Significance
The basal ganglia process cortical information that controls purposeful movements and appropriate behavior via the striatopallidal and striatonigral pathways. Despite their importance, little is known about the developmental mechanisms that control the formation of these pathways. We show here that telencephalic progenitors expressing the transcription factor Islet1 give rise to striatonigral neurons and that this factor is required for normal development of the striatonigral pathway. Moreover, Islet1 mouse mutants exhibit hyperactivity and a paradoxical response to psychostimulants. Given that the underlying causes of basal ganglia disorders such as attention deficit hyperactivity disorder (ADHD) are unknown, these findings may implicate possible alterations in neural circuitry.
Keywords: caudate-putamen, direct pathway, indirect pathway
Abstract
The mammalian striatum controls the output of the basal ganglia via two distinct efferent pathways, the direct (i.e., striatonigral) and the indirect (i.e., striatopallidal) pathways. The LIM homeodomain transcription factor Islet1 (Isl1) is expressed in a subpopulation of striatal progenitors; however, its specific role in striatal development remains unknown. Our genetic fate-mapping results show that Isl1-expressing progenitors give rise to striatal neurons belonging to the striatonigral pathway. Conditional inactivation of Isl1 in the telencephalon resulted in a smaller striatum with fewer striatonigral neurons and reduced projections to the substantia nigra. Additionally, conditional inactivation in the ventral forebrain (including both the telencephalon and diencephalon) revealed a unique role for Isl1 in diencephalic cells bordering the internal capsule for the normal development of the striatonigral pathway involving PlexinD1-Semaphorin 3e (Sema3e) signaling. Finally, Isl1 conditional mutants displayed a hyperlocomotion phenotype, and their locomotor response to psychostimulants was significantly blunted, indicating that the alterations in basal ganglia circuitry contribute to these mutant behaviors.
The basal ganglia control many aspects of human behavior, including purposeful movements and appropriate behaviors, that are affected in childhood neurological disorders such as Tourette’s syndrome and attention deficit hyperactivity disorder (ADHD) (1, 2). The striatum, also known as the caudate-putamen, represents the major component of the basal ganglia and is central in the processing of cortical information (reviewed in ref. 3). Moreover, the striatum controls the output of the basal ganglia through two distinct efferent pathways: the direct (i.e., striatonigral) pathway and the indirect (i.e., striatopallidal) pathway. Both of these projection neuron subtypes use the inhibitory neurotransmitter gamma aminobutyric acid (GABA) (4). In addition to their different axonal targets, these two projection neuron subtypes express distinct neurochemical characteristics. The striatonigral projection neurons contain the neuropeptide substance P and express the dopamine D1 receptor at high levels (5, 6). Conversely, the striatopallidal projection neurons contain the neuropeptide enkephalin and high levels of the dopamine D2 receptor (5, 6). These two efferent pathways functionally oppose each other and thereby provide a balanced output from the basal ganglia (7). This balance is believed to be crucial for normal motor control (8).
Striatal-projection neurons are known to derive from the lateral ganglionic eminence (LGE) (9–12) whereas their interneuron counterparts are derived from an adjacent domain, the medial ganglionic eminence (MGE) (11, 13, 14). Within the LGE, two developmental compartments have been identified: the ventral (v)LGE and the dorsal (d)LGE (15, 16). The vLGE is proposed to be the source of striatal-projection neurons (16) whereas the dLGE is thought to contribute interneuron subtypes to the olfactory bulb and amygdala (16–18). Little is known, however, about the molecular mechanisms that underlie formation of the direct (striatonigral) versus the indirect (striatopallidal) pathway from vLGE progenitors.
The only information available relates to the role of the transcription factor early B-cell factor 1 (Ebf1) in the formation of the striatonigral pathway. By performing a differential subtraction between genes expressed in striatopallidal or striatonigral neurons, Lobo et al. (19) identified a novel zinc finger protein, Zfp521, that was enriched in the striatonigral neurons. This factor is known to interact with Ebf1 and in fact, Ebf1 mutants show reduced striatonigral pathway formation, particularly those neurons normally located in the matrix compartment (19, 20).
The LIM homeodomain transcription factor Islet 1 (Isl1) is known to be expressed in at least a subset of striatal progenitors during embryogenesis (16, 21, 22). Although most of these progenitors are thought to give rise to striatal-projection neurons, a portion of them also develop into cholinergic interneurons (22, 23). Despite their expression of Isl1, the cholinergic striatal interneurons are known to derive from the MGE (11, 13, 24–27). In this study, we have addressed the progeny of Isl1-expressing striatal progenitors as well as the role of this factor in the development and function of striatal output pathways.
Results
Forebrain Fate Map of Isl1-Expressing Cells.
To determine the fate of Isl1-expressing LGE cells, we performed a genetic fate map to label the striatal neurons that derive from Isl1-expressing cells. Unlike the broad striatal-projection neuron marker forkhead box protein p1 (Foxp1) (28) (Fig. S1A), Isl1 is expressed in only a subpopulation of newly generated striatal neurons at embryonic stages, with a strong medial-to-lateral gradient (Fig. S1D). Indeed, in fate mapping with the Dlx5/6-cre-IRES-EGFP(CIE) mice (16) and CC-EGFP reporter mice (29), less than half of all recombined (i.e., EGFP-expressing) striatal cells at embryonic day (E) 18.5 were Isl1 containing (Fig. S1 E and F) whereas the vast majority expressed Foxp1 (Fig. S1 B and C). Again, in contrast to Foxp1, the expression of Isl1 within the striatum is dramatically down-regulated shortly after birth, with only the cholinergic interneurons retaining detectable expression into adulthood (22).
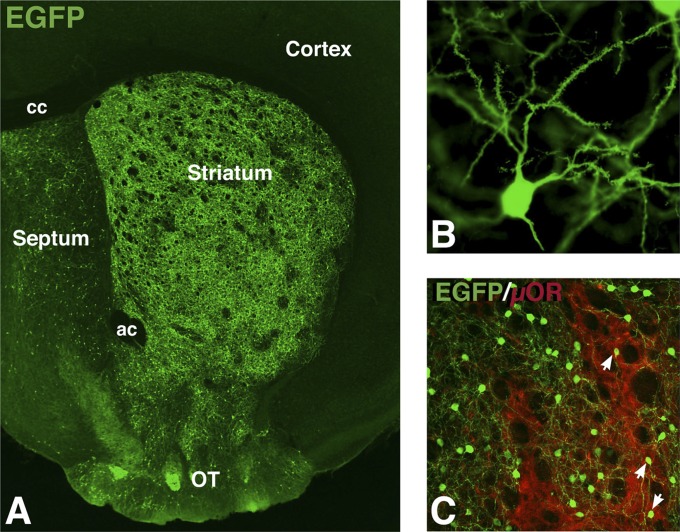
After crossing Isl1-cre mice (30) with CC-EGFP reporter mice, we found large numbers of recombined cells in the adult striatum (Fig. 1A). Upon closer examination, the vast majority of these EGFP-expressing neurons exhibited medium-sized spiny neuronal morphologies (Fig. 1B), characteristic of the GABAergic striatal-projection neurons (4). A small population of the recombined cells exhibited large cell bodies and aspiny dendrites, typical of the striatal cholinergic interneurons (31). Using µ opiate receptor (µOR) staining to mark the striatal patch compartment (32), we found that the Isl1 fate-mapped cells were distributed in both the patch and matrix compartments (Fig. 1C). As previously reported (33, 34), however, the dendrites of neurons in the matrix compartment do not infiltrate the patch and vice versa.
Fig. 1.
Isl1 progenitors generate striatal-projection neurons. (A) Isl1 fate-mapped cells are distributed throughout the striatum and found in scattered cells of the septum at P30. (B) Majority of recombined neurons are medium-sized spiny striatal-projection neurons. (C) Isl1 fate-mapped neurons are located in the patch, as marked by µ opiate receptor (µOR, red), and matrix (µOR negative) compartments of the striatum. ac, anterior commissure; cc, corpus callosum; OT, olfactory tubercle.
