Abstract
Purpose of review
We review recently published literature concerning the optimum time to start antiretroviral therapy (ART) in patients with HIV-associated opportunistic infections (OIs).
Recent findings
In addition to data from observational studies, results from six randomised controlled clinical trials were available by July 2010. The collective findings of these trials were that patients with CD4 cell counts <200 cells/μL who start ART within the first two weeks of treatment for OIs including Pneumocystis jirovecii pneumonia, serious bacterial infections or pulmonary tuberculosis have lower mortality when compared to patients starting ART at later time-points. Moreover, patients with pulmonary tuberculosis and CD4 counts of 200-500 cells/μL who started ART during TB treatment had improved survival compared to those who deferred ART until after the end of treatment. In contrast, in two separate studies, immediate ART conferred no survival benefit in patients with TB meningitis and was associated with substantially higher mortality risk in patients with cryptococcal meningitis.
Summary
Initiation of ART during the first 2 weeks of treatment for serious opportunistic infections has been shown to be associated with improved survival with the exception of patients with tuberculous meningitis and cryptococcal meningitis. Further clinical trials are ongoing.
Keywords: HIV, opportunistic infection, when to start, timing, antiretroviral
Introduction
Remarkable progress has been made over the past 15 years in the treatment of HIV infection such that average additional life expectancy of young adults initiating antiretroviral therapy (ART) in high-income countries is estimated to be in the region of 50 years [1]. However, mortality remains unacceptably high among those patients who present to ART services with advanced immunodeficiency and serious opportunistic infections (OI) and especially in resource-limited settings [2-4]. It is as yet unclear when patients should start ART during the treatment for their OI to minimize this mortality risk. In this paper, we discuss the rationale for either early or deferred initiation of ART. We review insights provided by observational cohort studies and the key evidence that is now emerging from randomised controlled trials.
Rationale for early or late initiation of ART
The rationale for either early or deferred initiation of ART is defined by a range of potential factors (Table 1). Early ART halts progressive immunodeficiency and rapid immune recovery may reduce the risks of further opportunistic infections and mortality. It may also promote more rapid immune clearance of the OI and reduce the risk of relapse [5]. Moreover, for some infections such as cryptosporidiosis, microsporidiosis and progressive multifocal leucoencephalopathy, ART represents the most important component of treatment and should therefore not be delayed at all.
Table 1.
Potential advantages and disadvantages of starting antiretroviral therapy (ART) early in the course of treatment for serious opportunistic infections (OIs)
| Potential advantages of early initiation of ART | Potential disadvantages of early initiation of ART (overlapping treatment) |
|---|---|
| Prevent progressive immunodeficiency | High pill burden |
| More rapid immune recovery | Co-toxicity |
| More rapid resolution of OI | Pharmacokinetic drug interactions |
| Rapid reduction in mortality risk | Immune reconstitution disease |
| Prevention of further OIs and other morbidity | More difficult to identify drug causing toxicity |
For other serious OIs such as tuberculosis (TB) and cryptococcal meningitis, however, there are several adverse consequences associated with early initiation of ART, which may favour delaying treatment (Table 1). The high pill burden with overlapping regimens may reduce treatment tolerability and undermine adherence. In addition, there may be pharmacokinetic drug-drug interactions and co-toxicities. An important example of this is the concurrent use of rifampicin-containing TB treatment and ART regimens containing either non-nucleoside reverse transcriptase inhibitors (NNRTIs) or protease inhibitors (PIs) [6, 7]. Moreover, in patients who develop suspected adverse reactions to drugs, identifying the cause is more complex when multiple drugs are started simultaneously.
Paradoxical immune reconstitution disease (IRD; also known as immune reconstitution inflammatory syndrome or IRIS) and its consequences is one of the most important considerations and is frequently cited as a key reason for treatment deferral. This complication arises as an inflammatory response to residual microbial antigen during rapid immune recovery [8, 9]. The risk of IRD is therefore higher the earlier ART is started [10, 11]. A major concern is that IRD associated with OIs of the central nervous system (CNS) may be associated with higher mortality than extra-cranial disease [12].
Thus, the optimal timing of ART depends on a number of important competing risks. For many years this has been associated with considerable clinical uncertainty and yet data from many observational studies and a number of controlled clinical trials are now available.
Observational studies
Studies from South Africa have highlighted the high mortality risk of adults and children waiting to start ART [13-15]; even short delays of a few weeks may be associated with substantial mortality risk. Mortality risk before and during ART are higher for patients initiating treatment in resource-limited settings [16]. In sub-Saharan Africa, for example, between 8% and 26% of patients die in the first year of ART [4]. A high proportion of these deaths occur in the first 3 months of ART and mortality risk in this period is several-fold higher than that of patients treated in high-income settings even after adjustment for the degree of immunodeficiency and other baseline patient characteristics [3]. Deferral of ART may therefore be associated with greater risks for patients treated in sub-Saharan Africa and the optimum timing for ART initiation during OIs may differ between settings [17].
