Abstract
Objective
To determine the short-term and long-term risks of tuberculosis (TB) associated with CD4 cell recovery during antiretroviral therapy (ART).
Design
Observational community-based ART cohort in South Africa.
Methods
TB incidence was determined among patients (n = 1480) receiving ART for up to 4.5 years in a South African community-based service. Updated CD4 cell counts were measured 4-monthly. Person-time accrued within a range of CD4 cell count strata (CD4 cell strata) was calculated and used to derive CD4 cell-stratified TB rates. Factors associated with incident TB were identified using Poisson regression models.
Results
Two hundred and three cases of TB were diagnosed during 2785 person-years of observation (overall incidence, 7.3 cases/100 person-years). During person-time accrued within CD4 cell strata 0–100, 101–200, 201–300, 301–400, 401–500 and more than 500 cells/µl unadjusted TB incidence rates were 16.8, 9.3, 5.5, 4.6, 4.2 and 1.5 cases/100 person-years, respectively (P < 0.001). During early ART (first 4 months), adjusted TB rates among those with CD4 cell counts 0–200 cells/µl were 1.7-fold higher than during long-term ART (P = 0.026). Updated CD4 cell counts were the only patient characteristic independently associated with long-term TB risk.
Conclusion
Updated CD4 cell counts were the dominant predictor of TB risk during ART in this low-resource setting. Among those with baseline CD4 cell counts less than 200 cells/µl, the excess adjusted risk of TB during early ART was consistent with ‘unmasking’ of disease missed at baseline screening. TB incidence rates at CD4 cell counts of 200–500 cells/µl remained high and adjunctive interventions are required. TB prevention would be improved by ART policies that minimized the time patients spend with CD4 cell counts below a threshold of 500 cells/µl.
Keywords: Africa, antiretroviral, CD4 cell, HIV, immune reconstitution, resource-limited country, tuberculosis
Introduction
In recent years, access to antiretroviral therapy (ART) has been rapidly scaled up in the countries of sub-Saharan Africa, which have borne the brunt of the dual tuberculosis (TB) and HIV epidemics. ART is associated with a 70–90% decrease in TB incidence rates among treated individuals [1–6] and is therefore a potentially important intervention to address the HIV-associated TB epidemic. Despite this, however, studies from within the region have reported that rates of TB during ART persist at levels much higher than background rates [7–11].
This high burden of TB is a key challenge to ART services in sub-Saharan Africa as this is a major cause of morbidity and mortality and concurrent administration of TB treatment and ART is problematic [9,11–13]. These congregate clinical settings are also associated with substantial risk of transmission of both drug-susceptible and multidrug-resistant TB [14,15]. Furthermore, high persisting rates of TB during treatment may substantially undermine the potential for ART to effect TB control at the population level [9,16,17].
Potential means to reduce this burden of TB might include initiation of ART at higher CD4 cell counts [17] and use of the interventions included within the World Health Organization (WHO) ‘3Is policy’ [18]. However, these approaches must be based on a clear understanding of clinical epidemiology of TB during ART. How TB risk changes over time in association with immune recovery in the short-term and long-term has not been clearly defined. Although some reports suggest that TB rates are particularly high during the initial weeks of ART due to immune-mediated ‘unmasking’ of subclinical disease [19,20], this phenomenon has not previously been quantified. Of particular importance, the CD4 cell count threshold above which TB rates are minimized during ART-induced immune recovery is not known. To address these questions, we analysed data collected prospectively over 4.5 years of follow-up of a large ART cohort in Cape Town, South Africa.
Methods
Study population
The ART service in Gugulethu township in Cape Town has been described previously [21,22]. The district has a predominantly African population of over 300 000, the vast majority of whom live in conditions of low socioeconomic status. At the time of the study, the antenatal HIV seroprevalence was approximately 30% and the annual TB notification rate exceeded 1500/100 000. National ART guidelines were based on WHO 2002 recommendations [23], providing free treatment for those with a prior AIDS diagnosis (WHO stage 4 disease) or a blood CD4 cell count less than 200 cells/µl. First-line ART consisted of stavudine, lamivudine and a nonnucleoside reverse transcriptase inhibitor (predominantly efavirenz). Treatment compliance was high with over 90% of patients achieving viral load suppression less than 400 copies/ml [22] and with a virological failure rate of approximately 2% of patients per year [24]. In keeping with current national practice, patients receiving ART did not receive isoniazid preventive therapy (IPT).
A TB screening questionnaire was used routinely at baseline to identify symptomatic patients for TB investigations, and during ART investigations for TB were conducted when clinically indicated. Available tests include sputum smear fluorescence microscopy, automated liquid culture of sputum (MGIT 960; Becton Dickinson, Sparks, Maryland, USA), sputum induction, chest radiology, abdominal ultrasonography and fine needle lymph node aspiration for cytology. Microbiological specimens were processed within nationally accredited laboratories.
Blood CD4 cell counts (CD4 cell counts) and plasma viral load measurements were done routinely at baseline and 4-monthly during ART together with clinical review. Patients had open access to the clinic in the event of intercurrent illnesses. Detailed structured clinical and laboratory records were maintained for every patient visit. Data were transferred on a weekly basis to an electronic database. Patients requiring hospital admission were referred to a nearby 200-bed facility. Information on in-patient care was gained from discharge letters, hospital and laboratory records and post-mortem examinations. Deaths and losses to follow-up were ascertained by active community-based follow-up as previously described [25].
