Abstract
Background
Antiretroviral therapy (ART) has been proposed as an intervention for reducing tuberculosis (TB) burdens in areas with high HIV prevalence. However, little data is available on the impact of ART on population-level TB.
Methods
Trends in adult TB case fatality and notifications were assessed prior to and during increasing ART coverage in a well-defined peri-urban community, from 1997 to 2008. Mean changes in TB rates were measured using linear autoregression models. ART coverage increased from 1% in 2003, to 5%, 13% and 21% of HIV-infected population in 2004, 2005 and 2008 respectively.
Results
From 1997 to end of 2004 TB notification rates increased by an average of 187 cases/100,000/yr (p<0.001), reaching a peak of 2,536/100,000 in 2005. From 2005 to 2008, TB notification rates declined by approximately 183 cases/100,000/yr (p<0.001). TB rates were initially stable in HIV-uninfected individuals, but declined moderately from 2005. TB rates declined in HIV-infected adults from 6,513/100,000 in 2005 to 4,741/100,000 in 2008. The predominant decline in TB notifications occurred among HIV-infected patients receiving ART (1,156 cases/100,000/yr) and was less marked in those not receiving ART (416cases/100,000/yr). Similarly, TB case fatality was constant for HIV-uninfected individuals but declined in HIV-infected individuals from 23% in 2002 to 8% in 2008 (p=0.01).
Conclusions
In this community heavily affected by both HIV and TB epidemics, rapid and high ART coverage was associated with significant reductions in TB notifications and TB-associated case fatality.
Keywords: tuberculosis, notification rates, HIV, antiretroviral, community
Introduction
The current World Health Organisation (WHO)-recommended TB control strategy is failing to contain the tuberculosis (TB) epidemic in high HIV prevalence countries, and TB rates continue to escalate in countries such as South Africa1. The STOP TB Partnership has proposed adjunctive strategies to address this problem, including the “Three I’s” strategy for reducing the burden of TB in HIV-infected patients: intensified case finding, isoniazid preventative therapy, and infection control2. However this strategy has not been widely implemented to date. Due to the reduction in TB risk in HIV-infected patients on highly active antiretroviral therapy (HAART)3;4, HAART has also been proposed as an adjunctive strategy for controlling the TB epidemic in low and middle-income countries with generalised HIV epidemics5. This strategy has been more extensively implemented, and through programs such as the “3 by 5” initiative6, the President’s Emergency Plan for AIDS Relief (PEPFAR)7 and the Global Fund8 there has been substantial progress in patients’ access to HAART9.
However, the impact of HAART on TB rates at a population level remains uncertain. While incidence of active TB disease in HIV-infected patients is reduced by 70–90% by HAART3;10, patients still have a 5–10 times higher risk of TB disease 3 years into HAART treatment compared to HIV-uninfected individuals11;12. The combination of prolonged survival and residual increased risk of TB incidence in HIV-infected patients on HAART will result in an increased number of highly susceptible individuals in the population. Therefore even substantial population coverage with HAART may have a limited impact on TB incidence at a population-level. However, empirical data addressing this issue are sparse and most evidence comes from mathematical modelling13.
Despite the well-described TB benefits of HAART use for HIV-infected individuals, there are no population level studies describing the impact of increased access to HAART on community TB rates in areas with generalised HIV epidemics. Therefore we assessed the impact of increasing antiretroviral provision on TB notification rates in a community with high HIV prevalence.
Methods
Study community
The study took place in a well-defined South African peri-urban township with a population of approximately 15,000 people, and an HIV prevalence of 23% in 200514. The community is served by a single primary care clinic that follows the National TB control program guidelines15, based on WHO-recommended DOTS program. The clinic manages all TB patients resident in the community and the protocol for diagnosis and management of TB patients did not change significantly from 1997 to 2008. The main change to the national TB protocol has been the addition, since 2004, of active TB screening for patients initiating HAART. This screening was based on sputum investigation, and did not include testing for latent TB infection. IPT has not been implemented in this community. Despite an apparently well-functioning TB program16, we have previously reported escalating TB notification rates in this community prior to rapid, high coverage HAART availability17. HAART provision began in 2003 with patients in the community accessing anti-retroviral treatment at the local clinic or local hospital, but the HAART program was only scaled-up in 2005.
TB and HAART data
TB notification data were obtained from the local TB clinic from 1997 to 2008. HIV status, antiretroviral treatment status and CD4 count data were obtained from the TB register, clinical folders as well as clinic and hospital HAART databases. Adults were defined as patients ≥15 years of age.