In patients with HIV-associated TB, there was concern that poor tolerability of concurrent treatment regimens and reduced plasma concentrations of NNRTIs and PIs due to induction of hepatic metabolism by rifampicin would undermine virological outcomes [6, 7]. However, excellent virological outcomes have subsequently been reported, regardless of whether patients were treated using a simplified public health approach in resource-limited settings or with highly individualised treatment in high-income settings [18-20]. Similarly, despite fears of co-toxicity from concurrent use of ART and TB treatment, treatment-limiting toxicity is not common in cohorts receiving NNRTI-based ART [21-23].
Perhaps the greatest concern among those favouring deferred initiation of ART in patients with TB was that of IRD [8]. A small proportion of patients with TB IRD die, and yet this risk has to be understood in the perspective that those who are most likely to develop this complication are the very patients who have the highest pre-existing mortality risk [8]. A recent systematic review and meta-analysis reported a pooled incidence estimate of TB IRD of 15.7% (95% confidence intervals, 9.7-24.5) among patients receiving overlapping TB treatment and ART [24*]. Of those developing this complication, 3.2% (0.7-9.2) died [24*], indicating that approximately 1 in 200 (0.5%) patients receiving concurrent TB treatment and ART die from (or with) this complication.
The optimal timing of ART initiation may depend on the OI and it anatomical location. IRD involving the CNS, for example, is generally more severe and associated with higher mortality risk [12]. A meta-analysis of studies of patients with cryptococcal meningitis starting ART found a pooled incidence of IRD of 19.5% (95%CI, 6.7-44.8) and of these patients 20.8% (5.0-52.7) died [24*]. Thus, approximately 1 in 25 patients starting ART during treatment for cryptococcal meningitis died of immune reconstitution disease. A South African study of 23 patients with paradoxical TB IRD involving the CNS, 87% required hospital admission, 91% received corticosteroids and 13% died during the 6 month follow-up period [25*]. Thus, early ART initiation in patients with CNS OIs may be associated with adverse overall outcomes.
Researchers have attempted to delineate the optimal timing of ART from observational cohorts of patients with TB. A retrospective analysis of 1003 Thai patients demonstrated that patients with HIV-associated TB who delayed ART for ≥6 months after TB diagnosis had a higher mortality rate than those who initiated ART <6 months after TB diagnosis (hazard ratio 2.65, 95%CI 1.15-6.10) [26]. Velasco and colleagues in Spain studied 313 adult patients with HIV-associated TB who received overlapping therapy [27*]. Groups of patients who started ART either within the first 2 months of TB treatment or after completing ≥3 months of TB treatment had similar median baseline CD4 cell counts but the adjusted hazards of death among those starting ART within 2 months of TB treatment was 0.37 (95%CI, 0.17-0.66). A major weakness in this analysis, however, is that it did not account for patients who died before starting ART nor biases inherent in ART allocation.
Observational data have also been derived from an analysis of a large paediatric cohort of children with HIV-associated TB (n=573) in South Africa [28*]. Mortality risk was calculated stratified according to the timing of ART during TB treatment. The authors report a statistically non-significant trend in results; delay in ART for more than 60 days compared to less than 60 days was associated with a hazards of death of 1.32 (95%CI 0.55-3.16) and a hazards of viral suppression of 0.84 (95%CI 0.61-1.15). Such analyses remain fundamentally flawed even after adjustment for baseline immunodeficiency and other characteristics. The timing of ART initiation is a clinically based decision and so sicker patients with more advanced immunosuppression (and higher mortality risk) are much more likely to start ART early in the course of TB treatment. How this decision is made cannot be fully adjusted for in observational data, which therefore remain confounded by indication.
Randomized controlled trials
Observational studies have proven useful in defining the competing risks inherent in the clinical decision making, informing early versions of treatment guidelines and shaping the subsequent design of controlled clinical trials. Well conducted randomised clinical trials are, however, needed to provide definitive data to underpin public health policy. By July 2010, data were available from five randomised controlled trials (RCTs) in which mortality was included in the primary outcome [29**,30**,31**,32**,33**]. These studies enrolled patients with a range of OIs in different geographical settings (Table 2) and below we discuss each of these in turn.
Table 2.