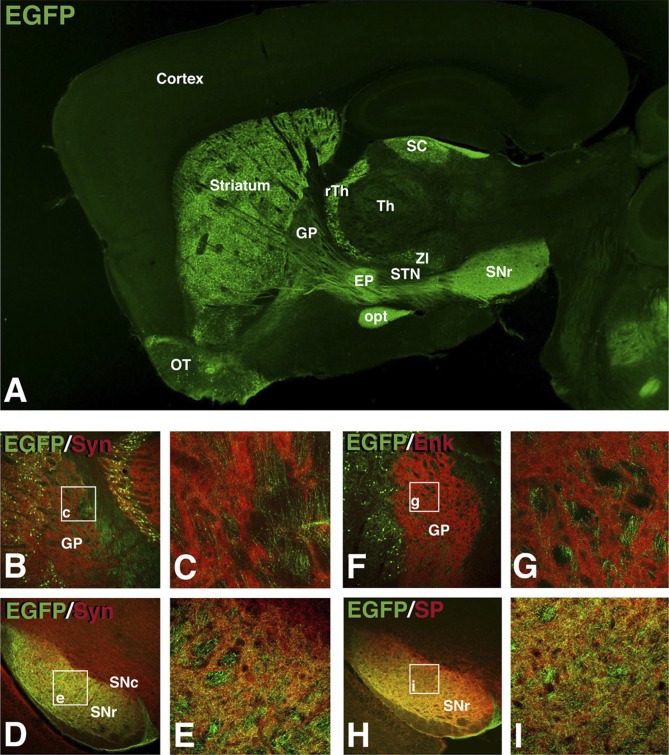
Not only were the dendrites and spines of recombined neurons filled with EGFP but also the axons and terminals (Fig. 2A). EGFP expression in the axons and terminals allowed us to examine the target nuclei of the recombined neurons. Interestingly, the terminal staining was strongest in the entopeduncular nucleus (EP) and the substantia nigra pars reticulata (SNr) (Fig. 2A), which constitute the targets of the direct output pathway. To confirm that Isl1-derived striatal neurons terminated in the EP and SNr, we double stained for EGFP and synapsin. In the globus pallidus (GP), no double labeling with synapsin was observed (Fig. 2 B and C). In fact, the EFGP-positive axons were mostly confined to the synapsin-negative myelin bundles. In contrast, the SNr (Fig. 2 D and E) showed extensive colocalization of EGFP and synapsin. To exclude the possibility that the CC-EGFP reporter mice show a bias toward labeling the direct pathway, we examined Dlx5/6-CIE; CC-EGFP mice and found that both the GP and SNr are innervated by EGFP-positive terminals, which colocalize synapsin (Fig. S2). To further confirm that the recombined striatal neurons belong to the direct pathway, we stained for the neuropeptide enkephalin (Enk), which marks the indirect (striatopallidal) pathway, and, for the direct (striatonigral) pathway, we used the marker substance P (SP). Enk staining did not show colocalization with the EGFP in the GP (Fig. 2 F and G). However, SP showed extensive coexpression with EGFP in the SNr (Fig. 2 H and I). Therefore, it appears that Isl1-derived striatal neurons consist of cholinergic interneurons as well as projection neurons belonging specifically to the direct (i.e., striatonigral) output pathway located in both the patch and matrix compartments.
Fig. 2.
Isl1 fate-mapped synaptic terminals are found in the SNr and not the GP. (A) Recombined cells (EGFP+) are distributed throughout the telencephalon and diencephalon of a fate-mapped brain at P30 with synaptic terminal staining in the EP and SNr and not the GP. A lack of coexpression of EGFP with synapsin (B and C) and enkephalin (F and G) in the GP indicates that fate-mapped neurons do not synapse with the GP. Fate-mapped synaptic terminals are found in the SNr as indicated by double labeling of EGFP with synapsin (D and E) and with substance P (H and I) in the SNr. EP, entopeduncular nucleus; GP, globus pallidus; opt, optic tract; OT, olfactory tubercle; rTh, reticular thalamic nucleus; SC, superior colliculus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Th, thalamus; ZI, zona incerta.
Telencephalon-Specific Inactivation of Isl1.
To determine whether Isl1 is required for the normal development of the direct striatal output pathways, we generated conditional Isl1 mutants using cre-loxP technology. Germ-line Isl1 mutants are lethal around E10 (35). Using an Isl1fl/fl mouse line with loxP sites flanking the third exon containing the second LIM domain (36), we generated telencephalon-specific inactivation by crossing with Foxg1tTA (37) and tetO-cre mice (38). Using this approach, Isl1 was lost in the telencephalon of Foxg1tTA;tetO-cre;Isl1fl/fl (i.e., conditional) mutants by E14 (Fig. S3 A and B). Isl1 remains expressed in the cholinergic neurons of the telencephalon as well as in the reticular thalamic nucleus (rTh) of control adult brains (22) (Fig. S3C). In the conditional mutant mice, Isl1 staining remained in the rTh but was not detected in any cells of the telencephalon (Fig. S3D).
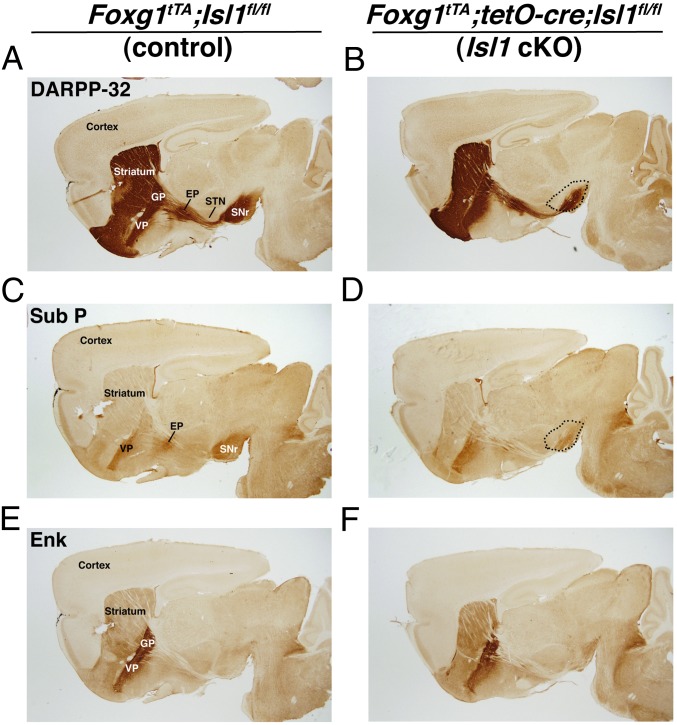
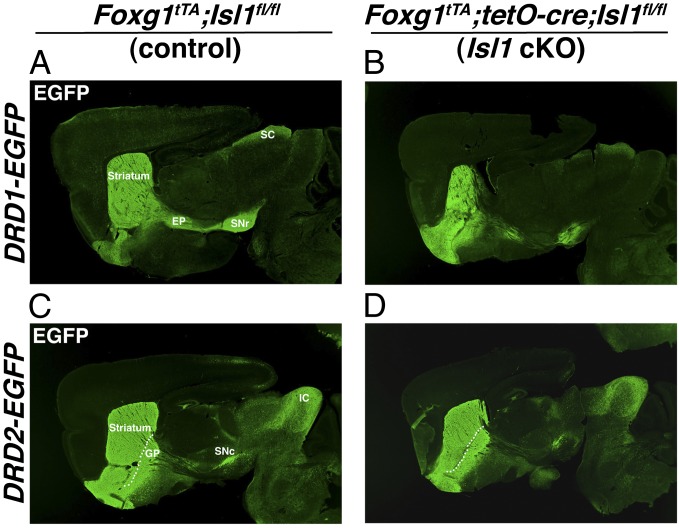
Dopamine- and cAMP-regulated neuronal phosphoprotein 32 (DARPP-32) is a phosphoprotein that marks essentially all striatal-projection neurons but is excluded from the interneuron populations (39). To assess the development of striatal-projection neurons, we stained the telencephalon-specific Isl1 mutant brains for DARPP-32 at postnatal day (P) 21. In these conditional mutants, DARPP-32 staining revealed a 43% reduction in striatal size, compared with control (Fig. 3 A and B; n = 3, P < 0.001). In addition to somal and dendritic staining in the striatum, DARPP-32 marks the axons and terminals of striatal-projection neurons of both the direct and indirect pathways. We noticed that, despite apparently normal innervation of the GP in the conditional mutants, the DARPP-32 staining along the striatonigral tract and in the SNr was considerably reduced compared with control brains (Fig. 3 A and B). In fact, DARPP-32 terminal staining in the SNr of Isl1 conditional mutants occupied only 45% of the area of that observed in controls (n = 3; P < 0.001). No significant difference was observed in the GP. To further confirm the alterations to the direct striatonigral pathway, we examined the expression of SP (Fig. 3 C and D) and EGFP in dopamine D1 receptor (DRD1)-EGFP BAC transgenic mice (Fig. 4 A and B). In agreement with the DARPP-32 results, SP and DRD1-driven EGFP expression in the SNr of Isl1 conditional mutants was severely reduced (Figs. 3D and 4B) compared with controls (Figs. 3C and 4A). Conversely, the striatopallidal markers Enk and dopamine D2 receptor (DRD2)-driven EGFP did not show overt differences between the telencephalon-specific Isl1 mutant and control brains (Figs. 3 E and F and 4 C and D). Thus, Isl1 not only marks the striatal progenitors that form the striatonigral pathway but it is also required for the correct development of a significant portion of these projection neurons.
Fig. 3.
Telencephalic-specific deletion of Isl1 leads to a reduced striatonigral pathway. Immunostaining with DARPP-32 shows reduced striatal size and reduced innervation of SNr in the Isl1 conditional knockout (B) versus control mice (A) whereas innervation of the GP appears normal in the conditional mutant. Substance P staining in the SNr and the EP nucleus was also reduced in the Isl1 conditional knockout (D) versus control mice (C). Enkephalin staining in the GP of Isl1 conditional mutants (F) looks similar to controls (E). EP, entopeduncular nucleus; GP, globus pallidus; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; VP, ventral pallium.
Fig. 4.