Population model
Annual and age-specific TB rate calculations were based on population denominators obtained from the 1996 South African national census, and community household censuses performed in 2002, 2004, 2006 and 2008. Linear population growth was assumed between each census. Community HIV prevalence from 1996 to 2004 was estimated using the Actuarial Society of South Africa (ASSA) 2003 AIDS and Demographic model for the African population18;19. In 2005 and 2008, the Desmond Tutu HIV Centre performed two community-based, random cross-sectional HIV prevalence surveys among adults ≥15 years of age in the study community14;20. When the ASSA2003 model predictions were compared to the 95% confidence intervals from the community surveys, the 2005 survey data14 matched the model’s predictions for the HIV prevalence in the community. However, the 2008 survey showed a shift towards higher HIV prevalence among 30–45 year olds, and particularly among women. This is in keeping with the impact of antiretroviral programs on community HIV epidemics, and the ASSA model estimates for the study community HIV prevalence from 2005 to 2008 were adjusted based on the survey results. The number of HIV-infected individuals derived from this model was used as the denominator for calculation of TB rates among HIV-infected population. Numbers of patients on HAART in each year, derived from the anti-retroviral program registers, were used as denominators for TB notification rates on HAART. HAART coverage was calculated as the proportion of the adult HIV-infected population in the community receiving HAART in each year.
HIV and TB rates calculations
All TB rates are reported as cases/100,000. HIV testing was routinely offered to TB patients from 2002, and therefore HIV-associated rates were only available from that year. To account for missing HIV test results, extreme case scenarios for HIV-associated and non HIV-associated TB rates were calculated assuming 100% of patients with unknown HIV status were HIV-infected and HIV-uninfected, respectively. Analyses in HIV-infected and HIV-uninfected strata were performed on patients with known HIV status.
Direct standardisation method, was used to calculate the age standardised annual TB rates for the population using the 1997 population age distribution as the reference population. Similarly using HIV-infected population as the reference population, direct standardisation was used to calculate age standardised rate ratio (RR) of TB in HIV-infected adults off HAART versus adult patients on HAART. Mean changes in TB rates and significance of trends prior to (pre-2005) and following (post-2004) the implementation of a high coverage HAART program were assessed using linear regression models. An autoregressive model with a one year lag was used to account for the autocorrelation of the data, and the impact of HAART was examined through an interaction term. We a priori chose 2005 as the first year of HAART availability, as this was the first year that an appreciable number of patients were receiving HAART. The median baseline CD4 counts of patients initiating HAART in each year were calculated from the most recent CD4 count in the 3 month period prior to HAART initiation.
