts from EEG/ERP. Psychophysiology. 2010;47(6):1142–1150. doi: 10.1111/j.1469-8986.2010.01015.x. [DOI] [PubMed] [Google Scholar]
- 36.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.