Collection of data on this study population for research purposes was approved by the Research Ethics Committee of the University of Cape Town and all patients enrolled gave written informed consent.
Definitions
Incident TB was defined as the first new clinical episode of TB diagnosed during ART for which the date of onset of overt symptoms occurred after ART initiation; TB episodes were dated according to symptom onset. The terms ‘early’ and ‘short-term’ ART were used interchangeably and were defined as the first 4 months of treatment. ‘Long-term’ ART was defined as treatment beyond 4 months. The terms ‘updated CD4 cell count’ and ‘updated viral load’ were used to refer to follow-up measurements during ART. TB diagnoses fulfilled WHO criteria for smear-positive pulmonary TB, smear-negative pulmonary TB or extrapulmonary TB [26].
Data analysis
Data were analysed using STATA version 10.0 (College Station, Texas, USA). Data from all patients who initiated ART between September 2002 and March 2006 were included. Person-time at risk of TB was accrued from the date of starting ART until either occurrence of incident TB, death, loss to follow-up, transfer to another ART programme or censoring of observation in early 2007. All person-time of observation accrued during treatment of prevalent TB present at baseline and during treatment of incident TB during ART was excluded.
As CD4 cell count and viral load measurements were routinely made every 4 months, person-time was subdivided into 4-month intervals for analysis. Each interval was defined by the CD4 cell count measurement at the start of the interval; in the event of missing CD4 cell count values (<5% of all intervals), we used the mean of the two values immediately before and after the start of the interval. These intervals were categorized into CD4 cell count strata (CD4 cell strata) 0–100, 101– 200, 201–300, 301–400, 401–500 and more than 500 cells/µl. Total person-time accrued within each of the CD4 cell count strata during follow-up of the cohort was calculated.
TB incidence rates in the overall cohort and within CD4 cell count strata were calculated and Kaplan–Meier (product limit) calculations were used to estimate TB-free survival. We compared TB incidence during early (0–4 months) and long-term (>4 months) ART and the incidence of TB within CD4 cell count strata using Poisson regression; results are presented as incidence rate ratios (IRRs) with 95% confidence intervals (CIs). In these analyses, variances were adjusted for clustering of person-time on individual patients using the Huber–White sandwich (robust) estimator.
In other analyses, Fisher's exact and Wilcoxon rank-sum tests were used to compare proportions and medians, respectively, and all statistical tests are two-sided at [alpha] value of 0.05.
Results
Cohort characteristics and follow-up
During the study period, 2000 consecutive patients were enrolled in the programme. Those aged less than 15 years (n = 161) and those who were non-naive to ART (n = 85) were excluded. At data censorship, 274 (15.6%) patients had not received ART because they had died (n = 91, 5.2%), were alive and awaiting treatment (n = 3, 0.2%) or had been deferred from the programme for a variety of reasons (n = 180, 10.3%). ART was received by 1480 (84.4%) patients; these typically had advanced immunodeficiency and many had previous TB diagnoses (Table 1).
Table 1.
Baseline characteristics of patients who initiated antiretroviral therapy and of those who did or did not develop tuberculosis during treatment.
| Total (n = 1480) | Incident TB (n = 203) | No incident TB (n = 1277) | |
|---|---|---|---|
| Mean age (years) | 34.0 | 33.2 | 34.1 |
| Men | 448 (30) | 56 (28) | 392 (31) |
| Baseline WHO stage 1 and 2 | 294 (20) | 35 (17) | 259 (20) |
| 3 | 825 (56) | 112 (55) | 713 (56) |
| 4 | 360 (24) | 56 (28) | 304 (24) |
| Baseline CD4 cell count | |||
| Median (IQR) | 97 (47–155) | 95 (46–143) | 97 (47–155) |
| ≥150 | 388 (27) | 45 (23) | 343 (28) |
| 100–149 | 308 (22) | 50 (25) | 258 (21) |
| 50–99 | 351 (25) | 49 (25) | 302 (25) |
| <50 | 375 (26) | 54 (27) | 321 (26) |
| Baseline viral load | |||
| Median (IQR) | 4.84 (4.44–5.25) | 4.86 (4.50–5.26) | 4.85 (4.44–5.26) |
| ≥5.0 | 570 (41) | 92 (44) | 478 (40) |
| <5.0 | 829 (58) | 118 (56) | 711 (60) |
| History of previous TB | 686 (46) | 116 (57) | 570 (45) |
| TB treatment at baseline | 448 (30) | 32 (16) | 416 (33) |
Values represent numbers (%) unless otherwise stated. CD4 cell counts in cells/µl. Viral load in log10copies/ml. IQR, interquartile range; TB, tuberculosis.
Of those who received ART, 155 (10.5%) died during treatment, 165 (11.2%) were lost to follow-up and 91 (6.1%) were transferred or moved out of area. The remaining 1069 (72.2%) patients were alive and receiving ART at the time data were censored. Patients were followed up for a median of 2.1 years [interquartile range (IQR), 1.5–2.7; range, 1.0–4.5 years]. Overall 2785 person-years of observation accrued during follow-up with person-time during TB treatment excluded.
