Significance
Insect wings are a core example of morphological novelty, yet their acquisition remains a biological conundrum. More than a century of debates and observations has culminated in two prominent hypotheses on the origin of insect wings. Here, we show that there are two separate wing serial homologs in the wingless first thoracic segment of a beetle, Tribolium. These two tissues are merged to form an ectopic wing structure in homeotic transformation. Intriguingly, the two wing serial homologs may actually be homologous to the two previously proposed wing origins, hence supporting the dual origin of insect wings. The merger of two unrelated tissues may have been a key step in developing this morphologically novel structure during evolution.
Keywords: serial homology, morphological novelty, Hox, appendage evolution
Abstract
Despite accumulating efforts to unveil the origin of insect wings, it remains one of the principal mysteries in evolution. Currently, there are two prominent models regarding insect wing origin: one connecting the origin to the paranotal lobe and the other to the proximodorsal leg branch (exite). However, neither hypothesis has been able to surpass the other. To approach this conundrum, we focused our analysis on vestigial (vg), a critical wing gene initially identified in Drosophila. Our investigation in Tribolium (Coleoptera) has revealed that, despite the well-accepted view of vg as an essential wing gene, there are two groups of vg-dependent tissues in the “wingless” first thoracic segment (T1). We show that one of these tissues, the carinated margin, also depends on other factors essential for wing development (such as Wingless signal and apterous), and has nubbin enhancer activity. In addition, our homeotic mutant analysis shows that wing transformation in T1 originates from both the carinated margin and the other vg-dependent tissue, the pleural structures (trochantin and epimeron). Intriguingly, these two tissues may actually be homologous to the two proposed wing origins (paranotal lobes and exite bearing proximal leg segments). Therefore, our findings suggest that the vg-dependent tissues in T1 could be wing serial homologs present in a more ancestral state, thus providing compelling functional evidence for the dual origin of insect wings.
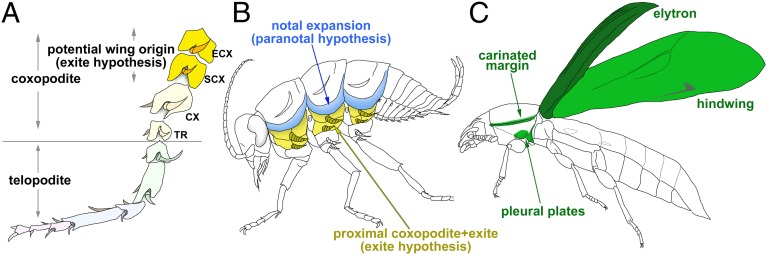
The insect wing is an extremely diverse structure, which has fascinated scientists for centuries. The paranotal hypothesis of insect wing origin proposes that wings evolved from lateral extensions of the notum (the dorsal portion of thoracic body wall), which helped ancient insects to glide and, when eventually articulated, to fly (1, 2; reviewed in refs. 3 and 4) (Fig. 1B). The presence of the wing-like paranotal lobes (or winglet) on the first thoracic segment of Paleozoic insects, in addition to similar vein patterning between these lobes and wings, is often used to support this hypothesis (2, 3, 5). The gill or exite hypothesis proposes that insect wings originated from exites (outer leg branches), which stemmed from ancestral proximal leg segments (proximal coxopodites such as epicoxa) (6, 7) (Fig. 1A). These ancestral proximal leg segments appear to have fused into the body wall to form the pleural plates in extant insects (5) (Fig. 1B and Fig. S1). The exite hypothesis states that these exites evolved into wings, while ancestral proximal leg segments provided a series of sclerotized plates as well as preexisting muscle attachment, allowing the quick acquisition of insect wing articulation (6). Shared expression of some genes between crustacean coxopodite exites and insect wings provides evidence to support the exite hypothesis from an evo-devo point of view (8).
Fig. 1.
Two wing origin hypotheses and the combinational wing origin model. (A) Arthropod leg ground plan. The proximal coxopodites (ECX and/or SCX) and their exites have been proposed to be a possible wing origin in the gill/exite hypothesis. CX, coxa; ECX, epicoxa; SCX, subcoxa; TR, trochanter. Annotation based on ref. 7. (B) The locations of two proposed wing origins (blue and yellow) in an ancestral insect ground plan. Blue, notal expansions; yellow, proximal coxopodites (pleural plates in extant insects) with their exites. (C) Wing serial homologs in Tribolium (green). The two wing serial homologs in T1 appear to be homologous to two proposed wing origins (blue and yellow tissues in B). The merger of these two tissues in Tribolium produces ectopic elytra in homeotic transformation, suggesting the combinational wing origin model.