Telencephalic specific deletion of Isl1 leads to a reduced striatonigral pathway. The striatonigral pathway as visualized by breeding the mice onto a DRD1-EGFP background is reduced in Isl1 conditional mice (B) compared with control mice (A). The striatopallidal pathway as visualized by breeding the mice onto a DRD2-EGFP background is similar between Isl1 conditional (D) and control mice (C). EP, entopeduncular nucleus; GP, globus pallidus; IC, inferior colliculus; SC, superior colliculus; SNr, substantia nigra pars reticulata; SNc, substantia nigra pars compacta.
To assess the effect of telencephalon-specific inactivation of Isl1 on striatal interneurons, we stained the conditional mutant brains at P21 for choline acetyl transferase (ChAT), to detect cholinergic interneurons, and, to reveal the two main GABAergic interneuronal subtypes, we probed for neuropeptide Y (NPY) and parvalbumin (PV). As previously shown by Elshatory and Gan (23), loss of Isl1 resulted in a nearly complete loss of striatal cholinergic interneurons (Fig. S4 A and B). In contrast, the GABAergic interneuron populations marked by NPY and PV were not obviously affected by the loss of Isl1 (Fig. S4 C and F).
Striatal development has been shown to depend on transcription factors that control patterning and differentiation, such as Gsx2, Ascl1, and Dlx genes (40–45). To determine whether loss of Isl1 affects the expression of these genes, we examined the expression of Gsx2, Ascl1, and Dlx in E14.5 Isl1 mutants. As shown in Fig. S5, the loss of Isl1 does not appear to alter expression of these transcription factors, which is in line with the fact that they are likely upstream of Isl1 expression (40, 42).
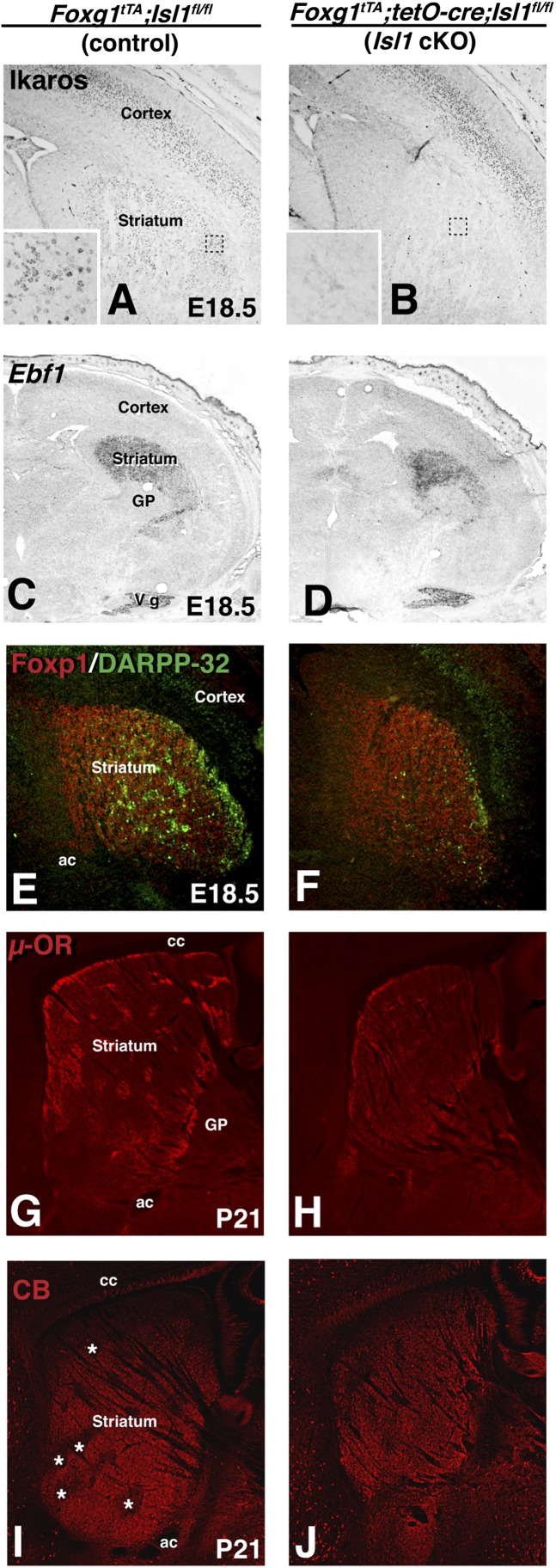
The zinc finger transcription factors Ikaros and Helios have also been implicated in striatal neuron development downstream of Gsx2 and Dlx genes (46, 47). Moreover, Ikaros has been associated with the differentiation of a subpopulation of Enk-expressing striatopallidial neurons (46, 48). Surprisingly, Isl1 conditional mutants exhibited a nearly complete loss of Ikaros expression within the developing striatal complex (Fig. 5B), compared with control (Fig. 5A). To determine whether Isl1 and Ikaros are coexpressed in the LGE subventricular zone (SVZ) and developing striatum, we double stained for these two factors and found extensive colocalization at E14.5 (Fig. S6 A and B). Although there are many more LGE cells expressing Isl1 than Ikaros, a considerable number of the Ikaros cells coexpress Isl1. By E18.5, however, the number of colabeled cells is reduced and there is an increase in Ikaros-only cells (Fig. S6C). Given our fate-mapping results for Isl1-expressing cells, it seems that Ikaros is expressed in, at least, a portion of striatonigral progenitors and that its expression in the developing striatal complex depends on Isl1. Unlike Ikaros, Helios remains expressed in many striatal neuron progenitors of the Isl1 mutant (Fig. S7), despite the reduced size of the LGE and striatal complex (see Figs. 3–5).
Fig. 5.
Striatal neuron differentiation and patch-matrix organization of Isl1 conditional mutant mice are disrupted. Ikaros is expressed in the developing cortical plate and in scattered cells of the striatum (A). Inset in A is a 25× magnification of the dashed box in striatum. Isl1 conditional mutants (B) show a nearly complete loss of Ikaros expression in the striatum whereas cortical plate expression appears unaffected. Inset in B is a 25× magnification of the dashed box in the mutant striatum. The domain of Ebf1 expression, which has been reported to be involved in the development of striatonigral projection neurons mainly in the matrix compartment, is reduced in conditional mutant mice with a telencephalic (D) deletion of Isl1 compared with control mice at E18.5 (C). At E18.5, double immunofluorescence with FoxP1 and DARPP-32 shows a reduction of both patch and matrix in the Isl1 conditional mice (F) compared with controls (E). µ-OR staining confirms a reduction in the patch compartment in adult Isl1 conditional mutants (H) compared with controls (G). Immunostaining with calbindin (CB), a marker of the matrix compartment that outlines the patch compartment, indicates a reduction in both the matrix and patch compartments (starred areas in I) of adult Isl1 conditional mutants (J) compared with controls (I). ac, anterior commisure; cc, corpus callosum; GP, globus pallidus; vg; Trigeminal ganglion.
The Ebf1 transcription factor has been shown to be required for the correct differentiation of striatonigral neurons (19, 20). An earlier study showed that Ebf1 is required for correct development of the striatal matrix compartment (49), and accordingly the observed requirement for striatonigral neuron differentiation is most pronounced within the matrix (20). We examined the expression of Ebf1 in the Isl1 conditional mutants at E18.5. The reduced size of the conditional mutant striatum is already evident at this stage (Fig. 5 C and D). Despite this reduction, the level of Ebf1 expression in the mutant striatum appears similar to that in controls. Our fate-map data showed Isl1-derived neurons in the patch compartment as well as the matrix (Fig. 1C), which prompted us to examine the development of the patch compartment in the telencephalon-specific Isl1 mutants. At E18.5, DARPP-32 marks the patch compartment in the forming striatum (50). Using Foxp1 to mark the majority of striatal neurons together with DARPP-32 (to reveal the nascent patch compartment), we found that loss of Isl1 leads to a dramatic loss of DARPP-32–positive neurons at E18.5 (Fig. 5F). This loss does not simply reflect a developmental delay because μOR staining reveals a severe loss of patch identity in P21 brains (Fig. 5H) compared with controls (Fig. 5G). The reduced size of the Isl1 conditional mutant striatum cannot be accounted for simply by the loss of the patch compartment. Indeed, the matrix marker calbindin (CB) also shows a notable reduction in staining in the Isl1 conditional mutant striatum (Fig. 5J) compared with controls (Fig. 5I). Therefore, loss of Isl1 leads to striatonigral defects in both the patch and matrix compartments.
The reduced size of the striatum in Isl1 conditional mutants is already evident at E18.5, supporting the notion that Isl1 is required during embryonic stages for the correct number of striatal-projection neurons to develop. To examine whether reduced proliferation may contribute to this phenotype, we examined phosphoHistone 3 (pH3) expressing (i.e., M-phase) cells in the LGE of E14.5 controls and Isl1 mutants. Few, if any, pH3 cells colocalized with Isl1 in the LGE (Fig. S8 A and B). We calculated the average number of pH3 cells per LGE section both at the apical surface [i.e., ventricular zone (VZ)] and in basal regions (i.e., SVZ) and found no difference between controls (n = 3) and mutants (n = 3) (Fig. S8; Apical pH3 cells, control, 24.9 ± 1.9; Isl1 mutant, 25.2 ± 1.8; Basal pH3 cells, control, 36.3 ± 0.9; Isl1 mutant, 36.0 ± 1.4). Conversely, we observed a 42% increase in cell death as marked by activated caspase-3 staining within the mutant LGE and forming striatum (207.5 ± 25.3 cells) already at E14.5, compared with controls (146.3 ± 15.8 cells; n = 3, P < 0.05). Thus, Isl1 is required, at least in part, for the survival of newborn striatonigral progenitors/neurons in both the patch and matrix compartments.