Summary of data available from randomized controlled trials (RCTs) to date
| Study | Study name / acronym and registration | Country | N | Opportunistic infection(s) | Comparison | Median (IQR) CD4 counts (cells/μL) | Outcome |
|---|---|---|---|---|---|---|---|
| Zolopa et al. 2009 [29**] | ACTG A5164 NCT00055120 | USA, Puerto Rico, South Africa (multicentre) | 282 | Acute AIDS-related OIs or severe bacterial infections excluding TB (63% PCP, 12% bacterial infections, 12% cryptococcal disease, 5% toxoplasmosis, 8% other) | Early arm: started ART within 14 days of starting treatment for OI (median=12 days). Deferred ART: started ART after OI treatment completed (median=45 days). |
Early: 31 (12-54) Deferred: 28 (10-56) |
No difference in primary composite end-point. But early ART associated with low risk of progression to AIDS or death (OR=0.51, 95%CI 0.27-0.94) and no increase in adverse events or IRD |
| Abdool Karim et al. 2010 [31**] | SAPIT NCT00398996 | South Africa | 642 | Smear positive pulmonary TB and CD4 cell counts <500 cells/μL | Early: 2 ‘integrated’ arms started ART with first 3 months of TB treatment. Late: the deferred group started ART within 1 month of the end of TB treatment. |
Integrated: 150 (77-254) Sequential: 140 (69-247) |
The hazards of death in the early ‘integrated’ groups was 0.44 (95%CI, 0.25-0.79) overall, 0.54 (0.30-0.98) in those with CD4 counts ≤200 cells/μL and 0.16 (0.03-0.79) in those with a CD4 count >200 cells/μL |
| Blanc et al. 2010. [33**] | CAMELIA NCT00226434 | Cambodia | 661 | Smear positive pulmonary or extrapulmonary TB and CD4 cell counts <200 cells/μL | Early arm: within 2 weeks Late arm: after 2 months |
Early: 25 (11-56) Late: 25 (10-55) |
35% lower risk of mortality in the early arm |
| Torok et al. 2009 [30**] | NCT00433719 | Vietnam | 253 | Tuberculous meningitis | Immediate ART versus ART deferred for 2 months | Early: 39 (18-116) Late: 43.5 (16-84) |
Hazards of death in immediate arm were 1.12 (95%CI 0.81-1.55; p=0.52) |
| Makadzange et al. 2010 [32**] | NCT00830856 | Zimbabwe | 54 | Cryptococcal meningitis | Early arm: within 72 hours of diagnosis. Late arm: after 10 weeks of treatment with fluconazole |
Early: 27 (17-69) Late: 51.5 (25-69.5) |
The hazards of death in the early arm was 2.85 (95%CI, 1.1-7.23) |
A sixth study was a pilot study in Tanzania that assessed the impact of delayed versus early initiation of a triple-nucleoside regimen on adverse events including IRD in patients (n=70) being treated for TB [34*]. Both early and late ART initiation was well tolerated and no IRD events were diagnosed.
Acute opportunistic infections excluding tuberculosis
The AIDS Clinical Trials Group (ACTG) study A5164 was the first randomized controlled trial to be reported and enrolled most of its participants in the USA (Table 2) [29**]. Patients had very advanced immunodeficiency (median CD4 cell count, 29 cells/μL) and a range of OIs, excluding TB. A majority (63%) had Pneumocystis jirovecii pneumonia (excluding severe disease), with the other most frequent OIs being bacterial infections, cryptococcosis and toxoplasmosis (Table 2). Patients were randomized to start ART within the first 14 days of OI treatment (median 12 days) or to start ART after completion of OI treatment (median 45 days; IQR 41-55) and were followed-up for 48 weeks.
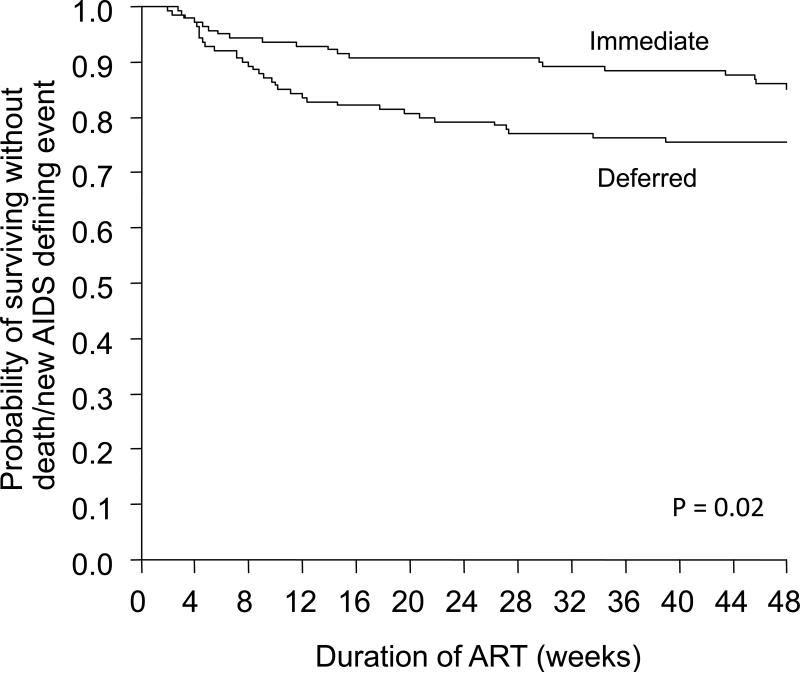
The primary end-point was a composite 3-level ordered categorical variable that included, death, progression to AIDS and virological response. A trend favouring earlier treatment, however, was not statistically significant. Since virological responses at 48 weeks in the two groups were equivalent, this effectively ‘diluted’ the observed difference in clinical outcomes. The simpler (and perhaps more appropriate) secondary end-point of death or progression to AIDS was strongly associated with the timing of ART (Figure 1). The early arm had fewer patients with progression to AIDS or death compared to the late arm (14.2% versus 24.1%; odds ratio = 0.51, 95%CI 0.27-0.94). Early ART was also strongly associated with a shorter time to achieving a CD4 cell count >50 cells/uL (4.0 weeks versus 8.6 weeks, P<0.001) and no increase in adverse events or immune reconstitution disease.