Insect wing development has been studied most thoroughly in a dipteran insect, Drosophila melanogaster. These studies have led to an excellent understanding of the molecular basis of the important steps in wing development including induction, differentiation, proliferation, and patterning (see ref. 9 for review). vestigial (vg), initially identified in Drosophila, is an interesting candidate to trace the origin of wing structures. In Drosophila embryos, vg expression identifies a special set of cells that later becomes the wing disc (10, 11). vg is also essential for the proliferation and survival of future wing cells throughout larval development (12), which is exemplified by the Drosophila vg null mutant lacking entire wing and haltere structures (13). Proper wing margin formation also depends on vg function at the dorsal-ventral (D-V) compartmental border of the wing disc (12). Furthermore, vg overexpression in Drosophila induces ectopic wing structures, defining vg as the wing “master gene” (14). Although the ectodermal function of vg in Drosophila appears to be specific to wing formation, it is yet to be determined to what extent the function of vg is conserved among other insect species. Therefore, analyzing vg function in various insects will be useful to gain unique insights into the origin of insect wings.
Results and Discussion
vg Function in Wing Development Is Conserved Between Tribolium and Drosophila.
The wing structures of Drosophila and Tribolium have become vastly different over evolutionary time. Drosophila have flight wings on their second thoracic segment (T2) and modified wing structures, called halteres, on their third thoracic segment (T3) for balance. In contrast, Tribolium have modified, hardened protective wing structures on T2, called elytra, and hindwings used for flight on T3 (Fig. 2 A, D, and F). Despite their modification, elytra still maintain wing identity, as disruption of wing genes [such as vg, apterous (ap), and nubbin (nub)] reduce or remove both wings and elytra in Tribolium and other beetles (15, 16).
Fig. 2.
vg is essential for proper wing formation in Tribolium. (A–C) Reduction of wing and elytron in vg RNAi. (A) Wild type. (B) Late vg RNAi. (C) Early vg RNAi. (D–G) Elytron and hindwing phenotypes caused by vg RNAi. (D and F) Wild type. (E and G) vg RNAi.
In Drosophila, proper wing margin formation depends on vg function (12). To investigate whether vg is important for wing margin formation in Tribolium, we performed vg RNAi in the last larval stage. Disruption of vg in the last larval stage led to reduction of elytra and hindwings (Fig. 2B). The reduction was the result of margin structure deletion, as interior structures and their relative positions remain fairly intact (Fig. 2 F and G and Fig. S2 A–D). For example, vg RNAi elytra lack marginal hair structures normally present on the wild-type elytra (Fig. S2 B and D) while retaining intact vein and sensory structure patterns (Fig. S2 A and C). Similarly, in vg RNAi hindwings, the margin structures are deleted while the vein pattern is fairly unaffected (Fig. 2 F and G). These results indicate that vg is responsible for wing margin formation in Tribolium.
We next investigated whether the induction and proliferation functions of vg are also conserved in Tribolium. We disrupted vg function in the penultimate stage, just before the onset of wing proliferation. Penultimate vg RNAi led to a complete lack of hindwing and elytron discs in the subsequent last larval stage, as well as complete deletion of hindwings and elytra in the resulting adults (Fig. 2C). The lack of hindwing and elytron discs during the last larval stages in vg RNAi suggests that vg plays an important role in the induction of wing structures in Tribolium. Alternatively, it is also possible that penultimate vg RNAi might be inducing cell death, causing the complete deletion of dorsal appendages.
vg Is Essential for Proper Body Wall Development in Tribolium.
Although vg has various functions in other tissues (17, 18), vg function in the ectoderm appears to be restricted to only dorsal appendage development in Drosophila (13). To determine whether the ectodermal function of vg is restricted to wing structures in Tribolium, we examined the nonwing structures in the vg RNAi adults. Interestingly, we noticed several disruptions in the thoracic body wall that were not expected based on previous Drosophila studies (Fig. 3). The insect thoracic body wall can be subdivided into three distinct regions; notum or thoracic tergum (dorsal), pleural plates (lateral), and sternum (ventral) (Fig. 3 A–C). In polyphagan beetles (including Tribolium), the dorsal tissue extends ventrally, forming the hypomeron that covers most of the pleural plates (Fig. 3 A–C; also see Fig. S1) (19, 20). We noticed that vg RNAi resulted in a deletion of several pleural plates, one of the trochantin plates (posterior trochantin or trochantin P) and the epimeron, in T1, producing a gap near the base of the T1 leg (Fig. 3 D–F and I–K). The other trochantin (anterior trochantin or trochantin A) and the endopleuron were unaffected (Fig. 3 E and J and Fig. S2F). The hypomeron failed to cover the coxa possibly because of the lack of these pleural plates that scaffold the hypomeron from inside (Fig. 3 E and J). In addition, the lateral ridge of T1 body wall (carinated margin, a part of the dorsal body wall) (20) was missing in the vg RNAi beetles (Fig. 3 G and L). vg RNAi also led to the formation of dents on the ventral side of T3 directly above the transverse groove (arrow in Fig. 3 H and M). This region does not correspond to any previously described structures (20). Although there is no reported function of vg in the ectoderm other than wings in Drosophila, it is possible that function of vg in the body wall may have been overlooked. However, vg RNAi in Drosophila did not cause any noticeable body wall abnormalities, indicating that vg does not hold an important function in adult body wall development in Drosophila (Fig. S3). Taken together, these results indicate that vg has an important role in the formation of Tribolium body wall, a function that is absent in Drosophila.
