Significance
We present a unique study of a liver-induced systemic tolerance using an intact virus, other than a single protein component-induced liver-persistent model. Liver-educated regulatory cells migrate into peripheral sites and mediate the systemic tolerance. Kupffer cells induce the generation of type 1-like regulatory cells in an IL-10–dependent manner. A possible role of type 1-like regulatory cells in suppressing germinal center formation via secreting IL-10 is demonstrated. We observed that a “regulatory cell-based” tolerizing mechanism, rather than a “clonal deletion” mechanism, provides the possibility of reversing the tolerance by immunotherapeutic approaches.
Keywords: peripheral tolerance, unresponsive
Abstract
The liver plays a critical role in inducing systemic immune tolerance, for example, during limiting hypersensitivity to food allergy and in rendering acceptance of allotransplant or even hepatotropic pathogens. We investigated the unknown mechanisms of liver tolerance by using an established hepatitis B virus (HBV)-carrier mouse model, and found that these mice exhibited an antigen-specific tolerance toward peripheral HBsAg vaccination, showing unenlarged draining lymph node (DLN), lower number of germinal centers (GC), and inactivation of GC B cells and follicular T helper (Tfh) cells. Both in vivo and in vitro immune responses toward HBsAg were suppressed by mononuclear cells from HBV-carrier mice, which were CD4+ Foxp3− type 1 regulatory T (Tr1)-like cells producing IL-10. Using recipient Rag1−/− mice, hepatic Tr1-like cells from day 7 of HBV-persistent mice acquired the ability to inhibit anti-HBV immunity 3 d earlier than splenic Tr1-like cells, implying that hepatic Tr1-like cells were generated before those in spleen. Kupffer cell depletion or IL-10 deficiency led to impairment of Tr1-like cell generation, along with breaking HBV persistence. The purified EGFP+CD4+ T cells (containing Tr1-like cells) from HBV-carrier mice trafficked in higher numbers to DLN in recipient mice after HBsAg vaccination, and subsequently inactivated both Tfh cells and GC B cells via secreting IL-10, resulting in impaired GC formation and anti-HB antibody production. Thus, our results indicate Tr1-like cells migrate from the liver to the DLN and inhibit peripheral anti-HBV immunity by negatively regulating GC B cells and Tfh cells.
The liver is a unique organ that favors induction of immune tolerance rather than immune activation (1–3). It was reported 40 y ago that primary liver transplant induced a recipient pig to accept other allograft organs from the same donor (4), which suggested that the liver not only maintained the self-tolerance, but also induced systemic tolerance in an antigen-specific manner. However, the precise mechanisms of how the liver induces systemic tolerance are not very clear. Previous studies showed that regulatory T cells (Tregs) are considered to be major players in mediating peripheral tolerance by suppressing both cellular and humoral immune response (5, 6). In vivo, Tregs are developmentally classified into natural Tregs (nTregs) and adaptive Tregs (iTregs). nTregs, identified by a CD4+CD25+Foxp3+ phenotype, differentiate in the thymus and function generally to prevent autoimmune disease. iTregs, comprised of several different subsets, are generated in secondary lymphoid organs or tissues and mediate tolerance toward foreign antigens; they can be subdivided into Foxp3+ Tregs, generated by de novo conversion from mature peripheral naive CD4+Foxp3− T cells, and Foxp3− Tregs, which includes the type 1 regulatory T (Tr1) cell, which suppresses via an IL-10–dependent mechanism as well as the T helper (Th) 3 cell, which mediates suppression via TGF-β production (7).
Tr1 cells have previously been shown to play a role in suppressing tissue inflammation, graft-versus-host disease, and autoimmunity by producing IL-10 in an antigen-specific manner (8–10). In diabetic mice, for example, IL-10 plus rapamycin prevented allograft rejection by inducing Tr1 cells that mediated stable antigen-specific tolerance in vivo (9). Furthermore, adoptive transfer of antigen-specific Tr1 cells induced an IL-10–dependent tolerance in the stringent mouse model of islet transplantation (11). The soluble peptide, myelin basic protein (MBP), could induce Ag-specific Tr1 cell generation, which plays a pivotal role in regulating T-cell tolerance that reverses ongoing experimental autoimmune encephalomyelitis disease in a rat model (12), which is consistent with the observation that MBP expression in mouse liver induces antigen-specific Tregs that protect from experimental autoimmune encephalomyelitis (13). Tr1 cells, in particular, are increased in the livers of patients with recurrent hepatitis C virus (HCV) infection after liver transplantation (14) and also mediate oral tolerance to BSA-induced systemic anaphylaxis (15). Interestingly, tolerogenic antigen-presenting cells in the liver also play a critical role to promote tolerance, but their relationship to Tr1-like cells is currently unclear (16). Therefore, a precise study to explore how viral persistence in the liver induces systemic tolerance is particularly needed.