During ART, 203 patients developed TB and they had broadly similar baseline characteristics as patients who did not develop TB except that those developing incident TB were less likely to have prevalent TB at baseline (Table 1). In those in whom disease site was specified (n = 198), 147 (74%) had pulmonary and 51 (26%) had extrapulmonary disease. Overall 64% of cases were microbiologically confirmed. Kaplan–Meier estimates of TB-free survival proportions at 1, 2 and 3 years were 0.89, 0.85 and 0.82, respectively.
Association between tuberculosis incidence rates and CD4 cell counts
The overall TB incidence rate during follow-up was 7.28 cases/100 person-years (95%CI, 6.32–8.36). Rates in the 1st, 2nd, 3rd, 4th and 5th years of the cohort were 12.5 (10.6–14.7), 4.5 (3.2–6.1), 3.2 (1.8–5.5), 4.5 (1.8–9.2) and 2.2 (0.1–12.5) cases/100 person-years, respectively.
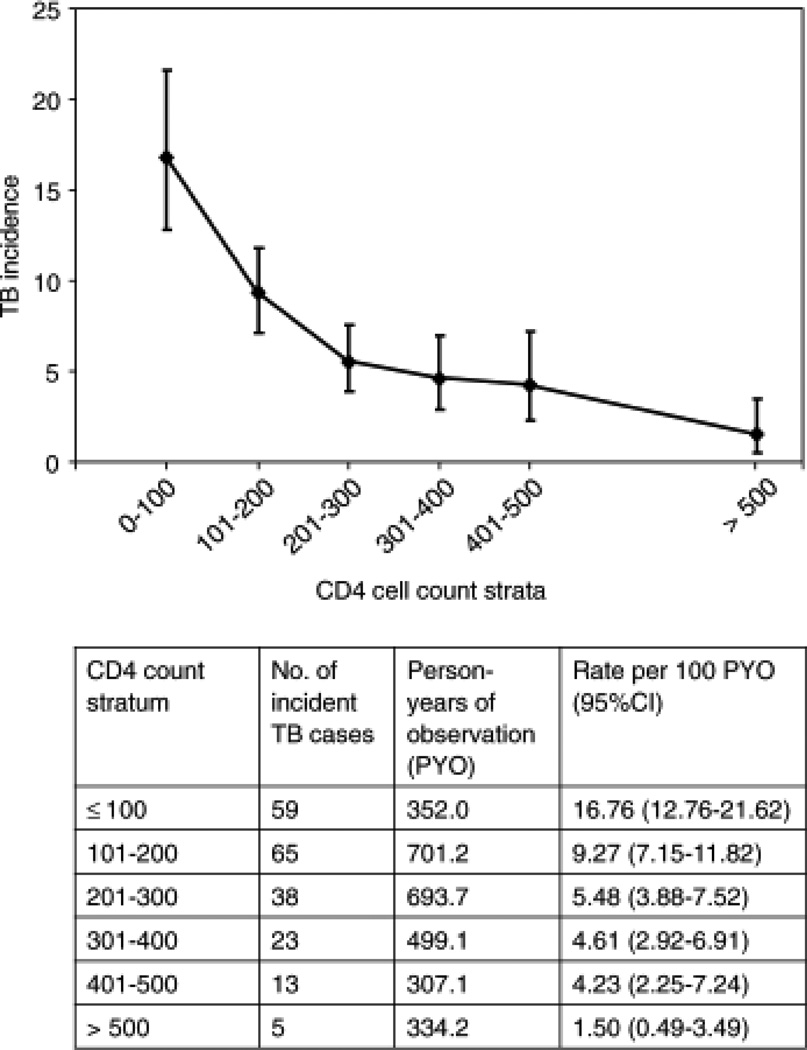
To provide greater insight into changes in TB incidence rates and the association with underlying immune recovery, we next calculated TB rates stratified according to updated CD4 cell counts categorized into 0–100, 101–200, 201–300, 301–400, 401–500 and more than 500 cells/µl CD4 cell count strata. A strong graded association was observed with the highest TB rates during person-time accrued within the less than 100 cells/µl CD4 cell stratum and the lowest during person-time accumulated within the more than 500 cells/µl CD4 cell stratum (Fig. 1).
Fig. 1. Graph of tuberculosis incidence rates (95% confidence interval, cases/100 person-years) plotted against serially updated CD4 cell counts measured during total duration (early and late) of antiretroviral therapy.
CD4 cell counts (cells/µl) were measured at baseline and 4-monthly during antiretroviral therapy (ART). Tuberculosis (TB) incidence rates are seen to decrease with increasing CD4 cell counts. Data used to derive these rates are displayed in the table beneath. Median CD4 cell counts within 0–100, 101–200, 201–300, 301–400, 401–500 and more than 500 CD4 cell strata were 60, 157, 250, 345, 446 and 700 cells/µl, respectively. CI, confidence interval.