Fig. 3.
vg RNAi leads to improper body wall formation. (A–C) Tribolium T1 body wall structures. (D–H) Wild type. (I–M) vg RNAi. Trochantin P (arrowhead in E and J), the epimeron (arrow in E, F, J, and K), and the carinated margin (arrow in G and L) are affected in vg RNAi T1. Trochantin A (asterisk in E and J) remains unaffected. An arrow in M indicates a dent in vg RNAi T3.
Overlapping of Gene Networks Responsible for Wing and Carinated Margin Development in Tribolium.
The vg RNAi phenotypes in T1 are especially intriguing for several reasons. First, although T1 belongs to the same tagma as the other two thoracic segments, it does not appear to possess wing-related structures (5). The vg dependency of the carinated margin and two pleural plates (trochantin P and the epimeron) in T1 may indicate that these tissues are actually related to the wings on T2 and T3 (i.e., serially homologous to wings). Second, these two vg-dependent tissues can be homologized to the tissues proposed as the possible sources of wing origin in the two prominent wing origin hypotheses (1, 6) (reviewed in ref. 3). For instance, pleural plates (including the trochantin plates and epimeron) are considered to have originated from the subcoxa, one of the ancestral proximal leg segments that possessed exites (5, 6). The other vg-dependent tissue, the carinated margin, is an expansion stemming from the lateral portion of the pronotum. This type of outgrowth is present in many insect orders (5) and appears to be homologous to paranotal lobes. Therefore, it is intriguing that both the carinated margin and the two pleural plates, tissues homologous to two proposed wing origins, are vg dependent.
To gain more insight into the relationship between the T1 vg-dependent tissues and wings, we next asked whether other wing genes are also responsible for the formation of these tissues. In Drosophila, ap has the central role in the formation of wings through induction of organizer activity along the D-V boundary (9). The establishment of this D-V organizer is followed by the induction of Wingless (Wg) morphogen, which subsequently patterns wings along the D-V axis (9). The ap function in the wing is conserved in Tribolium, because depletion of ap genes in Tribolium causes a similar wing reduction phenotype to that seen in Drosophila (there are two ap genes in Tribolium; ref. 15).
Our RNAi analysis for ap genes in Tribolium has revealed that ap is also important for the formation of the carinated margin. Double RNAi for apA and apB in the last larval stage resulted in adults with reduced wing and elytron structures as reported (15). Intriguingly, these beetles also lacked the defined carinated margin structure (Fig. 4B). In addition, although apA single RNAi did not significantly affect wing and elytron structures, it was sufficient to reduce the carinated margin (Fig. S2G). In contrast, apB RNAi did not produce any noticeable disruption in the carinated margin or wing structures (Fig. S2H), suggesting that apA might have a dominant role in the formation of the carinated margin. We also analyzed the pleural plates of the resulting adults; however, we did not detect any noticeable abnormalities caused by apAB RNAi.
Fig. 4.
ap, dsh, and the nub enhancer are active in the carinated margin formation. (A–C) Carinated margin in ap and dsh RNAi. (A) Wild type. (B) apAB RNAi. (C) dsh RNAi. Carinated margin is reduced in both apAB and dsh RNAi (arrows in B and C). (D and E) nub enhancer activity in the developing carinated margin in vg RNAi. (D) Control (dsRed dsRNA injection). (E) vg RNAi larvae. D and E Insets are magnified images of T1. The carinated margin expression (asterisks in D) is affected by vg RNAi, whereas the neuronal expression (arrowheads in D and E) remains intact.
To further investigate the involvement of wing genes in the carinated margin formation, we performed RNAi to inhibit Wg signal in Tribolium. Because there are multiple Wg ligands in Tribolium (21), we decided to target disheveled (dsh) to avoid potential redundancy. dsh encodes an intracellular protein that is critical for transducing Wg signal (see ref. 22 to review Wnt pathway). There is only one dsh ortholog in Tribolium (based on BLAST result), making it an ideal target to inhibit Wg signal through RNAi. RNAi for dsh caused multiple defects (such as leg malformation and eye reduction) because of the pleiotropic effect of the Wg signal (Fig. S2 I and J). Interestingly, the dsh RNAi adults lacked the carinated margin structure (Fig. 4C). dsh RNAi also drastically affected the sternum (the ventral body wall structure), which may represent the conserved function of Wg signal in sternum formation (Fig. S2J) (23). Despite the severe reductions in the ventral body wall, the pleural tissues appear to be less affected in these dsh RNAi beetles (Fig. S2J).
Taken together, these results indicate that there is a significant genetic overlap between wing and carinated margin development, suggesting that the carinated margin and wings may share common ancestry (i.e., are serially homologous). In contrast, we could not find further genetic similarity, other than vg, between wings and the pleural plates.
nubbin Enhancer Has vg-Dependent Residual Activity in the Carinated Margin.