In the present study, we tried to elucidate the role of Tr1-like cells in creating host systemic liver tolerance to hepatotropic pathogens, including hepatitis B virus (HBV), which might possibly be the key reason why pathogens can escape from immune surveillance and then establish lifelong chronic infection. Using an established HBV-carrier mouse model, we found these mice exhibited an antigen-specific tolerance toward peripheral HBsAg vaccination, showing an unenlarged draining lymph node (DLN), a lower number of germinal centers (GCs), and inactivation of GC B cells and follicular Th cells (Tfh cells). Immune responses toward HBsAg were suppressed by CD4+ Foxp3− Tr1-like cells from HBV-carrier mice, which acquired the ability to inhibit anti-HBV immunity 3 d earlier than splenic Tr1-like cells, and impairment of Tr1-like cell generation led to breaking HBV persistence. The purified EGFP+CD4+ T cells (with Tr1-like cells) from HBV-carrier mice trafficked in higher numbers to DLN in recipient mice after HBsAg vaccination, and subsequently inactivated both Tfh cells and GC B cells via secreting IL-10, resulting in impaired GC formation and anti-HBs antibody production. To our knowledge, we reveal a previously undescribed mechanism of liver tolerance to HBV infection, which may be helpful to explore new approaches to reverse liver-induced systemic immune tolerance in liver or even systemic disease.
Results
HBV-Specific Regulatory Cells Mediate HBV-Induced Immune Tolerance.
To explore the mechanism involved in the liver-induced systemic tolerance, we established an HBV-carrier mouse model by hydrodynamic injection of pAAV/HBV1.2 plasmid into C57BL/6 mice (17), which has been extensively used (18, 19). In contrast to control (pAAV/Control-injected) mice, HBV-carrier (pAAV/HBV1.2-injected) mice did not secrete anti-HBs antibodies after peripheral immunization with HBsAg (20); indicating the tolerance toward HBsAg was developed in HBV-carrier mice. To test whether this tolerance was mediated by regulatory cells, we found splenocytes from HBV-carrier mice significantly inhibited the production of anti-HBs antibodies in an antigen-specific manner (Fig. 1 A and B).
Fig. 1.
HBV-specific regulatory cells mediate HBV-induced immune tolerance. (A) Splenocytes from HBV-carrier or control mice were transferred (intravenously) (2 × 107) into naive mice. Serum anti-HBs levels were measured 1 wk after HBsAg vaccination. (B) Mice were treated as in A, serum antiovalbumin IgG levels were determined by ELISA. (C) HBV-carrier WT or HBV-carrier Rag1−/− mice received a transfer (intravenously) of splenocytes from HBsAg-immunized mice (2 × 107) (called “anti-HBV” here). (D) HBV-carrier Rag1−/− mice received splenocytes from either HBV-carrier mice (2 × 107) (called “HBV” here), anti-HBV (2 × 107), or a mixture of HBV with anti-HBV at a 1:1 ratio (total 4 × 107 cells). (E) Naive mice received a transfer (intravenously) of splenocytes from control mice or anti-HBV, followed by 6 μg of HBV plasmid injection. Serum HBsAg levels were measured (C–E) at indicated time points. (F) 3H-TdR incorporation was used to detect specific inhibition by splenocytes from HBV-carrier mice. Results represent two to four independent experiments (n = at least 3 per group). *P < 0.05, **P < 0.01, and ***P < 0.001.
A cellular adoptive transfer experiment showed that splenocytes from HBsAg-vaccinated mice eliminated HBV within 2 wk in recipient HBV-carrier Rag1−/− mice (Fig. 1C), and this effect could be attenuated by splenocytes from HBV-carrier mice (Fig. 1D). Anti-HBV immune cells could not completely eliminate HBV in WT HBV-carrier mice, perhaps because of preexisting tolerant regulatory immune cells (Fig. 1C); however, anti-HBV immune cells could prevent against establishing an HBV-carrier state after HBV plasmid injection in WT mice without tolerant regulatory immune cells (Fig. 1E). Furthermore, splenocytes from HBV-carrier mice significantly inhibited proliferation of splenocytes from HBsAg-vaccinated mice after HBsAg rechallenge in vitro (Fig. 1F). These data indicate that splenocytes from HBV-carrier mice contain an immune cell population with an HBsAg-specific regulatory function.