Poisson regression models were used to examine risk factors for incident TB during long-term ART. A very strong independent association between TB risk and updated CD4 cell counts during ART was observed (Table 2). The adjusted TB rate associated with the lowest CD4 cell stratum was more than nine-fold higher than the rate associated with the highest CD4 cell stratum. TB risk was not, however, independently associated with baseline patient characteristics, updated viral load measurements or duration of ART, which was included to control for any survival effect not reflected by updated CD4 cell counts.
Table 2.
Risk factors for incident tuberculosis during long-term antiretroviral therapy, excluding the first 4 months of treatment.
| Crude association | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|
| Patient characteristics | IRR | 95%CI | P | IRR | 95%CI | P | |
| Age | 0.99 | 0.97–1.02 | 0.605 | 0.99 | 0.97–1.01 | 0.307 | |
| Sex | 1.04 | 0.73–1.48 | 0.845 | 0.99 | 0.67–1.46 | 0.969 | |
| Previous TB diagnosis | 1.23 | 0.87–1.75 | 0.240 | 1.29 | 0.85–1.96 | 0.239 | |
| Baseline WHO stage | 1 and 2 | 1 | 1 | ||||
| 3 | 1.06 | 0.68–1.64 | 0.800 | 1.07 | 0.63–1.83 | 0.797 | |
| 4 | 1.19 | 0.73–1.92 | 0.488 | 1.21 | 0.66–2.21 | 0.533 | |
| Baseline CD4 cell count (cells/µl) | >150 | 1 | 1 | ||||
| 101–150 | 1.18 | 0.76–1.84 | 0.466 | 1.12 | 0.70–1.79 | 0.632 | |
| 51–100 | 1.05 | 0.67–1.65 | 0.832 | 0.78 | 0.45–1.35 | 0.369 | |
| 0–50 | 0.94 | 0.59–1.50 | 0.795 | 0.66 | 0.37–1.18 | 0.162 | |
| Baseline viral load (log copies/ml) | 0.88 | 0.71–1.10 | 0.258 | 0.92 | 0.71–1.20 | 0.548 | |
| Cohort enrolment year | 1 | 1 | 1 | ||||
| 2 | 0.81 | 0.53–1.26 | 0.353 | 0.67 | 0.43–1.04 | 0.078 | |
| 3 | 0.89 | 0.61–1.30 | 0.545 | 0.63 | 0.41–0.96 | 0.030 | |
| 4 | 0.65 | 0.31–1.37 | 0.255 | 0.44 | 0.20–0.97 | 0.042 | |
| Duration of ART | 5–12 months | 1.0 | 1.0 | ||||
| 13–24 months | 0.52 | 0.35–0.76 | 0.001 | 0.66 | 0.42–1.03 | 0.069 | |
| >24 months | 0.43 | 0.27–0.67 | <0.001 | 0.62 | 0.34–1.12 | 0.116 | |
| Updated CD4 cell count (cells/µl) | >500 | 1 | 1 | ||||
| 401–500 | 2.82 | 1.01–7.90 | 0.048 | 3.59 | 1.17–11.03 | 0.025 | |
| 301–400 | 3.08 | 1.18–8.09 | 0.022 | 3.78 | 1.30–11.01 | 0.015 | |
| 201–300 | 3.65 | 1.43–9.30 | 0.007 | 4.13 | 1.45–11.81 | 0.008 | |
| 101–200 | 4.86 | 1.93–12.26 | 0.001 | 5.42 | 1.79–16.47 | 0.003 | |
| 0–100 | 7.39 | 2.74–19.92 | <0.001 | 9.21 | 2.69–31.52 | <0.001 | |
| Updated viral load (copies/ml) | <400 | 1 | |||||
| >400 | 1.86 | 1.33–2.61 | <0.001 | 1.29 | 0.88–1.87 | 0.192 | |
Age and baseline viral load included as continuous variables. For baseline viral load, the IRR for a 1.0 log decrease in viral load is shown. ‘Previous TB diagnosis’ includes all TB diagnoses established at any time prior to ART initiation. ‘Updated’ CD4 cell count and viral load values are the serial measurements made 4-months during follow-up on ART. ART, antiretroviral therapy; CI, confidence interval; IRR, incidence rate ratio; TB, tuberculosis.
Excess tuberculosis rates during early antiretroviral therapy
Thus far we have demonstrated that TB rates during ART were very strongly associated with updated CD4 cell counts. However, further analyses exploring the exceptionally high TB rates (18.8 cases/100 person-years; 95%CI, 15.2–23.3) during the first 4 months of ART found that the rate of TB during this period was much higher than that during long-term ART having adjusted for relevant covariates.