Ventral Forebrain-Specific Inactivation of Isl1.
In the adult brain, Isl1 is expressed in neurons of the rTh and zona incerta (ZI) (51) (Fig. 6A). Interestingly, these neurons border the internal capsule, which carries the striatonigral and corticofugal fibers. It is possible, therefore, that Isl1 may play a noncell autonomous role in the formation of the striatonigral pathway by regulating the development of these nonstriatal neurons or substances they may express/release. Although we do not have a cre mouse line that restricts recombination to the diencephalon, we can use the Dlx5/6-CIE mice (16) to inactivate Isl1 throughout the ventral forebrain including the striatum, rTh, and ZI, which is in contrast to the Isl1 conditional mutants generated with the Foxg1tTA;tetO-cre mice (see Telencephalon-Specific Inactivation of Isl1).
Fig. 6.
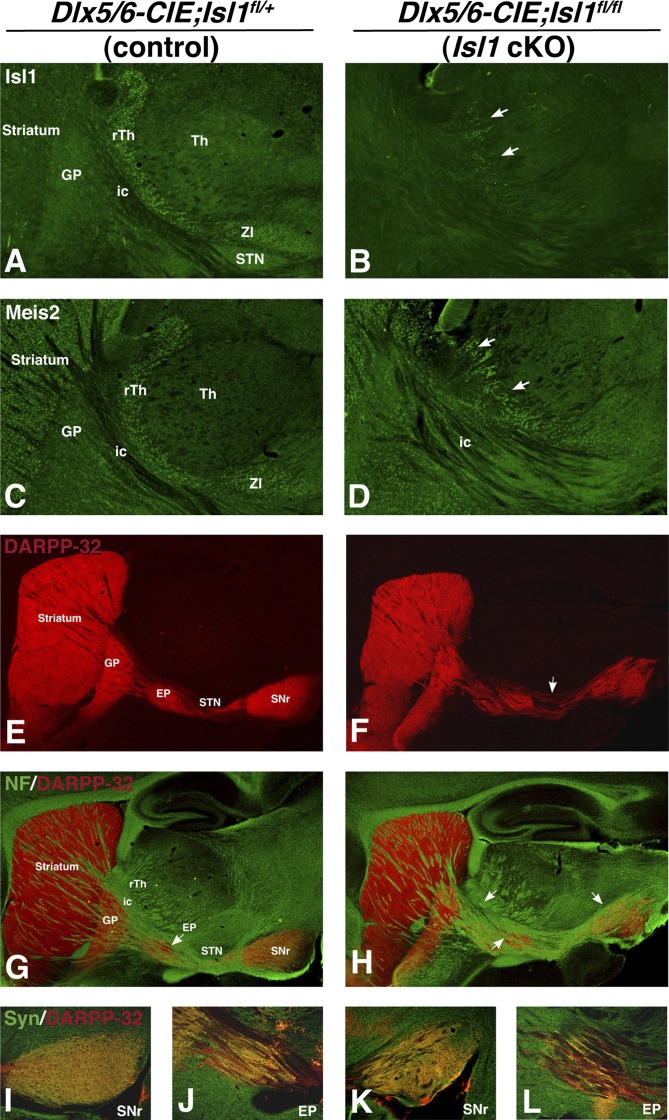
Ventral forebrain inactivation of Isl1 reduces Isl1 expression in the striatum as well as the reticular thalamus and leads to a disorganization of the striatonigral output pathway. At adult stages the ventral forebrain, inactivation of Isl1 causes a deletion of Isl1 in the striatum as well as a reduction of Isl1 in the reticular thalamus and zona incerta of the diencephalon of conditional mice (B) compared with controls (A). Meis2 immunostaining shows that the structure of the reticular thalamus and zona incerta is altered in Isl1 conditional mice (D) compared with controls (C). DARPP-32 immunostaining in Isl1 conditional mutants reveals a smaller striatal size as well as a disorganization of the striatonigral pathway to the SNr (F) compared with controls (E). Double immunofluorescence with neurofilament (green) and DARPP-32 (red) indicates an alteration in the structures of the internal capsule and the cerebral peduncle in Isl1 conditional mutants (H) compared with controls (G). High-power images of the SNr and EP double-labeled with synapsin-1 (green) and DARPP-32 (red) reveal fewer synapses in these two nuclei of the striatonigral pathway in conditional mutant mice (K and L) compared with controls (I and J). EP, entopeduncular nucleus; GP, globus pallidus; ic, internal capsule; rTh, reticular thalamic nucleus; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Th, thalamus; ZI, zona incerta.
Ventral forebrain inactivation of Isl1 in Dlx5/6-CIE; Isl1fl/fl mice led to a near complete loss of Isl1 protein in the telencephalon and diencephalon already at E14 (Fig. S3 E and F). In addition, Isl1 expression was severely depleted in the rTh, with only a few Isl1-positive cells remaining in the adult conditional mutants (Fig. 6B). The loss of Isl1 in this diencephalic region resulted in the disruption of rTh and ZI formation, as demonstrated by an independent marker, Meis2 transcription factor (Fig. 6 C and D). Thus, Isl1 is required for the normal formation of these diencephalic structures, which border the descending cortical and striatonigral axons. To determine the effect on the striatonigral pathway, we stained the Dlx5/6-CIE; Isl1fl/fl mice for DARPP-32. In agreement with the results obtained in the telencephalon-specific Isl1 mutants, we observed a 48% reduction in the size of the striatal complex (n = 5, P < 0.01) and a concomitant reduction of 45% in the area covered by DARPP-32 terminals in the SNr (n = 5, P < 0.01) (Fig. 6F), compared with controls (Fig. 6E). Again, no significant effect was observed on the DARPP-32 innervation of the GP. Unlike the Foxg1tTA;tet-O-cre; (i.e., telencephalon-specific) Isl1 mutants, the Dlx5/6-CIE; Isl1fl/fl mice showed abnormalities in the DARPP-32–positive axon trajectory along the striatonigral pathway. Notably, the fibers appeared disorganized and resulted in disrupted organization of both the EP and SNr (Fig. 6F). In controls, DARPP-32–positive fibers were observed to pass underneath the subthalamic nucleus (STN) before entering the SNr (Fig. 6E). However, in the Dlx5/6-CIE; Isl1fl/fl (i.e., ventral forebrain) mutants, dispersed DARPP-32 fibers were seen traveling through the STN (Fig. 6F). Neurofilament (NF) staining confirmed the abnormal axon projections through the internal capsule and along the striatonigral pathway (Fig. 6 G and H). In fact, it appears that NF-positive axon bundles are misplaced not only within the STN but also through the dorsal portion of the SNr (Fig. 6H). These axonal anomalies led to a considerable reduction in the number of DARPP-32 terminals that were Synapsin-positive in the SNr (Fig. 6K) and EP (Fig. 6L) in the ventral forebrain of Isl1 conditional mutants, compared with the controls (Fig. 6 I and J). Thus, the Dlx5/6-CIE; Isl1fl/fl mutants show the expected telencephalic defects in the striatal neurons forming the striatonigral pathway as well as diencephalic defects in the trajectories of striatonigral (and likely also corticofugal) axons traveling in the internal capsule and cerebral peduncle. These findings suggest an important role for the Isl1-expressing neurons that line the internal capsule, including those in the rTh and ZI.