HBV-Specific Regulatory Cells Are IL-10–Producing Tr1-Like Cells.
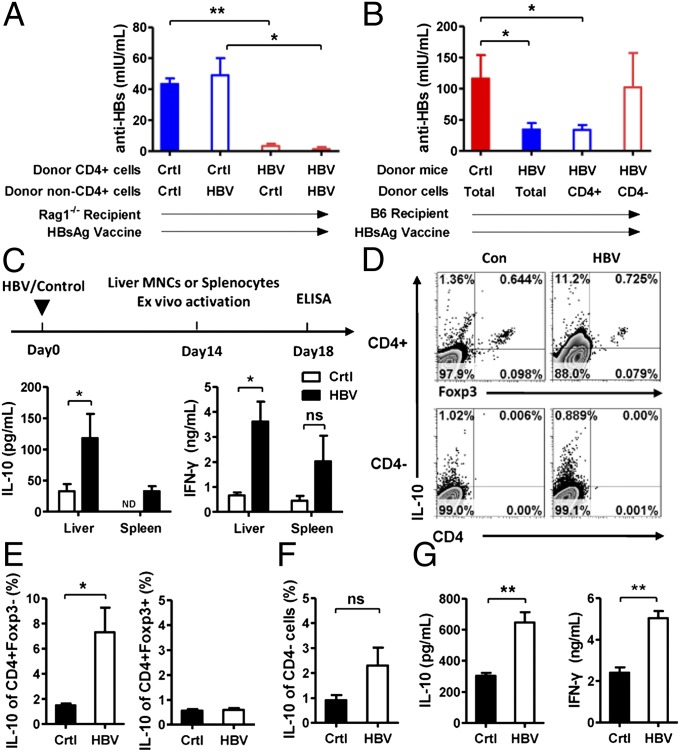
We next identified the regulatory cell population. Because splenocytes from HBV-carrier Rag1−/− mice did not confer any suppressive effect on anti-HBs production (Fig. S1), the regulatory cells were likely of T- or B-cell origin. Whereas control CD4+ T cells (purity was confirmed in Fig. S2) could trigger anti-HBs production independent of the non-CD4 cell origin, purified donor CD4+ T cells from HBV-carrier mice inhibited anti-HBs antibody production in recipient Rag1−/− mice (Fig. 2A). We next found purified CD4+ T cells from HBV-carrier mice inhibited anti-HBs production in recipient WT B6 mice (Fig. 2B), further suggesting that a subset of the CD4+ T-cell pool mediated HBsAg-specific tolerance (21).
Fig. 2.
HBV-specific regulatory cells are IL-10–producing Tr1-like cells. (A) CD4+NK1.1− cells were sorted from splenocytes of control or HBV-carrier mice, and the remaining cells without CD4+NK1.1− cells were called non-CD4+ cells. Recipient Rag1−/− mice received CD4+ cells and non-CD4+ cells in various combinations. (B) Naive recipient mice were divided into four groups: two groups received an intravenous transfer of total splenocytes (2 × 107) from control or HBV-carrier mice, and the others received an intravenous transfer of CD4+ cells (0.4 × 107) or non-CD4 cells (1.6 × 107) from HBV-carrier mice, respectively. Three days after cells transfer, recipient Rag1−/− mice were immunized with HBsAg vaccine twice within a 2-wk interval and then anti-HBs levels were detected for the treatment shown in A and B at day 7 after the last HBsAg vaccination. (C) Liver MNCs or splenocytes from control or HBV-carrier mice were cultured with anti-CD3 plus anti-CD28 antibodies. After 96 h, supernatants were assessed for IL-10 and IFN-γ levels. (D) Treatment was performed as in C. IL-10 expression levels in CD4+ or CD4− cells from liver MNCs of HBV-carrier or control mice was determined by flow cytometry. (E and F) Statistical analysis of IL-10 expression in CD4+Foxp3− or CD4+Foxp3+ cells (E) and in CD4− cells (F). (G) Treatment was performed as in C. Hepatic CD4+ T cells were purified from control or HBV-carrier mice, and then supernatant levels of IL-10 and IFN-γ were detected. ND, not detected; ns, no significance. Results represent two to three independent experiments (n = at least 3 per group). *P < 0.05 and **P < 0.01.