Figure 1.
Graph showing the probability of survival without death or development of an AIDS-defining illness in the randomised controlled trial comparing ART initiation within 14 days of starting treatment for acute opportunistic infections (excluding tuberculosis) versus delayed ART until after completion of the opportunistic infection. Graph adapted from Zolopa et al., 2009 [29**].
These data therefore provided important evidence to support early initiation of ART in patients presenting with acute AIDS-related OIs or severe bacterial infections with the exclusion of TB. There was, however, an insufficient number of patients with cryptococcal meningitis to inform management of this condition although there was a very strong trend towards lower progression to AIDS or death in those receiving early treatment.
Pulmonary tuberculosis in South Africa
In an open-label, randomised controlled trial in Durban, South Africa (the ‘SAPIT’ trial), HIV-infected patients with sputum smear-positive pulmonary TB were assigned to start ART within the first 4 weeks of the intensive phase of TB treatment (early integrated arm), within the first 4 weeks of the continuation phase (late integrated arm) or within 4 weeks of completing TB treatment (sequential arm) [31]. Patients with diagnoses of new or recurrent TB and with CD4 cell counts <500 cells/μL were included. The primary outcome was all-cause mortality (Table 2).
Following an interim analysis by the data safety and monitoring board, the sequential arm of the study was halted due to a high mortality rate. This initial report from the study compared the outcomes of patients in the two integrated arms combined with those of patients in the sequential arm [31]. Overall, the hazards of death in the integrated arms was 0.44 (95%CI, 0.25-0.79). For those with CD4 cell counts ≤200 cells/μL or 200-500 cells/μL, the hazards of death were 0.54 (0.30-0.98) and 0.16 (0.03-0.79), respectively. Thus, delay of ART initiation until after the completion of TB treatment was associated with significantly higher mortality risk for all patients regardless of CD4 cell count stratum.
Study limitations included the enrolment only of patients with smear-positive pulmonary TB. Smear-negative and extra-pulmonary TB are more frequent forms of disease at lower CD4 cell counts and are associated with higher mortality risk. The study was open-label and clinical judgement took precedence over the protocol-defined timing of ART, potentially undermining baseline randomization during follow-up. There were 36 withdrawals from the study and 41 patients were lost to follow-up, representing a total of 12% of patients enrolled.
This study has drawn criticism with regard to the inclusion of patients with CD4 cell counts <200 cells/μL into the sequential arm in which ART was deferred by up 7 months for new TB cases and up to 9 months for retreatment TB cases [35, 36]. It was predictable that such patients would have higher mortality and it is difficult to justify that equipoise existed when the study was designed in 2005. It was known that such patients had high case fatality rates of 16%-35% during 6 months TB treatment [37] and that ART substantially reduced mortality risk [38, 39]. Moreover, a systematic review of TB IRD in 2005 found not a single case that resulted in death [8] and there were no data to indicate drug co-toxicity resulted in appreciable mortality.
Notwithstanding this ethical concern, these data provide important evidence that all TB patients with CD4 cell counts in the range 0-500 cells/μL should receive concurrent (integrated) TB treatment and ART. The key remaining question is the optimal timing of ART during the initial 2-3 months of TB treatment.
Pulmonary tuberculosis in Cambodia
More recent data provided by an open label RCT conducted in Cambodia (the ‘CAMELIA’ trial) have provided more precise data for the optimum timing of ART in TB patients with CD4 cell counts <200 cells/μL (Table 2) [33]. Eligible patients (n=661) were ART-naive adults with newly diagnosed smear-positive pulmonary or extrapulmonary TB. ART initiation after 2 weeks and 8 weeks of TB treatment were compared. Follow-up was for a minimum of one year (mean, two years). The primary outcome was death.
The mortality rate in the early arm was 8.3 deaths/100 person-years (95%CI, 6.4-10.7) compared to 13.8 deaths/100 person-years (11.2-16.9) in the late arm (P=0.002). IRD events were twice as frequent in the early arm (33% versus 15%) and, although there were 5 IRD deaths in the early arm compared to 1 IRD death in the late arm, this did not off-set the benefits of early ART. In adjusted analyses, the hazards of death in the late arm was 1.52 (1.12-2.05; P=0.007) and early treatment was associated with a 35% lower mortality. Kaplan-Meier analyses showed that the survival benefit gradually accrued during long-term follow-up and was not a short-term effect. This was not related to long-term differences in the immunological and virological response to ART and the reasons for this remain unclear.
The benefit observed is entirely consistent with the data from Zolopa and colleagues as discussed above [29**]. However, it should be noted that in both these studies, patients had very advanced immunodeficiency and further data from patients with less advanced disease and from a range of settings are required.