To further investigate the genetic similarity between carinated margins and wings, we have analyzed the involvement of nub in the carinated margin development. nub is often used as a wing marker (8, 24–26), because of its strong expression that coincides with future wing tissues in Drosophila (27). Mutations in nub in Drosophila cause malformed wings, exemplifying the critical role of nub in wing development (27, 28). In Tribolium, nub is also expressed in the elytron and hindwing discs (15), and RNAi for nub induces a reduction of these structures (15). We have analyzed the carinated margin structure in nub RNAi beetles; however, we did not detect any abnormality (Fig. S2K). Unlike in Drosophila (which has two nub paralogs; ref. 27), this lack of abnormality cannot be explained by genetic redundancy as Tribolium have only one nub ortholog in their genome (15). Hence, our nub RNAi result indicates that nub is not functionally significant in the formation of the carinated margin in Tribolium.
Surprisingly, despite the lack of nub function in the carinated margin formation, we observed that a nub enhancer is active in the carinated margin during the larval stage. pu11 is a transgenic line that has a piggyBac transposon containing the 3xP3 enhanced yellow fluorescent protein (EYFP) construct in its genome (29, 30). 3xP3 is an artificial enhancer that drives the downstream gene (EYFP) in both larval and adult eyes (31). In addition to 3xP3, the pu11 insertion appears to have captured an endogenous enhancer near the insertion site, driving additional EYFP expression in the larval and pupal hindwing and elytron primordia as well as in some neurons (Fig. S4; refs. 29 and 30). Our inverse PCR analysis has revealed that the transposon is inserted near the nub locus (Fig. S4). Additionally, the EYFP expression pattern in the hindwing and elytron discs in pu11 accurately recapitulates the nub expression pattern in these tissues (Fig. S4) (15). Thus, pu11 is likely to be a nub enhancer trap line. Upon closer inspection of pu11 larvae, we noticed that there is a strip of weak EYFP expression in T1 in addition to the strong EYFP expression in the hindwing and elytron discs in T2 and T3. This T1 strip of expression appears to illuminate the future carinated margin (Fig. 4D). This expression is transient, visible only in the last 3 d of the last larval stage. The expression is not due to 3xP3 enhancer, because T1 reporter gene expression is absent from other 3xP3 transgenic lines (Fig. S5A) and because weak nub expression can be detected via in situ hybridization (Fig. S5B). Furthermore, vg RNAi in the last larval stage eliminated this strip of T1 EYFP expression (Fig. 4E), although the nearby neuronal expression remained intact (arrowheads in Fig. 4 D and E).
These analyses revealed that, although nub has no significant function in the carinated margin formation, the nub enhancer has residual activity in the future carinated margin. This activity is dependent on vg, further illustrating the similarity of the gene networks between the carinated margin and the wing.
Both the Carinated Margin and the Pleural Plates Contribute to the Homeotically Transformed T1 Elytron.
Between the two potential wing serial homologs in T1, the carinated margin appears to share more genes (and possibly the interaction among these genes) with wings. To investigate whether the T1 pleural plates are also serially homologous to wing, we have analyzed how these two potential wing serial homologs contribute to the ectopic elytron induced by homeotic transformation. Sex combs reduced (Scr, or Cephalothorax, Cx) is the Hox gene that represses wing development in T1 (30). RNAi for Scr in Tribolium causes a complete transformation of T1 external structures to those of T2 (30). In contrast, an allelic combination of Cx (Cx6/Cxapt, Cx6 is a null allele of Scr, while Cxapt is a hypomorphic allele; refs. 32–34) displays various degrees of transformation (Fig. 5 D–K and Fig. S6). Some of the beetles with this allelic combination show fairly complete transformation (Fig. 5 D–F and K), while others display much weaker transformation (Fig. 5 G–I). In the latter individuals, we noticed that the ectopic elytron originates from two distinct places (Fig. 5I). One origin of outgrowth occurs at the region close to the carinated margin (white arrow in Fig. 5I). In the weakest transformation, the carinated margin appears to be duplicated (or split into two margins), and a new tissue is induced between the two margins (Fig. 5I). The tissue interior to the two margins seems to correspond to the dorsal surface of a more completely transformed elytron (Fig. 5 D–F). The dorsal portion of the margin bordering this tissue corresponds to the dorsal hinge, while the ventral portion of the margin corresponds to the elytron D-V boundary (white arrow and arrowhead in Fig. 5I, respectively). The second outgrowth originates at a more ventral position (black arrow in Fig. 5I). Upon close observation, we found that a portion of this outgrowth originates from the base of the epimeron (one of the vg-dependent pleural plates) (Fig. 5 E and H). The epimeron in Cx6/Cxapt beetles expands laterally between the hypomeron and scutellum to form a part of the ectopic elytron (white arrows in Fig. 5 E and H). As the dorsal expansion of the epimeron gets larger, the ventral portion of the epimeron is more reduced (asterisks in Fig. 5 E and H), suggesting that more epimeron cells are recruited into the ectopic elytron in the strongly transformed individual. The fate of the vg-dependent trochantin in Cx6/Cxapt beetles is less clear, but it may also be recruited to the ectopic elytron, as the vg-dependent trochantin is reduced in the strongly transformed individual (arrowhead in Fig. 5K). We have analyzed the nub enhancer activity in Cx6/Cxapt pupae and noticed that both the carinated margin and the pleural outgrowths have EYFP expression (arrowhead and arrow in Fig. 5L, respectively), indicating that both outgrowths are nub expressing wing-related tissues. Furthermore, the endogenous carinated margin nub expression was absent when the outgrowth originating from the carinated margin was present (Fig. S5C), suggesting that the carinated margin cells are transforming into the ectopic elytron in Cx6/Cxapt beetles. In the strongly transformed individuals, the two outgrowths (the carinated margin and pleural outgrowths) are merged into one elytron (Fig. 5 D–F and Fig. S6 G–I). Although further analysis will be required to decipher the details of this merger, these observations suggest that both the carinated margin and the pleural plates are serially homologous to wings.