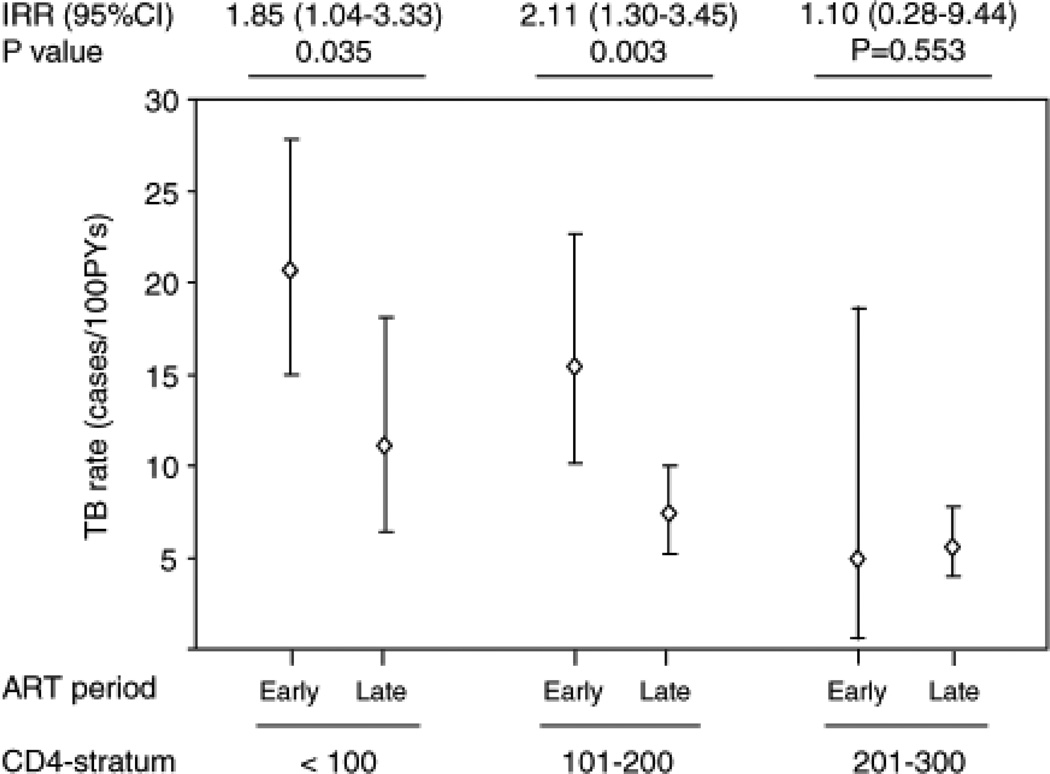
Initial unadjusted analyses showed that within the 0–100 and 101–200 cells/µl CD4 cell strata, TB rates during early ART were approximately double the rates during long-term ART (Fig. 2). In contrast, within the 201–300 cells/µl CD4 cell stratum, rates in two periods did not significantly differ. This excess TB incidence rate observed during early ART among those with CD4 cell counts 0–200 cells/µl was found to be confined to the first 4 months of treatment. After adjustment for covariates, including viral load, absolute CD4 cell count values, clustering and variable interval duration, the incidence rate during early ART remained significantly higher (adjusted IRR = 1.66; 95%CI, 1.06–2.59; P = 0.026). Thus, the excess proportion of TB cases presenting during early ART was 40% (95%CI, 6–61%).
Fig. 2. CD4 cell-stratified tuberculosis incidence rates during first 4 months of antiretroviral therapy (early antiretroviral therapy) and during person-time thereafter (late antiretroviral therapy).
Within each of the CD4 cell strata 0–100, 101–200 and 201–300 cells/µl, tuberculosis (TB) incidence rates during early antiretroviral therapy (ART) are compared with rates during late ART [cases/100 person-years, 95% confidence interval (CI)]. Incidence rates and incidence rate ratios (IRRS) for these CD4 cell strata are shown. Within the two lowest strata (0–100 and 101–200 cells/µl), TB incidence rates during early ART were approximately double the rates during long-term ART in unadjusted analyses and 1.7-fold higher in adjusted analyses (P = 0.026).
High persisting tuberculosis rates during long-term antiretroviral therapy
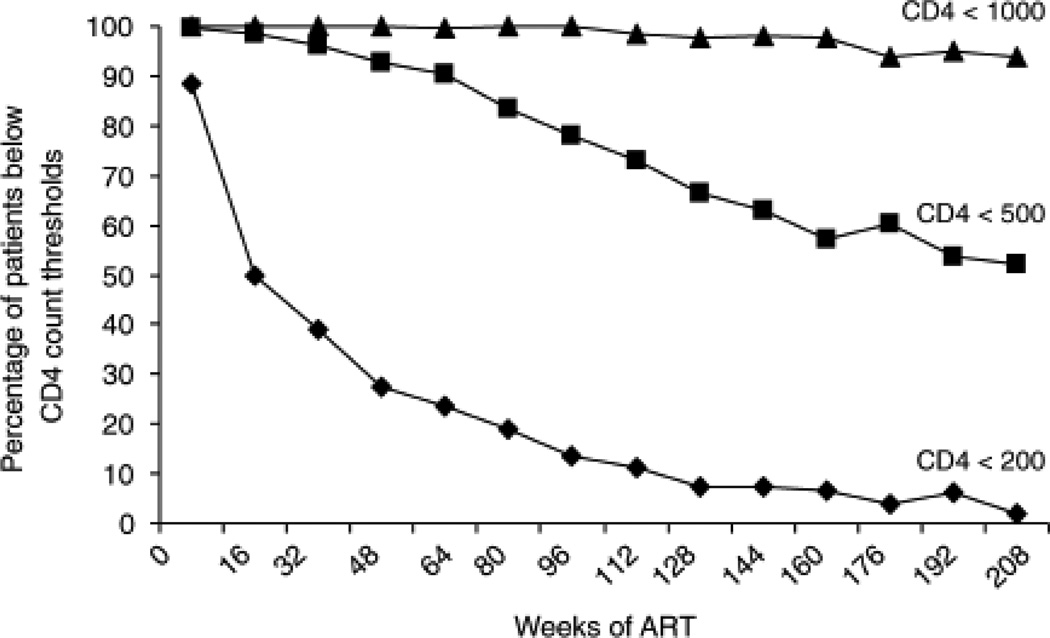
As the extent of CD4 cell count recovery was the dominant association with long-term TB incidence rates, we next examined how CD4 cell counts changed over 4 years of ART (Fig. 3). The proportion of patients with a CD4 cell count less than 200 cells/µl decreased steeply from 89% at baseline, reaching less than 10% of patients beyond 128 weeks. Conversely, the proportion of patients attaining a CD4 cell count more than 500 cells/µl steadily increased, representing 48% of patients after 4 years (Fig. 3).