Tuberculous meningitis in Vietnam
HIV-associated TB meningitis (Figure 2) has a devastating prognosis, with a mortality of 67% and median time to death of 20 days reported from a cohort patients without ART availability in Vietnam [40]. The risks and benefits of early versus delayed ART are unknown may differ from those in patients with other forms of TB. A randomised double-blind placebo-controlled trial of patients with HIV-associated TB meningitis (n=253) has now been completed in Vietnam [30**] (Table 2). The proportions with grade I, II and III meningitis were 32%, 38% and 29%, respectively, and the median CD4 cell counts in the early and late arms were 39 and 44 cells/μL. Patients received a 9-months TB treatment that contained rifampicin throughout. Either efavirenz-based ART (immediate arm) or placebo (deferred arm) were started at the same time as TB treatment and all patients subsequently received open label ART from 2 months. Adjunctive corticosteroids tapered over 6-8 weeks and co-trimoxazole were also standard of care for all patients. Mortality at 9 months was the primary outcome.
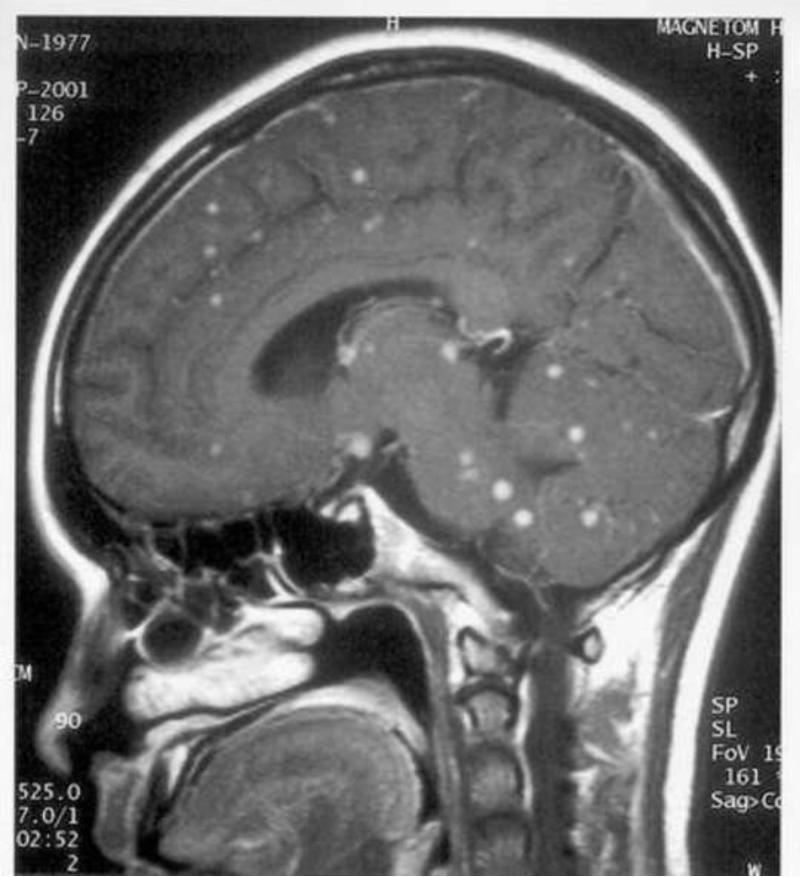
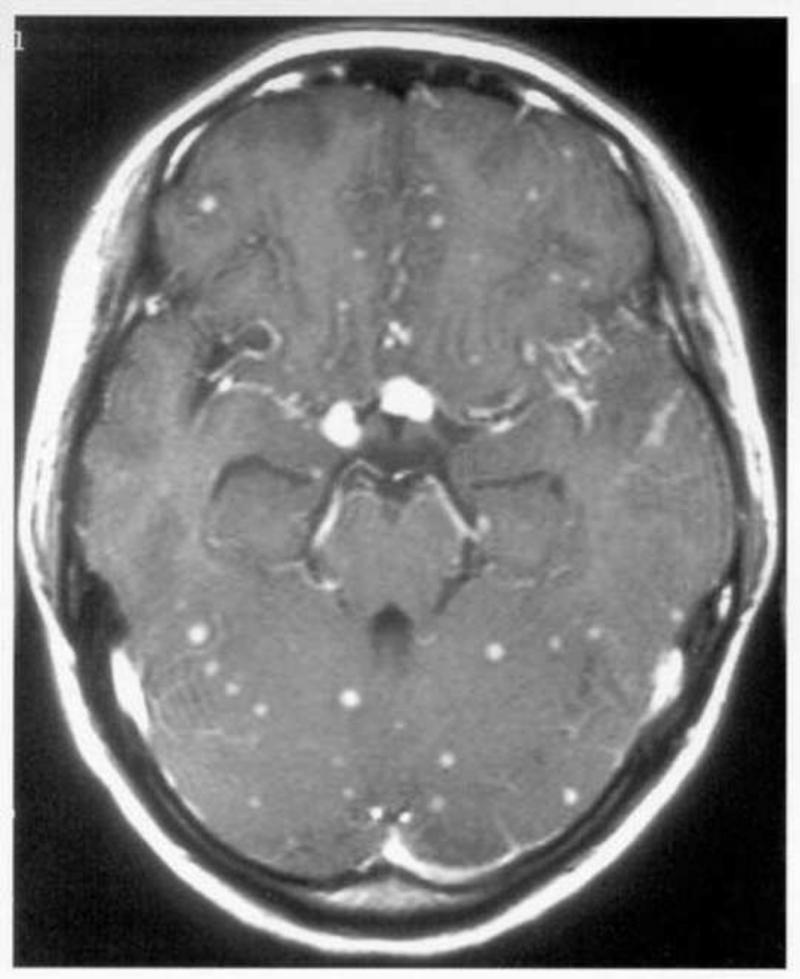
Figure 2.