Fig. 5.
Reduction of Scr leads to elytron-like outgrowths from two distinct regions of T1. (A–I) Ectopic elytra on T1. (A–C) Wild type. (D–F) Cx6/Cxapt strong. (G–I) Cx6/Cxapt weak. Two distinct outgrowths are most visible in weakly transformed individuals (white and black arrows in I). White arrows and arrowheads indicate the dorsal and ventral potion of the split carinated margin, respectively (I). (B, E, and H) The base of the epimeron (white arrow) invades into the space between scutellum (black arrow) and hypomeron, joining the elytron. The epimeron is reduced relative to the degree of transformation (asterisk in B, E, and H). (J and K) Reduction of trochantin P (arrowhead) and the epimeron (arrow) in Cx6/Cxapt. (J) Wild type. (K) Cx6/Cxapt. (L) nub enhancer activity in the carinated margin outgrowth (arrowhead) and the pleural outgrowth (arrow) of Cx6/Cxapt pupae.
Lobes, Gills, Both, or Neither? On the Origin of the Insect Wing.
When explaining the structures and development of insect wings, we often state that, in extant insects, wing formation in T1 is “repressed.” However, the fate of wing-related tissues in T1 is still elusive. We generally assume that wing-related tissues are never induced in T1. Our findings provide an alternative to this view, in which the wing-related tissues are present in T1, but maintained as (or reverted to) a more “ancestral” state.
Through the vg RNAi analysis, we have identified the carinated margin and the two pleural plates as potential wing serial homologs. Between these two groups of tissues, the carinated margin appears to share more genes (and possibly the interaction among these genes) with wings. Then, is the carinated margin the wing serial homolog in T1, not the pleural plates? Two lines of evidence suggest that the two pleural plates may also (at least partially) be serially homologous to wings. First, there are no vg-dependent body wall tissues in T2, and they are only residually present in T3 (the region corresponding to the dents induced by vg RNAi; Fig. 3M). This observation suggests that vg-dependent pleural tissues in T2 and T3 are either missing or have been recruited into other tissues, such as wings. The second line of evidence comes from our loss-of-function analysis for Scr (Cx mutants). We noticed that, in Tribolium, both the carinated margin and the pleural plates are transforming into the ectopic T1 elytron. Hence, the wingless T1 has two distinct wing serial homologs: the carinated margin and the pleural plates (Fig. 1C).
Recognizing these homologous relationships has a significant impact on our understanding of insect wing origin, as the carinated margin and the pleural plates appear to be homologous to two proposed wing origins (the paranotal lobe and paleozoic proximal leg segments, respectively) (Fig. 1) (1, 3, 5, 6). The shared position of the carinated margin and the paranotal lobe makes it likely that these tissues are homologous. Furthermore, the homologous relationship between ancestral proximal leg segments and pleural plates has also been well supported by fossil and morphological analyses (5, 6). Additionally, our RNAi screening for general developmental toolkit genes in Tribolium has identified odd-skipped family genes, critical leg genes (35), as an essential factor for pleuron development (Fig. S7), providing further evidence for the proximal leg segment origin of pleural plates. Therefore, although further developmental analysis must be performed to elucidate the evolutionary origin of the pleural plates, our interpretation favors the idea that insect wings have dual origins, namely paranotal lobes and the proximal leg segments.
This is not the first time this type of “combinational model” has been proposed. In fact, both the modified paranotal theory and the revised exite theory propose the dual origin of insect wings (notal expansion+subcoxa in Rasnitsyn, ref. 1; and notal expansion+epicoxa in Kukalova-Peck, ref. 6). More recently, based on expression analysis, Niwa et al. showed that there may be two potential “organ inductive fields” in basal insects and proposed that the fusion of these two fields might be the origin of insect wings (36). Our analysis has revealed a striking similarity in gene networks between wings and notal expansions (i.e., carinated margin) and also provides compelling functional evidence for the dual origin of insect wings.