Previous studies have shown that Tregs play a critical role in liver tolerance (13, 22, 23); however, in our study, Foxp3+ Treg numbers did not change in HBV-carrier mice, and CD25+ Treg depletion did not influence HBV persistence (Fig. S3). Evaluating the cytokine profile in cultured hepatic or splenic mononuclear cell (MNC) supernatants, concurrent significant increases in IL-10 and IFN-γ were observed from HBV-carrier liver MNCs (Fig. 2C), but TGF-β, IL-4, and IL-2 levels did not differ between the two groups (Fig. S4). Flow cytometric analysis demonstrated that CD4+Foxp3− T cells, but not CD4+Foxp3+ Tregs or CD4− cells were the main IL-10 source (Fig. 2 D–F). Furthermore, because purified CD4+ T cells from HBV-carrier mice secreted significantly more IL-10 and IFN-γ in culture supernatant (Fig. 2G), our results suggest that IL-10–producing Tr1-like cells play a major role in inducing liver immune tolerance. Previous study has suggested that coexpression of CD49b and LAG3 could uniquely identify Tr1 cells (24), and we also found that Tr1-like cells coexpressed LAG3 and CD49b increased significantly in HBV-carrier mice (Fig. S5).
Kupffer Cells Promote the Development of Tr1-Like Cells.
Antigen-specific regulatory CD4+ T-cell generation requires antigen-presenting cells help. Previous studies showed that liver resident Kupffer cells (KCs) have the potential to induce T-cell tolerance in liver (25, 26). In our model, we found IL-10 and IFN-γ production significantly decreased in hepatic MNCs from KC-depleted HBV-injected mice (Fig. 3A), raising the possibility that HBV-specific Tr1-like cell generation is dependent on KCs in this study. To address this possibility, naive CD4+ T cells were cocultured with KCs in vitro. Coculture with KCs from HBV-carrier mice increased both IL-10 productions and Tr1-like cell generation from naive CD4+ T cells (Fig. 3B), suggesting that KCs induced Trl-like cell generation. In addition, adding anti–IL-10R mAb or using KCs from IL-10−/−HBV mice in the coculture system blocked the generation of IL-10 and Tr1-like cells (Fig. 3C).
Fig. 3.
KC-derived IL-10 is critical for generation of Tr1-like cells. (A) IL-10 and IFN-γ levels in culture supernatant of hepatic MNCs from different mice. (B) Naive CD4+ T cells were cocultured with KC cells from control or HBV-carrier mice for 3 d. CD4+ T cells were isolated from culture and activated with anti-CD3/CD28 in vitro. Four days later, secreted IL-10 in supernatant and Tr1 phenotype (CD4+Foxp3−IL-10+) cells were assessed. (C) Naive CD4+ T cells were cocultured with KCs from HBV-carrier mice in the presence or absence of anti–IL-10R mAb (10 μg/mL) or IL-10−/− HBV mice and then treated as in B. (D) Naive mice were transferred with total splenocytes (2 × 107) or purified CD4+ T cells (4 × 106) from control, HBV-carrier, KC-depleted HBV, or IL-10−/− HBV mice. One day later, mice were immunized with HBsAg. (E) The treatment was performed as in Fig. 1D. Serum HBsAg levels were measured 1 wk after donor cell transfer. KCs depletion was obtained by intravenous injection of clodronate liposome (125 μL) once on day −2 before hydrodynamic injection of HBV plasmid. ND, not detected. Results represent two independent experiments (n = 3–6 per group). *P < 0.05 and **P < 0.01.
To further confirm this finding in vivo, we found that neither splenocytes nor CD4+ T cells from KC-depleted or IL-10−/− HBV-injected mice could significantly inhibit anti-HB antibody production in recipient mice following HBsAg vaccination (Fig. 3D). Moreover, donor splenocytes from KC-depleted HBV-injected mice lost this inhibitory effect on coinjected donor splenocytes from HBV-immunized mice, resulting in HBV elimination in Rag−/− mice (Figs. 1D and 3E). Although KCs in HBV-carrier mice did not exhibit any obvious tolerance-associated phenotypic changes (Fig. S6), which may be explained by residing in a sustained typical liver-tolerant environment (1, 25), these data strongly suggest that KCs contribute to systemic immune tolerance by inducing HBV-specific Tr1-like cell generation in an IL-10-dependent manner.
Tr1-Like Cells Contribute to Abnormal GC Formation in the DLN of HBV-Carrier Mice.