Fig. 3. Changes in CD4 cell counts during 4 years of antiretroviral therapy.
The graph shows the changes in the proportions (%) of patients with CD4 cell counts lying below thresholds of 200, 500 and 1000 cells/µl with increasing duration of antiretroviral therapy (ART). During the first year of ART, the proportion of patients with a CD4 cell count less than 200 cells/µl decreased steeply from 89% at baseline, eventually accounting for less than 10% of patients. In contrast, the proportion of patients with CD4 cell counts more than 500 cells/µl increased steadily over time reaching 48% after 47 years. Only data from patients with at least two serial measurements were included and the numbers of patients represented at 0, 48, 96, 144 and 208 weeks were 1313, 1129, 595, 237 and 97, respectively.
We next reasoned that the overall rate of TB in the cohort would be related to the proportions of person-time accrued at different CD4 cell count levels. Despite excellent CD4 cell count recovery resulting in a steadily increasing proportion of patients achieving a CD4 cell count more than 500 cells/µl, 88% of person-time was nevertheless associated with CD4 cell strata below 500 cells/µl. Thus, in this analysis, only 12% of person-time was associated with the lowest TB rates achievable during ART (1.5 cases/100 person-years).
Impact of baseline CD4 cell counts on person-time within low CD4 cell strata
We also hypothesized that patients with the lowest baseline CD4 cell counts would accrue substantially more person-time within the lowest CD4 cell strata and thereby remain at high risk of TB for long periods. Indeed, during long-term ART, those with baseline CD4 cell counts of 100 cells/µl or less accrued 40% of person-time with CD4 cell counts in the range 0–200 cells/µl compared with just 17% of person-time accrued by those whose baseline counts were more than 100 cells/µl (P < 0.001). Thus, patients with the lowest baseline counts remained at high risk of TB for a longer period of time.
Discussion
In this analysis, we calculated TB incidence rates stratified by serially updated CD4 cell counts during ART and compared TB rates during early and long-term treatment. Several key findings emerged. Among patients with CD4 cell counts less than 200 cells/µl, there was a 1.7-fold excess adjusted TB rate during early ART compared with rates during long-term treatment (P = 0.026). During long-term ART, a very strong independent association between TB rates and updated CD4 cell counts was observed. At CD4 cell counts of 200–500 cells/µl, TB incidence rates remained high but were significantly lower at CD4 cell counts exceeding a threshold of 500 cells/µl. However, despite excellent immune recovery, patients spent a large majority of time at CD4 cell counts less than 500 cells/µl and overall TB rates in the cohort were, therefore, high. These data substantially extend the findings of previous studies [7–11], providing important insights that will assist in the development of approaches to address the challenge of HIV-associated TB.
We suggest that the excess TB rates during early ART among those with CD4 cell counts less than 200 cells/µl may be due to ART-induced ‘unmasking’ of subclinical TB that was present but unrecognized at baseline [20,27]. In patients who develop ‘unmasking’ TB, rapid immune recovery is thought to trigger host inflammatory responses and development of symptomatic disease [20]. Although some overt cases of ‘unmasking TB’ have been described [19,20,28–31], this phenomenon lacks a clear clinical case definition [27] and has not previously been quantified.
Several lines of indirect evidence support our hypothesis. First, high rates of subclinical, culture-proven TB have been detected in patients enrolling for ART in this [32] and in other HIV cohorts in Africa [33–36]. Immune recovery in the first 4 months of ART in this cohort is very rapid, even in those with low baseline CD4 cell counts [37]. In keeping with ‘unmasking’ TB, excess rates were restricted to those with baseline CD4 cell counts less than 200 cells/µl and were confined to early ART [19,20,27,28]. Further corroboration is derived from a study of Ugandan children in whom a more than two-fold increase in TB rates during the initial months of ART was attributed to ‘unmasking’ TB [38].
These data suggest that ‘unmasking’ TB may account for over one-third of TB cases presenting during the initial months of ART in this setting. CIs around this estimate are wide, however, and confirmatory studies are required. Pre-ART investigations for TB were routinely done only in those with suggestive symptoms or clinical signs. These data suggest the potential need for routine microbiological screening for TB at baseline in all patients starting ART in this setting and this approach is supported by the findings of a more recent study in this cohort [32].