On the Origin of the Treehopper Helmet.
Recently, the helmet of treehoppers, a unique T1 expansion, was proposed to be serially homologous to wings (26). However, later this idea was rebutted, largely because of misinterpretations in the morphological analysis (37, 38). Detailed morphological analysis supports the idea that the treehopper helmet is an extreme posterior expansion of T1 itself (posterior, flattened, cuticular evagination; PFE) instead of an appendage on T1 (37). The widespread tendency for lateral and posterior pronotal expansions in hemipteran insects (such as lace bugs, Tingidae) also supports this idea (37). Nonetheless, the genetic similarity between the treehopper helmet and wings (such as nub and Dll expression) (26) is still remarkable. Miko et al. and Yoshizawa suggested that the genetic similarity was achieved via cooption of wing genes into body wall development (37, 38). Our findings provide yet another possible explanation for the origin of the treehopper helmet. The location of the pronotal expansion in hemipteran insects (at least its lateral portion) appears to correspond to the carinated margin in the beetle T1 (5, 37). Therefore, it is intriguing to speculate that the pads/helmets of hemipteran insects are homologous to beetle carinated margins (but not pleural plates), hence “partially” serially homologous to wings. Gaining (or regaining) some additional wing genes might have allowed hemipteran insects to quickly acquire impressively developed helmet structures. This scenario might also explain the superficial morphological similarities between pronotal pads and wings (such as vein-like structures in lace bugs), because the genetic networks responsible for making these two tissues may share common ancestry. Even bolder speculation can be made regarding the wing-like paranotal lobes of Paleodictyoptera, which were morphologically similar to wings, but lacked elaborate hinge structures (3, 4). These lobes may also have been partially serially homologous to wings. Genetic and developmental analyses in hemipteran insects that possess pronotal expansions (including the helmet of treehoppers) should provide more clues to the origin of these tissues.
Homology Among vg-Dependent Tissues.
Although identifying homologous structures (both serial homologs in one species and homologs among different species) is crucial to understand the history of morphological evolution, caution must be taken for this kind of homologization. For instance, vg function in several different tissues may also be the outcome of independent deployment of vg in evolutionarily unrelated tissues (i.e., deep homology via cooption). The extended conservation of the wing gene network in the carinated margin formation, in addition to the homologous positioning of the carinated margin to that of the paranotal lobe, may be used to argue against this possibility. Nonetheless, it will be a challenge to rule out the possibility of cooption. Yet another possibility is an “independent loss” of pleiotropy. It is plausible that vg was once important for a large portion of body parts (including wings and body wall), but the vg functional domain may have been limited to different regions of the body in different lineages. This scenario would also result in two nonhomologous tissues sharing expression and function of vg.
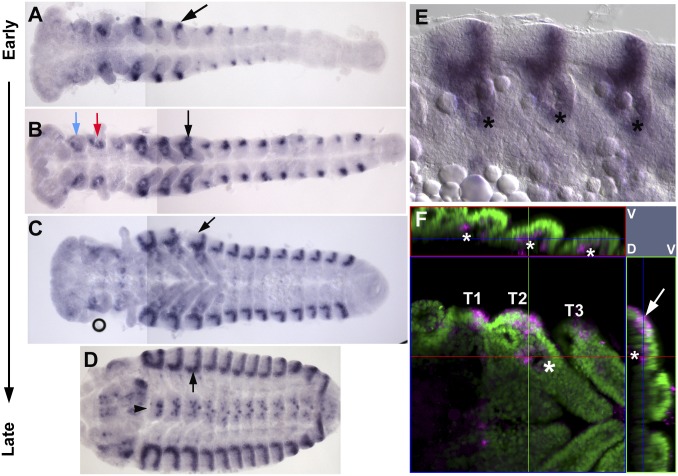
One way to discern homologous structures from linage-specific traits is to analyze multiple lineages. For example, if the vg function in T1 we identified in Tribolium represents a beetle-unique situation, we would expect to see variations of vg function/expression in T1 in different lineages. However, vg is expressed in both the edge of terga and the proximal leg segments of a basal insect (bristletail) (36), which appears to parallel the vg function in T1 of Tribolium. This observation may further support our interpretation that the wing-related tissues are maintained as (or reverted to) a more ancestral state in the Tribolium T1. Tracing the developmental origin of vg-dependent structures may also help us evaluate structural homology. Our expression analysis for vg has revealed extensive dorsal ectodermal expression of vg in the Tribolium embryo, which may correspond to the edge of the terga (including the future T1 carinated margin) (Fig. 6 A–D). Interestingly, we also identified invaginated vg-positive cell populations at the dorsal side of the base of leg primordia in the Tribolium embryo (Fig. 6 E and F). These invaginated “sacks” are found in all three thoracic segments. It would be enlightening to examine how these cells contribute to the pleural plates, the carinated margin, and the wing structures in Tribolium. Detailed developmental analysis of the vg-dependent structures in Tribolium, as well as other insect and arthropod species, should provide unique insights into the origin of insect wings.