Because we observed that anti-HBs antibodies decrease upon HBV-induced tolerance, antibody production in the GC of DLNs may be being repressed in HBV-carrier mice. We therefore examined GC formation as well as the phenotype and frequency of GC B cells, Tfh cells, and dendritic cells (DC) cells in the DLN. Although lymph nodes (LNs) were morphologically enlarged in normal mice after HBsAg vaccination, with markedly increased GC number and total MNC numbers, LNs from HBV-carrier mice remained small and relatively static in cell number (Fig. 4 A and B). The frequency of GC B cells expressing high Bcl-6 levels within total B220+ B cells (Fig. 4 C and D, and Fig. S7), as well as of Tfh cells and CD44/inducible T-cell costimulator (ICOS) double-positive CD4+ cells (Fig. 4 E and F, and Fig. S7) were lower in HBV-carrier mice. Although we observed the increased DC number and CD86 expression on these DCs at day 4 after HBV vaccination, these changes did not differ between the control and HBV-carrier mice (Fig. S8). Taken together, HBV persistence influences GC B cells and Tfh cells, but not DCs, in DLNs.
Fig. 4.
GC B cells and Tfh cells are low in the DLN of HBV-carrier mice after HBsAg vaccination. At 12 d after HBsAg vaccination at the tibialis anterior muscle, lymphocytes from the DLN (popliteal LN) were stained with different antibodies. (A) The DLN was photographed (Left) and total cells were counted (Right). (B) DLN sections were stained with Biotin-PNA; GCs are shown (Scale bar, 200 μm). (C) GC B cell (B220+Gl-7+Fas+) frequency and (D) Bcl-6 expression levels were examined. The MNC amount of the popliteal LN was too low from control mice without immunization with HBsAg (red symbol), so we put MNCs from two mice into one tube for intracellular staining by flow cytometry. (E) Tfh cell (CD4+CXCR5+PD-1+) frequency and (F) CD44+ ICOS+ cell percentage among CD4+ T cells were measured. Results represent three independent experiments (n = at least 3 per group). *P < 0.05, **P < 0.01, and ***P < 0.001.
To evaluate when the Tr1-like cell-containing MNCs acquired the ability to inhibit anti-HBV immunity, splenocytes or hepatic MNCs, isolated at different time points from HBV-injected mice, were transferred into recipient Rag1−/− mice. At day 10 after HBV-plasmid injection, both splenocytes and liver MNCs acquired a suppressive effect on anti-HB production (Fig. 5A) and GC B-cell development (Fig. 5B). However, hepatic MNCs obtained this function at least 3 d earlier than splenocytes (Fig. 5 A and B). Furthermore, we compared the suppressive effect of hepatic and splenic CD4+ T cells taken at day 7 after HBV plasmid injection. Fig. 5C shows that hepatic CD4+ T, but not splenic CD4+ T cells, delivered tolerance into naive Rag1−/− mice toward HBsAg vaccination. These data strongly suggested that Tr1-like cells appeared in liver MNCs before in splenocytes. Because Tr1-like cells were present in relatively low frequency, we purified EGFP+CD4+ T cells (containing Tr1-like cells) from liver MNCs of control or HBV-carrier mice to visually trace their migration after transfer. Cells from HBV-carrier mice trafficked in higher numbers than control cells to the DLN in recipient mice after HBsAg vaccination (Fig. 5D). We also tested the ability of purified hepatic CD4+ T cells from HBV-carrier mice to directly affect the generation and activation of GC B cells and Tfh cells by coculturing them with GC B cells and EGFP+ Tfh cells from the LNs of HBsAg-immunized mice. The generation and activation of both GC B cells and EGFP+ Tfh cells were inhibited after coculturing for 3 d (Fig. 5 E and F). This inhibition of GC B cells was IL-10–dependent, because anti–IL-10R antibody treatment attenuated this inhibition in the coculture (Fig. 5E). Furthermore, anti–IL-10R antibody treatment resulted in Tfh cells slightly increasing despite no statistical differences (Fig. 5F).
Fig. 5.