Although we have previously found that CD4 cell counts were strongly associated with TB incidence rates during ART [9], the present study used a novel analytic approach to derive CD4 cell-stratified TB rates, yielding important new insights. A steep risk gradient was observed between the highest and lowest CD4 cell strata with a more than 9-fold difference in adjusted rates. We have used a similar analytic approach to examine changing mortality risk in this cohort [39]. Whereas mortality risk was found to be largely minimized by the attainment of an updated CD4 cell count of more than 200 cells/µl, the present study shows that a threshold of more than 500 cells/µl has to be exceeded to minimize TB rates. Thus, eligibility criteria for ART initiation that aim to minimize mortality risk are not optimal for TB prevention.
Immune recovery in this cohort compared very favourably with that observed in ART cohorts in high-income countries [40]. Approximately half of the patients achieved a CD4 cell count more than 500 cells/µl after 4 years of ART (Fig. 3) and in these patients, TB rates (1.5 cases/100 person-years) remained approximately only two-fold higher than the rate among HIV-seronegative adults in a comparable neighbouring community (0.7 cases/100 person-years) [41]. However, despite excellent immune recovery, the large majority of person-time in this cohort accrued at CD4 cell counts less than 500 cells/µl, with TB rates ranging between 4.2 and 16.8 cases/100 person-years. As a result, the overall TB incidence rate in the cohort was high (7.3 cases/100 person-years), approximately 10-fold higher than the rate in HIV-seronegative adults in these communities [41].
Although this analysis only examined incident TB from the time of ART initiation, other factors occurring just prior to this may have influenced the findings. Many patients enrolling in this cohort had recently completed TB treatment, potentially conferring a relative protection against further TB episodes [9]. Although all person-time accrued during TB treatment was excluded from the analysis, a similar protective effect may also have been present during the period following TB treatment in the many patients with TB diagnoses at baseline. These effects may have reduced the unadjusted TB incidence rates among those with the lowest baseline CD4 cell counts.
In multivariate analysis, baseline CD4 cell counts did not have any predictive value for TB risk over and above that provided by the current CD4 cell count at any given time-point. This does not support the hypothesis that lower baseline CD4 cell counts are associated with increased risk of clinically significant persisting defects in TB-specific immune function during long-term ART [17]. Importantly, however, patients with low baseline CD4 cell counts accrued much greater person-time within low CD4 cell strata, thereby remaining at high TB risk for longer periods. Thus, whereas current CD4 cell counts are the key predictor of instantaneous TB risk, baseline CD4 cell counts are key predictors of cumulative long-term risk of TB during ART as was similarly found for mortality [39].
Data from this study provide insight into the strategies needed to reduce the long-term burden of incident TB. Most fundamentally, the time that patients spend at low CD4 cell counts less than 500 cells/µl needs to be minimized. This requires both earlier HIV diagnosis and initiation of ART at higher CD4 cell counts. Unfortunately, the current South African national ART policy restricts eligibility to those with AIDS or a CD4 cell count of less than 200 cells/µl and therefore greatly undermines the potential benefits of ART for TB prevention. A change in this policy is needed to reduce both high mortality rates [39] and to improve TB control.
Adjunctive TB prevention strategies such as the WHO ‘3Is policy’ [18] are also needed to reduce TB in ART services. Within this policy, intensified case finding (ICF) might be done not only at baseline but also serially (e.g. 6-monthly) during at least the first year of ART when TB rates are highest. This approach might particularly target those with persistently low CD4 cell counts. Use of IPT concurrently with ART is likely to reduce long-term TB rates [42] but data from randomized controlled trials are awaited [43]. However, initiation of IPT at the same time as ART may be problematic because high rates of subclinical active TB at baseline and ‘unmasking’ TB during the first 4 months of ART may inadvertently lead to many patients with active TB receiving isoniazid monotherapy. In light of our findings, a logical approach might be to consider initiating IPT after completion of the first few months of ART.
Strengths of this study include good patient retention and ascertainment of outcomes, frequent monitoring of CD4 cell counts and the novel analytic approach. Some person-time may have been misclassified as CD4 cell counts continuously change over time. Some TB disease may have remained unascertained among those who died, leading to underestimation of TB rates particularly in those with the lowest CD4 cell counts. Not all TB cases were proven by culture of Mycobacterium tuberculosis, although the rates and proportions of pulmonary and extrapulmonary disease reported are entirely consistent with other data from this setting [6,8,9,41]. The multiple lines of evidence for ‘unmasking’ TB during early ART are indirect and yet provide a coherent and biologically plausible explanation.
Baseline characteristics of the patients were typical of patients in ART roll-out programmes across Africa, but rates of loss to follow-up were comparatively low [44]. Such losses are not related to degree of immunodeficiency in this cohort [25] and so we do not suspect they affected the TB rates observed. The countries of southern Africa are the areas of the world hit hardest by the TB and HIV epidemics and the absolute TB rates recorded are likely to be higher than those in other regions. Nevertheless, the key relationship between TB risk and updated CD4 cell counts is likely to be applicable in other settings.