Fig. 6.
vg expression in the Tribolium embryo. (A–D) vg expression during Tribolium embryogenesis. Black arrow denotes T3. Mandible (blue arrow in B), maxilla (red arrow in B), and central nervous system (arrowhead in D) express vg. For A–C, two images were composed into one to show the whole embryo. (E) Close-up of the thoracic region of C focused dorsally, showing additional vg staining in all three thoracic segments (asterisks). (F) Confocal image of vg expression (purple) with DAPI nuclear staining (green) in the thoracic segments. Asterisks indicate additional vg expression in the thoracic segments dorsal to the leg primordia. Tergal vg expression is indicated by an arrow. Dorsal and ventral sides of the image are indicated in the upper right side of F by “D” and “V”, respectively.
While this manuscript was under review, Ohde et al. reported that the hypomeron in T1 and gin traps in the pupal abdominal segments are wing serial homologs in another beetle, Tenebrio molitor (39). Their finding of wing serial homologs in nonwinged segments, together with our detailed identification of T1 wing homologs, the carinated margin (a specific part of the hypomeron) as well as nonhypomeron structures such as the pleural plates, will further our understanding of insect wing origin and diversification.
Materials and Methods
Insect Cultures.
Beetles were cultured on whole wheat flour [+5% (wt/wt) yeast] at 30 °C. Detailed genotypes of the beetles and flies used in this study are in SI Materials and Methods.
Gene Cloning, dsRNA Synthesis, and RNAi.
Injection and dsRNA synthesis were performed as described (40). Detailed information including primer sequences, inverse PCR, RACE, off-target effect assessment, and GenBank accession numbers are in SI Materials and Methods and Table S1.
Tissue Staining and Documentation.
In situ hybridization was performed as described (15). The images were captured by using Zeiss AxioCam MRc5 with AxioPlan 2 or Zeiss Discovery V12. Confocal images were captured by using Zeiss 710. Detailed tissue dissection and fixation procedures are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank R. Beeman, S. Haas, and K. Leonard for Cx mutants; Bloomington Stock Center and Vienna Drosophila RNAi Center for fly stocks; the Center for Bioinformatics and Functional Genomics and Center for Advanced Microscopy and Imaging at Miami University for technical support; J. Parker for helpful comments; P. Ravisankar and H. Steigelman for technical assistance; and members of Y.T. laboratory for discussion. This work was supported by a Miami University start-up grant (to Y.T.) and National Science Foundation Grant IOS 0950964 (to Y.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession nos. KC688264–KC688267 and KF684967).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304332110/-/DCSupplemental.
References
- 1.Rasnitsyn AP. A modified paranotal theory of insect wing origin. J Morphol. 1981;168:331–338. doi: 10.1002/jmor.1051680309. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton KGA. The insect wing, Part 1. Origin and development of wings from notal lobes. J Kans Entomol Soc. 1971;44:421–433. [Google Scholar]
- 3. Grimaldi D, Engel MS (2005) Insects Take to the Skies. Evolution of the Insects (Cambridge Univ Press, New York), pp 155–187.
- 4. Kukalova-Peck J (1991) Fossil history and the evolution of hexapod structures. The Insects of Australia: A Textbook for Students and Research Workers, ed Naumann ID (Melbourne Univ Press, Carlton, Australia), 2nd Ed, Vol 1, pp 141–179.
- 5. Snodgrass RE (1935) The Thorax. Principles of Insect Morphology (Cornell Univ Press, New York), pp 157–192.
- 6.Kukalova-Peck Origin of the insect wing and wing articulation from the arthropodan leg. Can J Zool. 1983;61:1618–1669. [Google Scholar]
- 7.Kukalova-Peck J. Phylogeny of higher taxa in insecta: Finding synapomorphies in the extant fauna and separating them from homoplasies. Evol Dev. 2008;35:4–51. [Google Scholar]
- 8.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385(6617):627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 9.Brook WJ, Diaz-Benjumea FJ, Cohen SM. Organizing spatial pattern in limb development. Annu Rev Cell Dev Biol. 1996;12:161–180. doi: 10.1146/annurev.cellbio.12.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117(2):597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- 11.Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124(1):125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- 12.Zecca M, Struhl G. Control of Drosophila wing growth by the vestigial quadrant enhancer. Development. 2007;134(16):3011–3020. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- 13.Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991;5(12B):2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- 14.Halder G, et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12(24):3900–3909. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomoyasu Y, Arakane Y, Kramer KJ, Denell RE. Repeated co-options of exoskeleton formation during wing-to-elytron evolution in beetles. Curr Biol. 2009;19(24):2057–2065. doi: 10.1016/j.cub.