Saggital (a) and transverse (b) T1-weighted cranial magnatic resonance imaging (MRI) scans showing multiple tuberculomata in a patient with HIV-associated tuberculous meningitis.
Mortality was high at 9 months. Kaplan-Meier survival proportions were just 35.2% and 40.3% in the immediate and deferred arms, respectively. Compared to the deferred arm, immediate ART was not significantly associated with mortality (hazard ratio 1.12; 95%CI 0.81-1.55; P=0.50) or the time to new AIDS events or death (HR, 1.16; 0.87-1.55; p=0.31). The dominant independent predictor of mortality was the TB meningitis grade.
Grade 3 or 4 adverse events were observed in both immediate and deferred arms during the course of the study (90% versus 89%), but these were more common during the first 2 months in the immediate arm (86% versus 75%, respectively; P=0.04). Secondary end-points of immunological and virological responses to ART were, as expected, more rapid with immediate treatment. Overall, these data do not support immediate initiation of ART in patients with TB meningitis. Further data are needed from other settings.
Cryptococcal meningitis in Zimbabwe
Cryptococcal meningitis remains a major cause of HIV-associated morbidity. Mortality rates of 10-25% are reported from high-income settings, but may be considerably higher in resource-limited settings [41]. Little, however, is known about the optimal timing of ART. A randomised open-label clinical trial conducted in Zimbabwe, compared initiation of ART within the first 72 hours of antifungal treatment with initiation after 10 weeks [32**]. All patients presented with their first episode of cryptococcal meningitis and were ART-naive. They were treated with oral fluconazole (800 mg once daily) and nevirapine-based ART and followed up for 3 years. The trial was discontinued prematurely after an interim analysis by the data safety and monitoring board found a significantly higher mortality in the early treatment arm.
The baseline characteristics of the two groups were adequately matched. However, the three year mortality in the early and deferred arms was 88% versus 54% (P=0.006) and median survival in the two arms were 28 days and 637 days, respectively (P=0.031). In adjusted analyses, early ART was associated with an almost 3-fold greater mortality risk (adjusted HR, 2.85; 95%CI, 1.1-7.23). A large majority of deaths occurred in the first 2 weeks after diagnosis and almost all occurred within 4 weeks. Deaths were primarily ascribed from clinical observation to complications of cryptococcal meningitis.
The data show that under the circumstances studied, early ART was very strongly associated with higher mortality risk. However, a number of factors need to be considered when interpreting these data [42]. ‘Early ART’ was given very early indeed (<72 hours) and ‘late ART’ was given very late (>10 weeks). Many clinicians would perhaps not intuitively choose either of these timings for treating their patients. Patients received high-dose oral fluconazole (a fungistatic drug) for their cryptococcosis as this is the standard of care in most African countries. Use of amphotericin, a fungicidal drug, is thought to clear cryptococcal antigen from the CNS more rapidly [41] and may therefore be associated with a lower risk of IRD, which was potentially a key factor driving mortality in the early arm. Patients were also under routine care of local hospital physicians rather than the study team and did not have protocol-driven monitoring and interventions to reduce intracranial pressure, a very important component of the management of this condition. There was no clinical case definition nor management plan for cryptococcal IRD –a likely important cause of raised intracranial pressure and mortality [43]. Thus, the excess mortality risk associated with early ART might have been diminished by amphotericin use and appropriate management of raised intracranial pressure. Future studies will need to address alternative ART timing strategies, different antifungal regimens, management of raised intracranial pressure and use of adjunctive corticosteroids.
Current WHO guidelines
The WHO guidelines (2002) for the use of ART in adolescents and adults have been sequentially revised in 2003 and 2006 and most recently in 2010 [44*]. Data from early observational studies and later RCTs have resulted in a gradual shift in the guidelines towards earlier initiation of ART in those with TB. The most recent guidelines recommend that all patients with HIV-associated TB should start ART regardless of the CD4 cell count and this should be done as soon as possible within the first 8 weeks of TB treatment [44*]. Although the data from the CAMELIA trial emerged after publication of these guidelines, the data are nevertheless entirely supportive of the recommended timing [33]. No specific recommendations were made regarding the management of TB meningitis, however, but the currently available data [30] do not show an adverse impact of early treatment on survival as discussed above. No recommendations either were given in the current WHO guidelines regarding the timing of ART during treatment of other serious acute OIs. Future guidance will be particularly important with regard to the management of cryptococcal meningitis.