In conclusion, low baseline CD4 cell counts and ‘unmasking’ of subclinical TB are likely to explain the high burden of TB during the first 4 months of ART. This may potentially be reduced by initiation of ART at higher baseline CD4 cell counts and more effective screening for TB at baseline. The high long-term TB incidence is strongly associated with the proportion of person-time at CD4 cell counts less than 500 cells/µl and adjunctive TB prevention interventions are undoubtedly needed. However, the impact of ART on TB prevention in low-resource settings would be greatly improved by ART policies that minimize the time patients spend with CD4 cell counts less than 500 cells/µl.
Acknowledgements
S.D.L. is funded by the Wellcome Trust, London, UK with grant number 074641. R.W., L.M. and L.G.B. are funded in part by the National Institutes of Health through a CIPRA grant 1U19AI53217-01 and R.W. also receives support from RO1 grant (A1058736-01A1). The funding sources played no role in the decision to publish these data. The authors gratefully acknowledge the dedicated staff of the Hannan Crusaid ART clinic and the Desmond Tutu HIV Centre.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 2.Kirk O, Gatell JM, Mocroft A, Pedersen C, Proenca R, Brettle RP, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–872. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 3.Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteelli A, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. doi: 10.1097/00002030-200009080-00015. Ovid. [DOI] [PubMed] [Google Scholar]
- 4.Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. [PubMed] [Google Scholar]
- 5.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 6.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 7.Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med. 2005;172:123–127. doi: 10.1164/rccm.200410-1342OC. [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. Ovid. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. Ovid. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet M, Pinoges L, Varaine F, Oberhauser B, O'Brien D, Kebede Y, et al. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS. 2006;20:1275–1279. doi: 10.1097/01.aids.0000232235.26630.ee. Ovid. [DOI] [PubMed] [Google Scholar]
- 11.Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. Ovid. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. Ovid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn SD, Edwards DJ, Wood R. Concurrent drug therapy for tuberculosis and HIV infection in resource-limited settings: present status and future prospects. Future HIV Ther. 2007;1:387–398. [Google Scholar]
- 14.Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis. 2007;196(Suppl 1):S108–S113. doi: 10.1086/518661. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 16.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 17.Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS. 2005;19:1113–1124. doi: 10.1097/01.aids.0000176211.08581.5a. Ovid. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Report of a joint WHO HIV/AIDS and TB Department Meeting. Geneva: WHO; 2008. WHO three I's meeting. http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. [Google Scholar]
- 19.Breen RA, Smith CJ, Cropley I, Johnson MA, Lipman MC. Does immune reconstitution syndrome promote active tuberculosis in patients receiving highly active antiretroviral therapy? AIDS. 2005;19:1201–1206. doi: 10.1097/01.aids.0000176221.33237.67. Ovid. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and ‘unmasking’ of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. Ovid. [DOI] [PubMed] [Google Scholar]
- 22.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 23.World Health Organisation. Executive Summary. Geneva: World Health Organisation; 2002. Scaling up antiretroviral therapy in resource-limited settings: guidelines for a Public Health Approach. [PubMed] [Google Scholar]
- 24.Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker LG, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12:83–88. [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organisation. Recommendations for HIV-prevalent and resource-constrained settings. WHO/HTM/2007.379 & WHO/HIV/2007.1. Geneva: WHO; 2007. Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents. http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf. [Google Scholar]
- 27.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 29.Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79:337–338. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John L, Baalwa J, Kalimugogo P, Nabankema E, Castelnuovo B, Muhindo G, et al. Response to ‘Does immune reconstitution promote active tuberculosis in patients receiving highly active antiretroviral therapy?’. AIDS. 2005;19:2049–2050. doi: 10.1097/01.aids.0000191922.08938.12. Ovid. [DOI] [PubMed] [Google Scholar]
- 31.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organising pneumonia: the role of macrophages. AIDS. 2009;23:143–145. doi: 10.1097/QAD.0b013e32831d2a98. Ovid. [DOI] [PubMed] [Google Scholar]
- 32.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening prior to ART: diagnostic yield and association with immune reconstitution disease. AIDS. 2009 doi: 10.1097/qad.0b013e32832e05c8. in press. [DOI] [PubMed] [Google Scholar]
- 33.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 34.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, et al. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4:e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis. 2006;10:523–529. [PubMed] [Google Scholar]
- 37.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakeera-Kitaka A, Dhabangi A, Namulema E, Maganda A, Boulware DR. Programme and Abstracts of the 45th Annual Meeting of the Infectious Diseases Society of America. San Diego, California, USA: 2007. Oct, Immune reconstitution inflammatory syndrome and postantiretroviral tuberculosis among HIV-infected Ugandan children. [Google Scholar]
- 39.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–342. doi: 10.1097/QAD.0b013e328321823f. Ovid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battegay M, Nuesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 41.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 42.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. Ovid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Churchyard GJ, Scano F, Grant AD, Chaisson RE. Tuberculosis preventive therapy in the era of HIV infection: overview and research priorities. J Infect Dis. 2007;196(Suppl 1):S52–S62. doi: 10.1086/518662. [DOI] [PubMed] [Google Scholar]
- 44.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]