Hepatic Tr1-like cells inactivate Tfh and GC B cells in DLN of HBV-carrier mice after HBsAg vaccination. (A and B) Recipient Rag1−/− mice received liver MNCs (1 × 107) or splenocytes (1 × 107) isolated at different time points (days 1, 7, and 10) from HBV-plasmid–injected mice. Serum anti-HBs antibody levels (A) and GC B cells (B) were determined after multiple HBsAg vaccination. (C) Seven days after HBV plasmids injection, hepatic or splenic CD4+ T cells were purified from 56 HBV-injected mice by MACS. Recipient Rag1−/− mice received CD4+ cells (0.5 × 107) and non-CD4+ cells (2.0 × 107) in various combinations. Then, the recipient mice were treated as shown in Fig. S9A. (L, liver MNCs; ND, not detected; S, splenocytes). (D) EGFP+CD4+ T cells from liver MNCs of HBV-carrier or control mice were adoptively transferred into naive recipient mice, which were then immunized with HBsAg vaccine 1 d later. After 4 wk, flow cytometry was used to analyze EGFP+CD4+ T-cell number in the DLN. (E and F) Hepatic CD4+ T cells from HBV-carrier (2 wk) mice were cocultured with purified B cells (E) or sorted EGFP+CD4+ T cells (F) from DLN of HBsAg-immunized mice in presence of anti–IL-10R or not. GC B cell or EGFP+ Tfh cell frequency was measured after 3 d. Results represent two independent experiments (n = at least 3 per group). *P < 0.05 and **P < 0.01.
IL-10 from Tr1-Like Cells Plays a Crucial Role in Inducing Liver Tolerance.
CD4+Foxp3− Tr1 cells have previously been reported to suppress immune responses mainly via IL-10 production (7). Interestingly, IFN-γ also significantly decreased in the supernatant of cultured hepatic MNCs from HBV+ IL-10−/− mice (Fig. 6 A and B), suggesting a determinant role for IL-10 in Tr1 cell development. Consistent with the above result, the frequency of GC B cells also increased significantly in HBV-injected IL-10−/− mice after HBsAg vaccination (Fig. 6C), suggestive of the critical role of IL-10 in Tr1-like cell function. Furthermore, compared with Fig. 2B, we found that donor CD4+ T cells from HBV-injected IL-10−/− mice lost the ability to suppress anti-HB antibody production in recipient Rag1−/− mice after HBsAg vaccination (Fig. 6D), directly indicating IL-10 produced by Tr1-like cells plays a crucial role in inducing liver tolerance. Either hepatic CD4+ T cells or total splenocytes from HBV-carrier mice mediated tolerance toward HBsAg vaccination via secreting IL-10, because blockade of the anti–IL-10 receptor could reverse this tolerance (Fig. 5C and Fig. S9). These data indicated that IL-10 was required to induce systemic tolerance via inducing generation of Tr1-like cells, which eventually mediated tolerance through secreting IL-10 in HBV-carrier mice.
Fig. 6.
IL-10 plays a crucial role in Tr1-like cells-mediated systemic tolerance. (A and B) Treatment was performed as in Fig. 3E; IL-10 and IFN-γ levels were determined in culture supernatants of sorted CD4+ T cells from liver MNCs of control, HBV-carrier, or IL-10−/−HBV mice. (C) GC B-cell frequency was measured in DLN from HBV-carrier or IL-10−/− HBV-injected mice after HBsAg vaccination. (D) Splenic CD4+ T cells and splenocytes without CD4+ T cells (called non-CD4+ cells), from WT, HBV-carrier, or IL-10−/− HBV mice, were transferred to recipient Rag1−/− mice in various combinations. ND, not detected. Results represent two independent experiments (n = at least 3 per group). *P < 0.05.
Discussion
The liver induces antigen-specific tolerance by a series of mechanisms, including clonal deletion (similar to central tolerance), as well as induction of Tregs and inhibition of memory T-cell responses, which favor peripheral tolerance (6, 22, 27–29). However, the precise mechanisms underlining liver tolerance are not fully understood. In our HBV-carrier mouse model, we found specific responsiveness to HBsAg immunization was lost, showing systemic immune tolerance was induced by liver-persistent virus through generation of HBsAg-specific regulatory Tr1-like cells that migrated to the DLN and participated in inducing systemic tolerance by inhibiting GC formation upon HBsAg vaccination. These mechanisms provide fresh insight into the phenomenon of “liver tolerance” and may prove instrumental in exploring new approaches to reversing liver-induced systemic immune tolerance after chronic pathogen infection in the liver (e.g., HBV, HCV, and malaria).