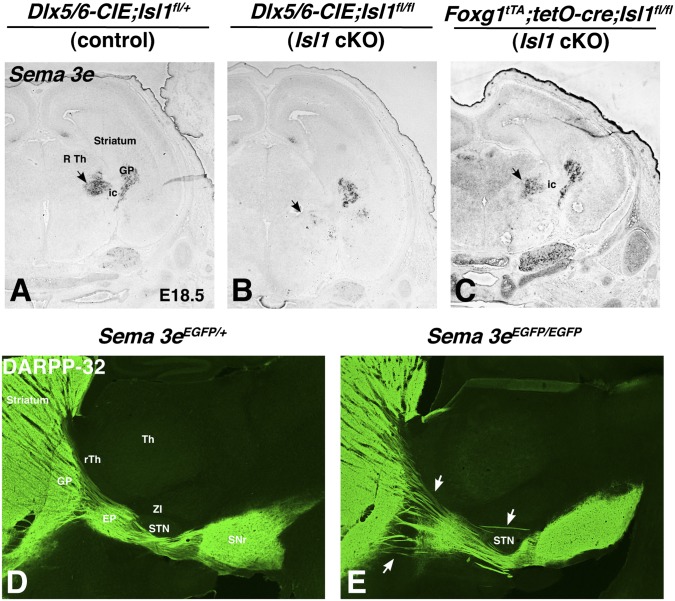
What remains unclear, is whether the rTh and ZI are physically required for the correct formation of the striatonigral pathway or whether they express/release a molecule that guides pathway formation. One group of molecules that are known to regulate axon pathfinding are the plexins and semaphorins (52). A previous study by Chauvet et al. (53) implicated PlexinD1 and Sema3e signaling in shaping the trajectories of corticofugal and striatonigral axons. Indeed, PlexinD1 is expressed highly in striatonigral neurons (54) whereas the gene encoding its repulsive ligand Sema3e is expressed in the GP and rTh/ZI during development (53) (Fig. 7A). We found that Isl1 is required for the expression of Sema3e in the rTh of the Dlx5/6-CIE;Isl1fl/fl mutants (Fig. 7B), in accordance with the disrupted rTh development in these mutants (see, e.g., Fig. 6 A–D). Sema3e expression in the diencephalon of Foxg1tTA;tet-O-cre;Isl1fl/fl mutants was not different from that in controls (Fig. 7C). Sema3e mutants have been shown to exhibit defects in axon pathfinding of descending (i.e., cortical and/or striatal) pathways, particularly, misrouting of fibers into the dorsal thalamus (53). However, these authors focused on early postnatal time points and did not discriminate between descending cortical or striatal fibers. To examine the effect on the striatonigral pathway, we stained Sema3e mutants for DARPP-32 at P21 and found that, although the misdirected fibers within the thalamus were not obvious at this stage, abnormal axon trajectories were observed passing through or just over the STN (Fig. 7E), very similar to what was observed in the Dlx5/6-CIE; Isl1fl/fl mice (Fig. 6F). However, unlike the Dlx5/6-CIE; Isl1fl/fl mice, the Sema3e mutants did not exhibit morphological defects in the rTh. These findings indicate that PlexinD1-Sema3e signaling contributes to the correct formation of the striatonigral pathway and that, in particular, Sema3e expression in the developing rTh/ZI regions is crucial for the normal trajectories of the striatonigral pathway to form.
Fig. 7.
In situ hybridization of E18.5 sections indicates that inactivation of Isl1 has a differential affect on factors implicated in striatonigral projection neuron development and axon pathfinding. The secreted ligand for the PlexinD1 receptor, Sema3e, is expressed in the RTh and GP surrounding the internal capsule (A) and acts as a repulsive signal. Expression of Sema3e in conditional mutants with a telencephalic inactivation of Isl1 (C) is similar to controls (A); however, in conditional mutants with a ventral forebrain deletion of Isl1 (B), the expression of Sema3e in the rTh is reduced compared with controls (A), which coincides with the reduction of Isl1 in rTh as well as in these mutants. DARPP-32 immunostaining of Sema3e mutants at adult stages indicates misguided fibers passing through and over the STN (E) compared with controls (D), where fibers normally travel under the STN.
Behavioral Analysis of Isl1 Conditional Mutants.
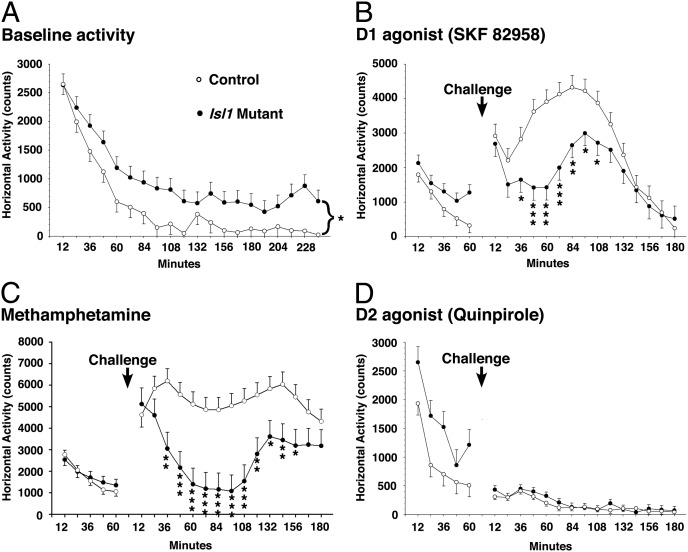
Given the importance of balanced activity in the striatal output pathways (55), the alterations in basal ganglia circuitry observed in the Isl1 conditional mutants (i.e., Foxg1tTA;tetO-cre;Isl1fl/fl and Dlx5/6-CIE; Isl1fl/fl mice) suggest that altered behavior may result. To begin to address this possibility, we examined baseline locomotor activity in the Dlx5/6-CIE; Isl1fl/fl mice between 3 and 9 mo of age. We examined these mice because the Foxg1tTA;tetO-cre;Isl1fl/fl mice did not survive well beyond 1 mo. Interestingly, the Isl1 conditional knockouts showed a hyperlocomotive phenotype compared with control littermates (Fig. 8A). Increased locomotor activity has been used as a measure of hyperactivity in rodents and even as a behavioral correlate of the hyperactivity phenotype observed in people with attention deficit hyperactivity disorder (ADHD) (see, e.g., refs. 56–58). One striking aspect of ADHD is the paradoxical response to stimulants (i.e., dopaminergic agonists) (59). We chose to examine the effects of stimulants on the hyper-locomotive phenotype observed in the Isl1 conditional mutant mice. The addition of the dopamine D1 agonist SKF 82958 (2 mg/kg) showed an almost immediate increase in the locomotor activity in the control mice; however, in the Isl1 conditional mutant mice, the response was significantly blunted (Fig. 8B). Of note, there was a delayed spike in activity around 90 min after the drug was administered. It may not be surprising that a D1 agonist would be less effective given the significant reduction in the DRD1-expressing direct pathway in the Isl1 conditional mice (Fig. 4B). To examine the effect of a more general dopamine agonist, we administered methamphetamine (4 mg/kg), and, unlike the controls, which showed a dramatic increase in locomotor activity that was sustained for more than 3 h, the Isl1 conditional knockouts again exhibited a blunted response to the drug, returning to predrug activity levels in less than 1 h (Fig. 8C). Finally, to examine the specificity of the phenotype in the Isl1 conditional mice, we examined the effect of quinpirole, a dopamine D2 agonist (1 mg/kg). In line with the fact that the indirect pathway is largely intact in these mutants, there was no difference in the response of control or Isl1 conditional mice to D2 receptor stimulation (Fig. 8D). The drug resulted in a significant reduction in locomotor activity despite the fact that the mutants started from a higher activity level than the controls. Thus, the observed alterations in the striatonigral pathway of Isl1 conditional mutants lead to hyperactivity and a blunted response to stimulants, compared with controls.
Fig. 8.
Isl1 conditional mice are hyperactive and have altered responses to pharmacological reagents that stimulate the dopamine D1 receptor pathway. Horizontal locomotor activity of Isl1fl/fl; Dlx 5/6-CIE and control (Isl1fl/fl or Isl1fl/+) mice is expressed as the total number of photobeam interruptions in 12-min intervals. (A) Isl1 conditional mutants are hyperactive over the 4-h period in the activity chamber compared with control mice (n = 9 mutants and 11 controls) [gene, F(1,16.2) = 5.97, P < 0.03]. (B) Isl1 conditional mice exhibited a significantly less hyperactive response than control mice to a 2 mg/kg dose of the D1 receptor agonist (SKF 82958) (n = 9 mutants and 11 controls) [prechallenge ANOVA, gene (1,16.9) = 4.48, P < 0.05; postchallenge ANOVA gene, F(1,30.3) = 8.98, P < 0.01; gene × Interval, F(14,216) = 1.85, P < 0.04]. (C) Isl1 conditional mice responded to a 4 mg/kg dose of methamphetamine with an uncharacteristic suppression in activity that was significantly different from the typical hyperactive response exhibited by control mice (n = 7 mutants and 11 controls) [prechallenge ANOVA, not significant; postchallenge ANCOVA, gene, F(1,18) = 12.9, P < 0.01; Gene × Sex, F(1,18) = 5.53, P < 0.04; Gene × Interval, F(14,191) = 2.98, P < 0.001; Gene × Sex × Interval, F(14,191) = 1.93, P < 0.03]. Values are mean ± SEM. (D) No significant differences in motor suppression were observed between Isl1 conditional and control mice following a challenge with a 1 mg/kg dose of the D2 receptor agonist, quinpirole (n = 7 mutants and 11 controls) [prechallenge ANOVA, gene, F(1,14.1) = 7.39, P < 0.02; postchallenge ANCOVA, not significant]. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Isl1-Expressing Striatal Cells Give Rise to Striatonigral Neurons.
Striatal-projection neurons in both the direct and indirect pathways share many characteristics, such as a medium spiny morphology and the utilization of GABA as a neurotransmitter (4). Furthermore, all striatal-projection neurons, but not the interneurons, express the phosphoprotein DARPP-32 (39), which is involved in dopamine receptor signaling. The most important difference between these neurons is their axonal targets, with the indirect pathway innervating the GP and the direct pathway innervating the EP and SNr (reviewed in refs. 3 and 55). Indeed, balanced activity in these two striatal output pathways is essential for normal motor control (see, e.g., ref. 7). However, despite their importance for normal brain function, little is known about the molecular mechanisms that underlie the development of these two output pathways.