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Ohde T, et al. Vestigial and scalloped in the ladybird beetle: A conserved function in wing development and a novel function in pupal ecdysis. Insect Mol Biol. 2009;18(5):571–581. doi: 10.1111/j.1365-2583.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Bell JB, Simmonds AJ. Vestigial is required during late-stage muscle differentiation in Drosophila melanogaster embryos. Mol Biol Cell. 2010;21(19):3304–3316. doi: 10.1091/mbc.E10-04-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guss KA, Mistry H, Skeath JB. Vestigial expression in the Drosophila embryonic central nervous system. Dev Dyn. 2008;237(9):2483–2489. doi: 10.1002/dvdy.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hlavac TF. The prothorax of Coleoptera: Origin, major features of variation. Psyche (Stuttg) 1972;79(3):123–149. [Google Scholar]
- 20.El-Kifl Morphology of the adult Tribolium confusum Duv. and its differentiation from Tribolium (Stene) castaneum Herbst. Bulletin de la Société Fouad Ier d'Entomologie. 1953;37:173–249. [Google Scholar]
- 21.Bolognesi R, et al. Tribolium Wnts: Evidence for a larger repertoire in insects with overlapping expression patterns that suggest multiple redundant functions in embryogenesis. Dev Genes Evol. 2008;218(3-4):193–202. doi: 10.1007/s00427-007-0170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 23.Shirras AD, Couso JP. Cell fates in the adult abdomen of Drosophila are determined by wingless during pupal development. Dev Biol. 1996;175(1):24–36. doi: 10.1006/dbio.1996.0092. [DOI] [PubMed] [Google Scholar]
- 24.Damen WG, Saridaki T, Averof M. Diverse adaptations of an ancestral gill: A common evolutionary origin for wings, breathing organs, and spinnerets. Curr Biol. 2002;12(19):1711–1716. doi: 10.1016/s0960-9822(02)01126-0. [DOI] [PubMed] [Google Scholar]
- 25.Jockusch EL, Ober KA. Hypothesis testing in evolutionary developmental biology: A case study from insect wings. J Hered. 2004;95(5):382–396. doi: 10.1093/jhered/esh064. [DOI] [PubMed] [Google Scholar]
- 26.Prud’homme B, et al. Body plan innovation in treehoppers through the evolution of an extra wing-like appendage. Nature. 2011;473(7345):83–86. doi: 10.1038/nature09977. [DOI] [PubMed] [Google Scholar]
- 27.Ng M, Diaz-Benjumea FJ, Cohen SM. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development. 1995;121(2):589–599. doi: 10.1242/dev.121.2.589. [DOI] [PubMed] [Google Scholar]
- 28.Cifuentes FJ, García-Bellido A. Proximo-distal specification in the wing disc of Drosophila by the nubbin gene. Proc Natl Acad Sci USA. 1997;94(21):11405–11410. doi: 10.1073/pnas.94.21.11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzen MD, et al. piggyBac-mediated germline transformation in the beetle Tribolium castaneum. Insect Mol Biol. 2003;12(5):433–440. doi: 10.1046/j.1365-2583.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 30.Tomoyasu Y, Wheeler SR, Denell RE. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature. 2005;433(7026):643–647. doi: 10.1038/nature03272. [DOI] [PubMed] [Google Scholar]
- 31.Berghammer AJ, Klingler M, Wimmer EA. A universal marker for transgenic insects. Nature. 1999;402(6760):370–371. doi: 10.1038/46463. [DOI] [PubMed] [Google Scholar]
- 32.Beeman RW, Stuart JJ, Haas MS, Denell RE. Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev Biol. 1989;133(1):196–209. doi: 10.1016/0012-1606(89)90311-4. [DOI] [PubMed] [Google Scholar]
- 33.Curtis CD, et al. Molecular characterization of Cephalothorax, the Tribolium ortholog of Sex combs reduced. Genesis. 2001;30(1):12–20. doi: 10.1002/gene.1027. [DOI] [PubMed] [Google Scholar]
- 34.Shippy TD, Rogers CD, Beeman RW, Brown SJ, Denell RE. The Tribolium castaneum ortholog of Sex combs reduced controls dorsal ridge development. Genetics. 2006;174(1):297–307. doi: 10.1534/genetics.106.058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263(2):282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Niwa N, et al. Evolutionary origin of the insect wing via integration of two developmental modules. Evol Dev. 2010;12(2):168–176. doi: 10.1111/j.1525-142X.2010.00402.x. [DOI] [PubMed] [Google Scholar]
- 37.Mikó I, et al. On dorsal prothoracic appendages in treehoppers (Hemiptera: Membracidae) and the nature of morphological evidence. PLoS ONE. 2012;7(1):e30137. doi: 10.1371/journal.pone.0030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshizawa K. The treehopper’s helmet is not homologous with wings (Hemiptera: Membracidae) Syst Entomol. 2012;37:2–6. [Google Scholar]
- 39. Ohde T, Yaginuma T, Niimi T (2013) Insect morphological diversification through the modification of wing serial homologs. Science 340(6131):495–498. [DOI] [PubMed]
- 40.Philip BN, Tomoyasu Y. Gene knockdown analysis by double-stranded RNA injection. Methods Mol Biol. 2011;772:471–497. doi: 10.1007/978-1-61779-228-1_28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.