Despite the finding that theories of “clonal deletion” or “regulatory cells” to explain systemic tolerance induced by liver persistent antigen were supported by different mouse models (23, 28), our data more strongly support regulatory cell mechanisms than clonal deletion in maintaining systemic tolerance toward HBsAg, because: (i) splenocytes from HBV-carrier mice can inhibit the immune response toward HBsAg stimulation (Fig. 1 A and F), (ii) splenocytes from HBV-carrier mice could greatly attenuate HBV elimination by splenocytes from anti-HB–positive mice (Fig. 1D), and (iii) immunotolerance in HBV-carrier mice could be reversed by immunostimulatory HBx-siRNA plasmid (20). These results indicate the real existence of HBsAg-specific clones in HBV-carrier mice. Because Tregs are developmentally divided into thymus-derived nTregs and peripheral-generated iTregs, HBV is a nonself antigen that is not expressed in the thymus, and CD4+CD25+ Treg depletion could not break tolerance in HBV-carrier mice (Fig. S3), the regulatory cells are unlikely to be Foxp3+ Tregs. Our results demonstrate that the unique cytokines observed in HBV-carrier mice, including IL-10 and IFN-γ, was consistent with the cytokine profile for Tr1 cells (Fig. 3), suggesting that Tr1-like cells are the major regulatory cell mediator functioning to maintain systemic tolerance. In Con A- and liver-targeting gene transfer of human α-1 antitrypsin by adenovirus-induced liver tolerance models (22), Breous et al. declared that Tregs played an important role in producing IL-10. However, the results also showed the probable presence of additional IL-10–producing cell type in liver, such as IL-10-producing Tr1-like cells.
Furthermore, we found that HBsAg-specific Tr1-like cells suppress the immune response to HBV vaccination in an IL-10–dependent manner. We found that IFN-γ significantly increased in purified CD4+ T cells from HBV-carrier mice, similar to IL-10. Although IFN-γ is typically considered to be an antiviral cytokine, it also plays an important role in maintaining immune tolerance in other studies, where IFN-γ may be related to antigen-specific regulatory cell differentiation, development, or clonal deletion (30, 31). The underlying mechanism for the role of IFN-γ, likely different from IL-10, remains elusive at present and needs further investigation in our model.
Previous studies have shown that Foxp3+ regulatory cells with a Tfh-like phenotype modulated the GC response in DLNs (32, 33). We found here that differentiation of GC B cells (Fig. 4 C and D) and Tfh cells (Fig. 4 E and F) was abnormal in DLN of HBV-carrier mice after HBsAg immunization. The enhanced Tr1-like cells-containing EGFP+CD4+ cells from HBV-carrier mice trafficked into the DLN of naive recipient mice after HBsAg vaccination (Fig. 5D). Furthermore, hepatic Tr1-like cells-containing CD4+ T cells directly decreased the number of GC B cells and Tfh cells in an in vitro coculture system in an IL-10–dependent manner (Fig. 5 E and F). These data indicate that Tr1-like cells migrated to the DLN to negatively modulate the differentiation of Tfh cells, GC B cells, or both. To our knowledge, this report of Tr1-like cells regulating antibody production is unique. Further experiments are needed to understand the mechanism by which Tr1-like cells inhibit GC reactions.
Taking these data together, our study provides evidence to support the hypothesis that Tr1-like cells migrate from the liver to the DLN and inhibit anti-HBV immunity by negatively regulating differentiation of GC B cells, Tfh cells, or both, although the underlying molecular mechanisms require further investigation.
Materials and Methods
Mice.
Male C57BL/6 mice (6-8 wk old) were purchased from the Shanghai Experimental Animal Center. IL-10−/− mice (B6.129P2-Il10tm1Cgn/J) were purchased from the Jackson Laboratory. Rag1−/− and EGFP-transgenic mice were obtained from the Model Animal Research Center. All mice were housed in a specific pathogen-free facility and used according to the guidelines for experimental animal use from the University of Science and Technology of China.
Immunohistochemistry.
To assess GC formation, sections of formalin-fixed and paraffin-embedded LN were stained with biotinylated PNA (Vector Laboratories) followed by streptavidin-HRP conjugates (Zhongshan Goldenbridge). The stains were all developed with a DAB kit (Vector Laboratories).
Cell Isolation.
Splenocytes and liver MNCs were separated as previously described (34). CD4+ T cells were separated by positive magnetic cell sorting using an anti-CD4 mAb according to the manufacturer’s instructions (Miltenyi Biotec). KC isolations were performed as previously described (35).
Cell Culture and Cytokine Assays.
Cytokine levels released into culture supernatant were measured as previously described (22). IL-10 and TGF-β1 levels in culture supernatants were measured with ELISA kits from R&D Systems. IL-2, IL-4, and IFN-γ levels were measured using a Cytometric Bead Array Kit (BD Biosciences). Anti–IL-10 receptor antibody was purchased from BD Biosciences.