Ongoing clinical trials
A number of ongoing RCTs also aim to address the optimum time to start ART in TB patients (Table 3). These studies are being conducted in a wide range of geographical locations and include patients with differing degrees of immunodeficiency. A variety of study primary outcomes include survival, disease progression, TB treatment outcomes and drug toxicity. However, none of these studies includes participants under the age of 13 years and thus a critical need exists for studies in children. A further RCT (NCT01075152) was also funded in 2010 to investigate the optimal timing of ART in patients receiving amphotericin treatment for cryptococcal meningitis at three sites in Uganda and South Africa (David Boulware, personal communication).
Table 3.
Summary of ongoing trials examining timing of antiretroviral (ART) initiation in HIV-associated tuberculosis (TB)
| Trial and sponsor | Setting and sample size | Type of tuberculosis | CD4 count at entry (cells/μL) | ART regimen | Treatment arms | Duration of follow-up | Primary outcome measure(s) |
|---|---|---|---|---|---|---|---|
| SAPIT (follow-up of arms 1 and 2): A study to compare three existing time points for starting ART in HIV/TB patients. Centre for the AIDS Programme of Research in South Africa (NCT00398996) | South Africa N=429 | Smear positive pulmonary TB | <500 | Didanosine, lamivudive efavirenz | Arm 1: ART within first 4 weeks. Arm 2: ART within 4 weeks of completion of intensive phase |
18 months | All cause mortality and progression to AIDS-defining illness |
| TB-HAART: An evaluation of the impact of early initiation of HAART on TB treatment outcomes for HIV/TB patients (ISRCTN77861053), WHO / TDR | South Africa, Uganda, Zambia, Tanzania N=1900 | Smear and culture positive pulmonary TB | 220 – 500 | Zidovudine lamivudine, efavirenz | Arm 1: ART initiation at 2 weeks Arm 2: Placebo for 6 months followed by ART |
24 months | Composite endpoint of TB treatment failure or death at 6 months after initiation of TB treatment |
| ACTG A5221: Immediate versus deferred start of ART in HIV-infected adults being treated for tuberculosis (NCT00108862), NIAID | USA, Brazil, Haiti, South Africa, Kenya, Malawi, India, Thailand. N = 800 | Confirmed or probable TB | <200 | Tenofovir, emtricitabine, efavirenz | Arm 1: ART initiation at 2 weeks Arm 2: ART initiation at 8 to 12 weeks |
48 weeks | Survival without progression to AIDS |
| Anti-HIV Drugs for Ugandan Patients with HIV and Tuberculosis (NCT00078247) NIAID | Uganda N=350 | Smear or culture positive pulmonary TB | >350 | Zidovudine, lamivudine, abacavir | Arm 1: immediate ART for 6 months Arm 2: ART delayed until CD4 count <250 cells/mm3 |
2 years | CD4 count decline and progression to AIDS |
| TIME: Appropriate timing of HAART in co-infected HIV/TB patients (NCT01014481), Bamrasnaradura Infectious Diseases Institute | Thailand N=210 | Clinical or smear or culture positive TB | <350 | Tenofovir, lamivudine, efavirenz | Arm 1: ART initiation after 4 weeks Arm 2: ART initiation after 12 weeks |
144 weeks | Composite endpoint of death rate, hospitalization rate and adverse drug reactions |
| Simultaneous versus sequential ART and TB treatment (NCT00737724), Instituto Nacional de Enfermedades Respiratorias | Mexico N=160 | Active pulmonary TB | <200 | Tenofovir, emtricitabine, efavirenz | Arm 1: immediate ART Arm 2: ART initiation after 2 months |
96 weeks | 1.Signs and symptoms of active tuberculosis 2.Mycobacterial load in body fluids or affected tissues |
Conclusions
Great progress has recently been made in defining the optimum timing of ART in patients with serious OIs. The overall conclusion from the accumulated data is that early ART (within the first 2 weeks) is associated with lower mortality for patients with Pneumocystis jirovecii pneumonia, serious bacterial infections and pulmonary TB compared to treatment at later time-points. However, RCTs have shown that immediate ART conferred no survival benefit in patients with TB meningitis in Vietnam and was associated with substantially higher mortality risk in patients receiving fluconazole for cryptococcal meningitis in Zimbabwe. In both these conditions, further data are required to determine the optimum management strategies.
Acknowledgements
SDL is funded by the Wellcome Trust, London, UK. RW is funded in part by the National Institutes of Health (NIH) through a CIPRA grant 1U19AI53217-01 and RO1 grant (A1058736-01A1).