Previous studies (19, 20) have implicated the transcription factor Ebf1 in the normal differentiation of striatonigral neurons located within the matrix compartment of the striatum. These authors showed that Ebf1 and Zfp521, a zinc finger transcription factor that interacts with Ebf1 (60), are restricted to the striatonigral pathway by P20. It is unclear, however, whether either of these two factors is expressed exclusively in striatonigral progenitors or whether they become restricted to striatonigral neurons at postnatal stages. In fact, Zfp521 increases its enrichment in striatonigral neurons between P20 and adulthood (19). Thus, it is possible that Ebf1 and Zfp521 are expressed in progenitors of both the direct and indirect pathway at embryonic stages and become restricted to the striatonigral pathway at postnatal time points. Indeed, the striatonigral axon defects observed at P0 in Ebf1 mutants were not as severe as those observed at P14 (19). In any case, Ebf1 and Zfp521 appear to play an important role in the differentiation/survival of striatonigral neurons at postnatal stages.
Our genetic fate-mapping results show that Isl1-expressing cells give rise to striatal neurons selectively belonging to the direct pathway, innervating both the EP and SNr but not the GP. In agreement with the fact that striatal cholinergic interneurons express Isl1 into adulthood (22, 23), we found that large-diameter aspiny neurons, presumably cholinergic interneurons (31), are also derived from Isl1-expressing cells. These interneurons are likely of MGE origin (11, 13, 14). At present, it is unclear whether all striatonigral projection neurons derive from Isl1-expressing LGE cells or whether it is only a subpopulation. If it is a subpopulation, they do not segregate between the patch and matrix compartments because fate-mapped striatonigral neurons were found in both of these striatal compartments.
Isl1 Is Required for Normal Development of the Striatonigral Pathway.
Ebf1 is required for the normal differentiation and/or survival of striatonigral neurons, particularly those in the matrix compartment of the striatum (19, 20). This compartment-specific phenotype is in line with the previously documented role for Ebf1 in the normal development of the striatal matrix (49). Therefore, other molecular mechanisms must underlie the formation of patch-compartment striatonigral neurons. In this respect, Ephrin A/EphA signaling has been shown to regulate correct development of the striatal patch and matrix compartments (61, 62); however, it is currently unknown whether Ebf1 or Isl1 control the expression of either of these factors.
Isl1-expressing cells give rise to striatonigral neurons belonging to both the patch and matrix compartment. Moreover, Elshatory and Gan (23) demonstrated that, in Isl1 conditional mutants made using Six3-cre (which recombines in progenitors of the MGE and a portion of LGE) (63), most striatal cholinergic interneurons and a subpopulation of the DARPP-32–positive projection neurons are lost. Our data show that it is specifically the striatonigral neurons in both the patch and the matrix compartment that are severely reduced in the Isl1 conditional mutants, leading to considerable reductions in the innervation of both the EP and SNr. This reduction in the striatonigral pathway is in contrast to the striatopallidal pathway, which appears to be largely intact in the conditional mutants based on DARPP-32 innervation of the GP. Despite the reduced size of the Isl1 conditional mutant striatum even at E18.5, Ebf1 remained expressed at detectable levels. Thus, it may be that striatonigral neurons can be divided into subpopulations that are differentially dependent on Isl1 and Ebf1 for their normal development and/or differentiation. The zinc finger transcription factor Ikaros has been implicated in the differentiation of a subpopulation of Enk-positive striatopallidal neurons (46, 48). Surprisingly, we found that Ikaros was almost completely lost in the developing striatum but not the overlying cortical plate. To investigate further, we double stained for Isl1 and Ikaros in the LGE. At E14.5, many Ikaros cells coexpressed Isl1; however, by E18.5 fewer cells were colabeled and more Ikaros-only cells were observed. The reduction in colabeling could be due to the down-regulation of Isl1 that occurs around birth or the de novo expression of Ikaros in distinct striatal (i.e., Enk-expressing striatopallidal) progenitors at perinatal stages. In either case, our data indicate that Isl1 is required for the normal expression of Ikaros in striatal neuron progenitors. Because there are no fate-mapping data regarding the progeny of Ikaros-expressing cells, it is unclear how tightly this factor is associated with the striatopallidal lineage. Our data suggest that the Enk-expressing striatopallidal pathway is largely intact in the Isl1 conditional mutants; however, it does appear that, similar to the striatonigral pathway, which has Isl1- and Ebf1-dependent subpopulations, the striatopallidal pathway has Ikaros-dependent and -independent subpopulations. Indeed, only about 1/3 of the Enk-expressing neurons are lost in the Ikaros mutant (46). In this case, Isl1 conditional mutants would be expected to have an increased proportion of Ikaros-independent striatopallidal neurons because the innervation of the mutant GP appears rather normal.
Our results indicate that Isl1 is required for the normal development of striatonigral neurons. In this respect, we observed increased cell death within the LGE and forming striatum already at E15 when these neurons are beginning to arise. Thus, Isl1 may serve to maintain neuronal survival in the direct pathway during the embryonic stages when these neurons are becoming postmitotic. In line with the observed role in neuronal survival, previous studies have shown that Isl1 is required for the development and survival of most retinal ganglion cells in the ganglion-cell layer and subpopulations of bipolar and amacrine cells in the inner nuclear layer in the mouse retina (36) as well as neurons in the dorsal root ganglia and trigeminal ganglia (64). A transient role in striatonigral neuron survival would fit the expression profile of Isl1 in the developing striatum as this factor down-regulates in striatal-projection neurons within days after birth (22). Because Isl1 remains expressed in the cholinergic interneurons of the adult striatum, it may be required for survival over a longer period in this neuronal subpopulation.
Diencephalic Regulation of Striatonigral Pathway Formation.
Striatonigral axons travel together with descending cortical axons through the internal capsule and farther in the cerebral peduncle. The internal capsule wraps around the rTh and ZI, and thus these nuclei may act as a “guardrail” for the descending cortical and striatal axon trajectories. Our data show that Isl1 is also required for the normal formation of the rTh. Unlike the Foxg1tTA;tetO-cre conditional mutants, the Dlx5/6-CIE conditional mutants appear to lack most of the rTh neurons. In addition, the internal capsule appears to be severely disrupted in these conditional mutants, suggesting that the normal formation of the rTh and ZI is crucial to the correct formation of the descending cortical and striatal trajectories. It is possible that either the neurons in these nuclei provide a physical boundary or that signaling between the descending (including both cortical and striatal) fibers and the bordering nuclei of the internal capsule/cerebral peduncle (e.g., GP, rTh, and ZI) contribute to the refinement of these pathways.
A previous study by Chauvet et al. (53) showed that semaphorin signaling contributes to the formation of the internal capsule, with the descending fibers expressing PlexinD1 and the rTh as well as the GP expressing its repulsive ligand Sema3e. Interestingly, PlexinD1 appears to be expressed by striatonigral neurons (54). However, in the cortex, it is largely expressed in layer 5a neurons (i.e., callosally projecting neurons) and not by layer 5b neurons, which give rise to descending cortical projections (65, 66). Thus, it seems likely that the majority of the PlexinD1-expressing fibers within the internal capsule and cerebral peduncle originate from striatonigral neurons. In the Foxg1tTA;tet-O-cre conditional mutants, Sema3e expression in the rTh and GP was normal, but, in the Dlx5/6-CIE conditional mutants, its expression was severely reduced, particularly in the rTh. The reduced Sema3e expression correlates well with the abnormalities observed in the formation of the internal capsule of Dlx5/6-CIE conditional mutants as well as the misrouting of axons through the STN and SNr in these mutants. Moreover, Sema3e mutants showed similar abnormalities in axon trajectories as the Dlx5/6-CIE conditional mutants, particularly in the STN. Chauvet et al. (53) showed that Sema3e responsive axons (presumably striatonigral axons) are abnormally misrouted through the dorsal thalamus at early postnatal time points. Abnormalities in axon trajectories were not as obvious by P21; however, DARPP-32–positive striatonigral axons were observed to abnormally pass over the STN in the Sema3e mutants very similar to that observed in the Dlx5/6-CIE conditional mutants. Thus, Isl1-expressing diencephalic cells regulate the formation of the striatonigral pathway, at least in part, by facilitating the expression of Sema3e in rTh cells.
Consequences of Altered Striatonigral Pathway Formation in Isl1 Conditional Mutants.
An imbalance in neuronal activity within the direct and indirect pathways has been proposed to underlie the alterations of motor control in basal ganglia disorders such as Parkinson disease (8, 67). In Parkinson patients, it is thought that the indirect pathway is overactive whereas the direct pathway exhibits decreased activity, which ultimately leads to reduced motor output. In support of this notion, recent optogenetic experiments in mice have shown that specific activation of the indirect (striatopallidal) pathway leads to decreased locomotor activity whereas selective activation of the direct (striatonigral) pathway enhances locomotor activity (68). In a different study (69), inhibition of neuronal activity in either of these striatal output pathways did not alter locomotor patterns under normal conditions. However, blocking neuronal activity in either the direct or indirect pathway prevented the hyperlocomotor response to psychostimulants (e.g., methamphetamine and cocaine).