Flow Cytometry.
Mouse MNCs from liver or DLN were isolated, blocked, and incubated with the indicated fluorescent mAbs. Foxp3 and IL-10 expression was evaluated using an intracellular staining set (eBioscience). The stained cells were analyzed using a FACSCalibur (Becton Dickinson) flow cytometer, and the data were analyzed with WinMDI 2.8 or FlowJo software (Tree Star).
3H-TdR Incorporation.
For detecting specific inhibition by splenocytes in HBV-carrier mice, splenocytes were cultured (5 × 105 cells per well) in a 96-well U-bottomed plate with 200 µL of completed RPMI 1640 for 52 h in the presence of 5 µg/mL HBsAg (adw subtype; Hytest). An unstimulated control was incubated under the same conditions in the absence of HBsAg. 3H-TdR (1 µCi per well; Amersham) was added for the last 16 h of culture. Data were collected using a β-liquid scintillation analyzer (Perkin-Elmer) and expressed as a proliferation index (PI = cpm of HBsAg-stimulated cells-blank/cpm of the unstimulated control-blank).
Statistical Analysis.
Unpaired two-tailed Student t test was used to compare variables between two groups. Data were expressed as means ± SEM, and significance was denoted as *P < 0.05, **P < 0.01, and ***P < 0.001. Calculations were performed using GraphPad Prism version 4.00 (GraphPad Software).
Supplementary Material
Acknowledgments
We thank Pei-Jer Chen (National Taiwan University) for the hepatitis B virus plasmid pAAV/HBV1.2 and N. V. Rooijen (Vrije Universiteit) for the clodronate liposomes. This work was supported by the Natural Science Foundation of China (91029303, 31021061), the Ministry of Science and Technology of China (973 Basic Science Project 2013CB944902, 2012CB519004), and the National Science and Technology Major Projects (2012ZX10002006, 2013ZX10002002-002).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306437110/-/DCSupplemental.
References
- 1.Crispe IN, et al. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect’. Immunol Cell Biol. 2002;80(1):84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 3.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47(2):729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 5.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 7.Cottrez F, Groux H. Specialization in tolerance: Innate CD(4+)CD(25+) versus acquired TR1 and TH3 regulatory T cells. Transplantation. 2004;77(1) Suppl:S12–S15. doi: 10.1097/01.TP.0000106471.23410.32. [DOI] [PubMed] [Google Scholar]
- 8.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 9.Battaglia M, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55(1):40–49. [PubMed] [Google Scholar]
- 10.Ahangarani RR, et al. In vivo induction of type 1-like regulatory T cells using genetically modified B cells confers long-term IL-10-dependent antigen-specific unresponsiveness. J Immunol. 2009;183(12):8232–8243. doi: 10.4049/jimmunol.0901777. [DOI] [PubMed] [Google Scholar]
- 11.Gagliani N, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010;59(2):433–439. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wildbaum G, Netzer N, Karin N. Tr1 cell-dependent active tolerance blunts the pathogenic effects of determinant spreading. J Clin Invest. 2002;110(5):701–710. doi: 10.1172/JCI15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lüth S, et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118(10):3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpentier A, et al. Increased expression of regulatory Tr1 cells in recurrent hepatitis C after liver transplantation. Am J Transplant. 2009;9(9):2102–2112. doi: 10.1111/j.1600-6143.2009.02743.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16(10):1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10(11):753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 17.Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103(47):17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YJ, et al. Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model. Proc Natl Acad Sci USA. 2010;107(20):9340–9345. doi: 10.1073/pnas.1004762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology. 2013;58(1):73–85. doi: 10.1002/hep.26339. [DOI] [PubMed] [Google Scholar]
- 21.Dobrzynski E, et al. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA. 2006;103(12):4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50(2):612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao O, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110(4):1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagliani N, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19(6):739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 25.Everett ML, Collins BH, Parker W. Kupffer cells: Another player in liver tolerance induction. Liver Transpl. 2003;9(5):498–499. doi: 10.1053/jlts.2003.50092. [DOI] [PubMed] [Google Scholar]
- 26.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48(3):978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrzynski E, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104(4):969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 28.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: Graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 29.Bertolino P, Bowen DG, Benseler V. T cells in the liver: There is life beyond the graveyard. Hepatology. 2007;45(6):1580–1582. doi: 10.1002/hep.21786. [DOI] [PubMed] [Google Scholar]
- 30.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AO, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37(3):511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J Hepatol. 2006;44(3):446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology. 2009;49(3):940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.