Abbreviation
- ACTG
AIDS Clinical Trials Group
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral treatment
- CNS
central nervous system
- HIV
human immunodeficiency virus
- IRD
immune reconstitution disease
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- OI
opportunistic infection
- PI
protease inhibitor
- RCT
randomised controlled trial
- TB
tuberculosis
- WHO
World Health Organization
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 3.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahid P, Gonzalez LC, Rudoy I, et al. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–S75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD, Edwards DJ, Wood R. Concurrent drug therapy for tuberculosis and HIV infection in resource-limited settings: present status and future prospects. Future HIV Ther. 2007;1:387–98. [Google Scholar]
- 8.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 9.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–27. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 11.Shelburne SA, III, Darcourt J, White AC, Jr., et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–52. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 12.Torok ME, Kambugu A, Wright E. Immune reconstitution disease of the central nervous system. Curr Opin HIV AIDS. 2008;3:438–45. doi: 10.1097/COH.0b013e328302ebd1. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 14.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn SD, Harries AD, Wood R. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5:18–26. doi: 10.1097/COH.0b013e328333850f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawn SD, Wood R. Optimum time to initiate antiretroviral therapy in patients with HIV-associated tuberculosis: there may be more than one right answer. J Acquir Immune Defic Syndr. 2007;46:121–3. doi: 10.1097/QAI.0b013e3181398d28. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 19.Breen RA, Miller RF, Gorsuch T, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–40. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]
- 20.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–22. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 22.Boulle A, Van CG, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 23.Breen RA, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax. 2006;61:791–4. doi: 10.1136/thx.2006.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [A systematic review and meta-analysis of the risks and mortality associated with immune reconstitution disease in patients initiating antiretroviral therapy during treatment of opportunistic infections.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48:e96–107. doi: 10.1086/598988. [An observational study from South Africa describing the outcomes of patients with TB immune reconstitution disease involving the central nervous system.] [DOI] [PubMed] [Google Scholar]
- 26.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 27*.Velasco M, Castilla V, Sanz J, et al. Effect of Simultaneous Use of Highly Active Antiretroviral Therapy on Survival of HIV Patients With Tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [An observational study from Spain comparing mortality among TB patients starting ART within the first 2 months TB treatment or after 3 months. Mortality was higher among those with late ART, but no account was made for deaths occurring pre-ART.] [DOI] [PubMed] [Google Scholar]
- 28*.Yotebieng M, Van RA, Moultrie H, et al. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010;24:1341–9. doi: 10.1097/QAD.0b013e328339e576. [An analysis of a large observational cohort of children in South Africa with a diagnosis of HIV-associated TB. These are the only paediatric data available but are likely to be substantially confounded by the clinical decision making process regarding the timing of ART initiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [Patients with a range of opportunistic infections (predominantly Pneumocystis jirovecii pneumonia and serious bacterial infections) had half the mortality risk if they started ART with the first 2 weeks of treatment compared to deferring treatment to the end of treatment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Torok ME, Yen NTB, Chau TTH, Mai NTH, Phu NH, et al. Randomised controlled trial of immediate versus deferred antiretroviral therapy in HIV-associated tuberculous meningitis.. 49th Interscience Conference on Antiomicrobial Agents and Chemotherapy (ICAAC); San Francisco, USA. September 2009; Abstract # H-1224. [These data investigate the timing of ART in patients with TB meningitis and advanced immunodeficiency and very high mortality risk. Mortality did not differ between early and late treatment arms.] [Google Scholar]
- 31**.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [These data from South Africa indicate that patients with pulmonary TB should not defer ART initiation until the end of TB treatment, regardless of the CD4 cell count.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50:1532–8. doi: 10.1086/652652. [Immediate initiation of ART in patients with cryptococcal meningitis in Zimbabwe had an almost 3-fold greater mortality risk compared to those starting ART after 10 weeks treatment with high-dose fluconazole.] [DOI] [PubMed] [Google Scholar]
- 33**.Blanc F-X, Sok T, Laureillard D, et al. Significant enhancement in survival with early (2 weeks) vs late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis.. Abstracts of the XVIII International AIDS Conference; Vienna, Austria. July 2010; [International AIDS Society. Abstract THLBB1. Patients in Cambodia with smear-positive TB who started ART after 2 weeks TB treatment had a 35% lower mortality compared to those starting after 8 weeks.] [Google Scholar]
- 34*.Shao HJ, Crump JA, Ramadhani HO, et al. Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Res Hum Retroviruses. 2009;25:1277–85. doi: 10.1089/aid.2009.0100. [This pilot study showed that TB patients receiving triple nucleoside ART tolerated the treatment well regardless of the timing of ART initiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson D, Meintjes G. Timing of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:2137–9. doi: 10.1056/NEJMc1003767. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. AIDS research. Bioethicists assail a celebrated TB/HIV treatment trial. Science. 2010;328:799–801. doi: 10.1126/science.328.5980.799. [DOI] [PubMed] [Google Scholar]
- 37.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–52. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 38.Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 39.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–6. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 40.Torok ME, Chau TT, Mai PP, et al. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS ONE. 2008;3:e1772. doi: 10.1371/journal.pone.0001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–29. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 42.Meintjes G, Wilkinson RJ. Optimum timing of antiretroviral therapy for HIV-infected patients with concurrent serious opportunistic infections. Clin Infect Dis. 2010;50:1539–41. doi: 10.1086/652653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 44*.World Health Organization . Recommendations for a public health approach (2010 revision) World Health Organization; Geneva: [19.07.10]. Antiretroviral therapy for HIV infection in adults and adolescents. at the following URL: http://www.who.int/hiv/pub/arv/adult/en/index.html. [The 2010 update version of the WHO guidelines for antiretroviral therapy (ART) in resource-limited settings recommends ART should be started as soon as possible with the first 8 weeks of TB treatment by all patients with HIV-associated TB.] [PubMed] [Google Scholar]