Our results show that the observed alterations in the striatonigral pathway in Isl1 conditional mutants leads to hyperlocomotion under normal conditions compared with littermate controls. Moreover, the hyperlocomotor response to methamphetamine or the D1 agonist SKF 82958 was largely missing in the Isl1 conditional mutants. These later results are reminiscent of those reported by Hikida et al. (69). However, the major difference between the above-mentioned studies in mature adult basal ganglia circuitry is that the Isl1 conditional mutants do not form a normal striatonigral pathway, which likely exhibits reduced activity from birth onward, and thus compensatory developmental mechanisms may further alter the circuitry. Dopamine D1 receptor (DRD1) mutants also show hyperlocomotion and a blunted or missing response to psychostimulants (70–73). This reduction is interesting because, in the Isl1 conditional mutants, it is the DRD1-expressing striatonigral pathway that is specifically compromised, suggesting that the effects observed in the DRD1 mutants are a result of dysfunction specifically within the striatonigral pathway despite global loss of DRD1 gene function.
Hyperlocomotion has been used as a model for the hyperactivity component of ADHD in rodents (56–58). Moreover, a striking aspect of ADHD treatment is the paradoxical response to psychostimulants (59). Thus, the Isl1 conditional mutants may model aspects of the hyperactivity phenotype in ADHD because they show hyperlocomotion under normal conditions and a blunted response to psychostimulants. It is presently unclear whether imbalances in direct and indirect pathway activity underlie the behavioral abnormalities that characterize the ADHD phenotype. However, our findings implicate a reduced activity in the striatonigral pathway as a contributing factor. It should be noted, however, that the loss of striatal cholinergic interneurons as well as the defects in the nucleus accumbens and/or rTh seen in the Isl1 conditional mutants may also contribute to the observed behavioral phenotype. It will be interesting to determine whether the Isl1 conditional mutants also exhibit attention deficits and whether these are ameliorated in any way by treatment with psychostimulants.
In summary, our findings indicate that Isl1 marks the progenitors of the striatonigral pathway and is required for their development and survival. Furthermore, Isl1 is required for the normal development of rTh/ZI neurons, and these diencephalic cells regulate the formation of striatonigral axon trajectories, at least in part, through PlexinD1-Sema3e signaling. Finally, the abnormal striatal circuitry observed in Isl1 conditional mutants leads to hyperlocomotion and a lack of response to psychostimulants, similar to that observed in DRD1 mouse mutants.
Materials and Methods
Animals.
For the fate-mapping studies, CagCat (CC)-EGFP (29) mice were mated to Isl1cre/+ (30) mice, generously provided by T. Jessell (Columbia University, New York) and to Dlx 5/6-CIE (16) mice. Isl1cre/+ and Dlx 5/6-CIE were genotyped as previously described (18). Double transgenic brains were collected on postnatal day 21 (P21). Sema3eGFP/+ mice were obtained from C. Henderson (Columbia University, New York).
Isl1fl/+ mice (36) were used for the conditional knockout studies. Foxg1tTa/+ (37) and tetO-cre (38) were genotyped as previously described (18). To generate the conditional deletion of Isl1 in the telencephalon, Isl1fl/fl; Foxg1tTa/+ mice were mated to Isl1fl/fl; tetO-cre female mice to produce Isl1fl/fl; Foxg1tTa/+ control mice and Isl1fl/fl; Foxg1tTa/+; tetO-cre conditional mutants. To obtain a deletion of Isl1 in the ventral telencephalon, Isl1fl/fl mice were bred with Isl1fl/+; Dlx 5/6-CIE mice to produce Isl1fl/fl; Dlx 5/6-CIE conditional mutants.
For the staging of embryos and adult mice, the morning of the vaginal plug was considered E0.5, and the day of birth was designated P0. Embryos were fixed in 4% (wt/vol) paraformaldehyde overnight, washed in PBS, cryoprotected in 30% (wt/vol) sucrose in PBS, and sectioned at 12 µm on a cryostat. Postnatal brains were fixed overnight in 4% (wt/vol) paraformaldehyde, washed in PBS, cryoprotected in 20% (wt/vol) sucrose in PBS, and sectioned at 35µm on a freezing sliding microtome. All of the mouse studies were approved by the Institutional Animal Care and Use Committee of the Cincinnati Children’s Research Foundation and were conducted in accordance with US National Institutes of Health guidelines.
Immunohistochemistry.
Primary antibodies were used at the following concentrations: rabbit anti-EGFP (1:500; Invitrogen), goat anti-EGFP (1:5,000; Abcam), guinea pig anti-µ opioid receptor (1:3,000; Millipore), rabbit anti-synapsin 1 (1:1,000; Millipore), rabbit anti-enkephalin (1:500; Millipore), rabbit anti-substance P (1:5,000; Millipore), rabbit anti-activated caspase 3 (1:250; Cell Signaling), rabbit anti-DARPP-32 (1:1,000; Millipore), goat anti-DARPP-32 (1:200; Santa Cruz), goat anti-Helios (1:200; Santa Cruz), goat anti-Ikaros (1:200; Santa Cruz), goat anti-Isl1 (1:2,000; R&D Systems), rabbit anti-phospoHistone 3 (pH3, 1:200; Millipore), guinea pig anti-Ascl1 (1:10,000; gift from J. Johnson, UT Southwestern, Dallas, TX), rabbit anti-DLX (1:500; gift from J. Kohtz, Northwestern University, Chicago, IL), rabbit anti-Gsx2 (1:5,000; Campbell Laboratory), rabbit anti-Isl1 (1:2,000; gift from T. Edlund, Umeå University, Umeå, Sweden), rabbit anti-Meis2 (1:2,000; gift from A. Buchberg, Thomas Jefferson University, Philadelphia, PA), rabbit anti-FoxP1 (1:4,000; gift from E. Morrisey, University of Pennsylvania, Philadelphia, PA), mouse anti-neurofilament 1 2H3 (1:200; developed by T. Jessell and J. Dodd and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242). Di-amino-benzidine colorimetric reaction for bright-field immunostaining was developed as previously described (17). The following secondary antibodies were used for immunofluorescence: donkey anti-rabbit antibodies conjugated to Cy2 or Cy3 (Jackson ImmunoResearch), donkey anti-goat antibodies conjugated to Cy2 (Jackson ImmunoResearch), donkey anti-guinea pig antibodies conjugated to Cy3 (Jackson ImmunoResearch), and donkey anti-mouse antibodies conjugated to Cy2 (Jackson ImmunoResearch).
Quantification.
For both Isl1 conditional mutants (i.e., Isl1fl/fl; Foxg1tTa/+;tetO-cre, n = 3 and Isl1fl/fl; Dlx 5/6-CIE, n = 5) and their appropriate controls (n = 3 and n = 5, respectively), the size of the striatal complex, globus pallidus, and substantia nigra in adult mice was estimated by computing the area of each structure on every relevant section using the National Institutes of Health ImageJ program. The area of each structure as delineated by DARPP32 expression was measured on every sagittal section, and the average area was computed based on the number of sections per animal. Data were analyzed using a Student’s unpaired t test (P < 0.05 required for significance) to compare control and Dlx 5/6-CIE; Isl1fl/fl or control and Foxg1tTa/+;tetO-cre; Isl1fl/fl animals. For the embryos with the telencephalon-specific deletion of Isl1, cell death was quantified by counting the total number of activated caspase-3–positive cells in all sections containing LGE and developing striatum of E14.5 controls (n = 3) and mutants (n = 3) (between 12–14 sections per embryo containing two LGE per section were analyzed for each). Cell proliferation was assessed by counting pH3 positive (i.e., M-phase) cells in the E14.5 LGE of controls (n = 3) and mutants (n = 3). pH3-positive cells in the VZ (i.e., apical) and SVZ (i.e., basal) were separately counted for each animal, and the average per section was calculated. Significance was determined using a Student’s unpaired t test with P < 0.05 required for significance.
In Situ Hybridization.
The in situ hybridization procedure was performed as previously described (74). Digoxigenin-labeled antisense probes against Ebf1 (75) and Sema3e (76) were used on 12-µm sections of E18.5 embryos.
Behavior.
Locomotor activity was measured in 41 × 41 × 30-cm Accuscan activity monitors equipped with 16 pairs of photodetector-LED beams along the x and y axes (Accuscan Electronics). Isl1fl/fl; Dlx 5/6-CIE conditional mutants and control mice (Isl1fl/fl or Isl1fl/+) between 3 and 9 mo of age were used for behavior. Baseline activity for Isl1 conditional and control mice was measured for 4 h. For the drug-challenged locomotor activity, Isl1 conditional and control mice were acclimated to the chamber for 1 h before each of the following challenges: 4 mg/kg methamphetamine HCl (free base); 2 mg/kg D1 receptor agonist SKF82958 (Sigma); and 1 mg/kg D2 receptor agonist, quinpirole (Sigma). Drugs were administered s.c. in either a volume of 5 or 10 mL/kg of body weight. Postchallenge activity was measured for 3 h. Horizontal activity and total distance were recorded in 3-min intervals during the pre- and postchallenge periods and analyzed in 12-min intervals.
Statistical Analysis.
Locomotor activity data without drug were analyzed by gene × time mixed linear ANOVA where time was a repeated measure factor. Postdrug activity data were analyzed by ANCOVA, with the last two predrug intervals used as the covariate to account for hyperactivity in the conditional KO mice. Significant interactions were sorted using Slice-effect ANOVAs for genotype at each time interval.
Supplementary Material
Acknowledgments
We thank S. Anderson, M. Colbert, C. E. Henderson, T. Jessell, A. Nagy, and J. Whitsett for mouse strains. We also thank A. Buchberg, T. Edlund, and E. Morrisey for providing antibodies. This work was supported by National Institutes of Health (NIH) Grant MH090740 (to K.C.). L.A.E. was supported, in part, by NIH Training Grant T32 ES007051. The Isl1-floxed mice were generated with support from NIH Grants EY011930 and EY010608-139005 and Robert A. Welch Foundation Grant G-0010 (to W.H.K.), assisted by the MD Anderson Genetically Engineered Mouse Facility supported by NIH Grant CA016672. Research in the lab of X.M. is supported by grants from the Whitehall Foundation, by National Eye Institute Grant EY020545, and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology of the State University of New York at Buffalo.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308275110/-/DCSupplemental.
References
- 1.Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 2.Galvez-Jimenez N. Tics and Tourette syndrome: An adult perspective. Cleve Clin J Med. 2012;79(Suppl 2):S35–S39. doi: 10.3949/ccjm.79.s2a.07. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kita H, Kitai ST. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: Their morphological types and populations. Brain Res. 1988;447(2):346–352. doi: 10.1016/0006-8993(88)91138-9. [DOI] [PubMed] [Google Scholar]
- 5.Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: An in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460(1):161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- 6.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 7.Rubin JE, McIntyre CC, Turner RS, Wichmann T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. Eur J Neurosci. 2012;36(2):2213–2228. doi: 10.1111/j.1460-9568.2012.08108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 9.Deacon TW, Pakzaban P, Isacson O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: Neural transplantation and developmental evidence. Brain Res. 1994;668(1-2):211–219. doi: 10.1016/0006-8993(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 10.Olsson M, Campbell K, Wictorin K, Björklund A. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience. 1995;69(4):1169–1182. doi: 10.1016/0306-4522(95)00325-d. [DOI] [PubMed] [Google Scholar]
- 11.Olsson M, Björklund A, Campbell K. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience. 1998;84(3):867–876. doi: 10.1016/s0306-4522(97)00532-0. [DOI] [PubMed] [Google Scholar]
- 12.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 13.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development. 1999;126(15):3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 14.Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20(16):6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128(2):193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: Implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23(1):167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waclaw RR, et al. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49(4):503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Waclaw RR, Ehrman LA, Pierani A, Campbell K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 2010;30(20):6944–6953. doi: 10.1523/JNEUROSCI.5772-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9(3):443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 20.Lobo MK, Yeh C, Yang XW. Pivotal role of early B-cell factor 1 in development of striatonigral medium spiny neurons in the matrix compartment. J Neurosci Res. 2008;86(10):2134–2146. doi: 10.1002/jnr.21666. [DOI] [PubMed] [Google Scholar]
- 21.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: Opposing roles for Pax6 and Gsh2. Development. 2000;127(20):4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 22.Wang HF, Liu FC. Developmental restriction of the LIM homeodomain transcription factor Islet-1 expression to cholinergic neurons in the rat striatum. Neuroscience. 2001;103(4):999–1016. doi: 10.1016/s0306-4522(00)00590-x. [DOI] [PubMed] [Google Scholar]
- 23.Elshatory Y, Gan L. The LIM-homeobox gene Islet-1 is required for the development of restricted forebrain cholinergic neurons. J Neurosci. 2008;28(13):3291–3297. doi: 10.1523/JNEUROSCI.5730-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, et al. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci USA. 2003;100(15):9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136(22):3841–3851. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Chatterjee M, Li JY. The mouse homeobox gene Gbx2 is required for the development of cholinergic interneurons in the striatum. J Neurosci. 2010;30(44):14824–14834. doi: 10.1523/JNEUROSCI.3742-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes R, Verhey van Wijk N, Neves G, Pachnis V. Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc Natl Acad Sci USA. 2012;109(8):3119–3124. doi: 10.1073/pnas.1109251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura S, Morikawa Y, Iwanishi H, Hisaoka T, Senba E. Foxp1 gene expression in projection neurons of the mouse striatum. Neuroscience. 2004;124(2):261–267. doi: 10.1016/j.neuroscience.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98(12):1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 30.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;291(5814):415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- 33.Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA. 1985;82(24):8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62(5):1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84(2):309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 36.Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci USA. 2008;105(19):6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22(15):6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99(16):10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson KD, Reiner A. Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: Implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 1991;568(1-2):235–243. doi: 10.1016/0006-8993(91)91403-n. [DOI] [PubMed] [Google Scholar]
- 40.Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128(23):4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- 41.Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461(2):151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- 42.Long JE, et al. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 2009;512(4):556–572. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Waclaw RR, Allen ZJ, 2nd, Guillemot F, Campbell K. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev. 2009;4:5. doi: 10.1186/1749-8104-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang B, Lufkin T, Rubenstein JL. Dlx6 regulates molecular properties of the striatum and central nucleus of the amygdala. J Comp Neurol. 2011;519(12):2320–2334. doi: 10.1002/cne.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, et al. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol. 2013;521(7):1561–1584. doi: 10.1002/cne.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martín-Ibáñez R, et al. Ikaros-1 couples cell cycle arrest of late striatal precursors with neurogenesis of enkephalinergic neurons. J Comp Neurol. 2010;518(3):329–351. doi: 10.1002/cne.22215. [DOI] [PubMed] [Google Scholar]
- 47.Martín-Ibáñez R, et al. Helios transcription factor expression depends on Gsx2 and Dlx1&2 function in developing striatal matrix neurons. Stem Cells Dev. 2012;21(12):2239–2251. doi: 10.1089/scd.2011.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agoston DV, et al. Ikaros is expressed in developing striatal neurons and involved in enkephalinergic differentiation. J Neurochem. 2007;102(6):1805–1816. doi: 10.1111/j.1471-4159.2007.04653.x. [DOI] [PubMed] [Google Scholar]
- 49.Garel S, Marín F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126(23):5285–5294. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- 50.Foster GA, et al. Development of a dopamine- and cyclic adenosine 3′:5′-monophosphate-regulated phosphoprotein (DARPP-32) in the prenatal rat central nervous system, and its relationship to the arrival of presumptive dopaminergic innervation. J Neurosci. 1987;7(7):1994–2018. doi: 10.1523/JNEUROSCI.07-07-01994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thor S, Ericson J, Brännström T, Edlund T. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron. 1991;7(6):881–889. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- 52.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 53.Chauvet S, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56(5):807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci. 2012;15(2):215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 56.Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods. 2007;161(2):185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Krapacher FA, et al. Mice lacking p35 display hyperactivity and paradoxical response to psychostimulants. J Neurochem. 2010;114(1):203–214. doi: 10.1111/j.1471-4159.2010.06748.x. [DOI] [PubMed] [Google Scholar]
- 58.Castelli M, et al. Loss of striatal cannabinoid CB1 receptor function in attention-deficit / hyperactivity disorder mice with point-mutation of the dopamine transporter. Eur J Neurosci. 2011;34(9):1369–1377. doi: 10.1111/j.1460-9568.2011.07876.x. [DOI] [PubMed] [Google Scholar]
- 59.Greenhill LL. Pharmacologic treatment of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 1992;15(1):1–27. [PubMed] [Google Scholar]
- 60.Mega T, et al. Zinc finger protein 521 antagonizes early B-cell factor 1 and modulates the B-lymphoid differentiation of primary hematopoietic progenitors. Cell Cycle. 2011;10(13):2129–2139. doi: 10.4161/cc.10.13.16045. [DOI] [PubMed] [Google Scholar]
- 61.Janis LS, Cassidy RM, Kromer LF. Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum. J Neurosci. 1999;19(12):4962–4971. doi: 10.1523/JNEUROSCI.19-12-04962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Passante L, et al. Temporal regulation of ephrin/Eph signalling is required for the spatial patterning of the mammalian striatum. Development. 2008;135(19):3281–3290. doi: 10.1242/dev.024778. [DOI] [PubMed] [Google Scholar]
- 63.Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26(2):130–132. [PubMed] [Google Scholar]
- 64.Sun Y, et al. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci. 2008;11(11):1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47(6):817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 67.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249(4975):1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 68.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Xu M, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79(6):945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 71.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: Cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74(3):813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 72.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852(1):198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 73.Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22(7):1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- 74.Toresson H, Mata de Urquiza A, Fagerström C, Perlmann T, Campbell K. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999;126(6):1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- 75.Garel S, et al. Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210(3):191–205. doi: 10.1002/(SICI)1097-0177(199711)210:3<191::AID-AJA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 76.Gu C, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.