TB completion rates were calculated as proportion of TB patients who completed TB treatment (TB treatment completion or cure), excluding those who were transferred out of the community during the treatment course. Retreatment TB was defined as a new TB diagnosis in a patient who had previously completed TB treatment. All cause case fatality rates in TB patients were calculated as proportion of TB patients who died on TB treatment each year, excluding those who were transferred out of the community during the treatment course. Trend analysis for TB treatment completion rates and case fatality rates were assessed using chi2 test for trend21.
Data were analysed using Stata Version 10.0 (Stata Corporation, Texas, USA). All statistical tests were 2-sided at alpha=0.05. This study analysis was performed on register data, and ethics approval for the study was obtained from the University of Cape Town Human Research Ethics Committee.
Results
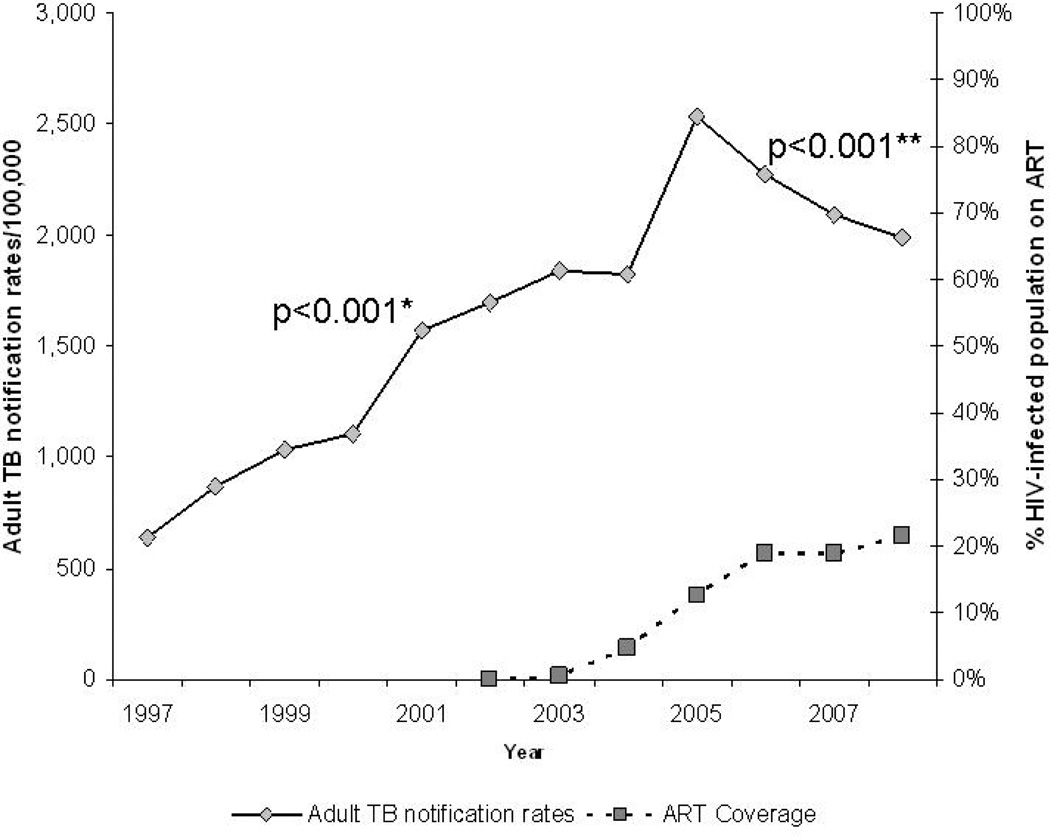
Over the 12 year study period, 1,974 TB cases were notified in the study community. Of these, 1,741 were adult cases, the median age of which was 32 years (interquartile range {IQR}: 26 – 40 years), and 45% were female. Overall, HIV testing was performed in 77% of adult cases, of which 69% were HIV-infected. From 2002, 87% of adult TB cases were tested for HIV, of which 70% were HIV-infected. In 2003, 1% of the HIV-infected population was receiving HAART. By end 2005 this proportion had increased to 13%, and by the end of the study period 21% of the estimated HIV-infected population were receiving HAART (Figure 1).
Figure 1.
Adult TB notifications rates and anti-retroviral coverage in study community, from 1997 to 2008
* p-value for autoregression model from 1997 to 2004
** p-value for autoregression model from 2005 to 2008
Adult TB notification
Table 1 shows total adult TB notification, TB case fatality and TB treatment completion data over the study period. From 1997 to end 2004 adult TB notification rates (per 100,000) increased by an average of 187 cases a year (95% confidence interval [CI]: 143 to 232; p<0.001). From 2005, the rate of adult cases decreased by an average of 183 cases per year (95% CI: −183 to −183; p<0.001) (Figure 1). The annual age standardised rates confirmed these trends, with an increase in annual rate from 1998 to 2005, followed by a decreasing trend to 2008 (Table 1). TB treatment completion rates averaged 80% over the study period, with no significant change over the study period (p=0.88).
Table 1.
Total adult TB notification and age standardised TB rates, and TB case fatality and treatment completion rates in the study community, from 1997 to 2008
| Year | Number of Adult TB cases | Adult Population |
Total TB Rates/100,000 |
Age- standardised TB rate/100,000 |
Case fatality (%) |
Treatment completion (%) |
||
|---|---|---|---|---|---|---|---|---|
| Total TB | TB case fatality |
TB Treatment completion |
||||||
| 1997 | 30 | 2 | 17 | 4,695 | 639 | Reference population | 7% | 61% |
| 1998 | 46 | 1 | 32 | 5,305 | 867 | 882 | 3% | 80% |
| 1999 | 61 | 1 | 53 | 5,916 | 1,031 | 1,060 | 2% | 98% |
| 2000 | 72 | 7 | 47 | 6,527 | 1,103 | 1,117 | 11% | 77% |
| 2001 | 112 | 9 | 75 | 7,138 | 1,569 | 1,582 | 10% | 80% |
| 2002 | 131 | 9 | 94 | 7,722 | 1,696 | 1,762 | 8% | 80% |
| 2003 | 160 | 17 | 122 | 8,714 | 1,836 | 1,876 | 11% | 79% |
| 2004 | 177 | 17 | 125 | 9,706 | 1,824 | 1,924 | 11% | 81% |
| 2005 | 252 | 20 | 176 | 9,935 | 2,536 | 2,784 | 9% | 79% |
| 2006 | 231 | 15 | 170 | 10,165 | 2,273 | 2,386 | 7% | 78% |
| 2007 | 231 | 13 | 181 | 11,062 | 2,088 | 2,171 | 6% | 85% |
| 2008 | 238 | 10 | 163 | 11,958 | 1,990 | 2,183 | 5% | 81% |
HIV-infected and HIV-uninfected TB notifications
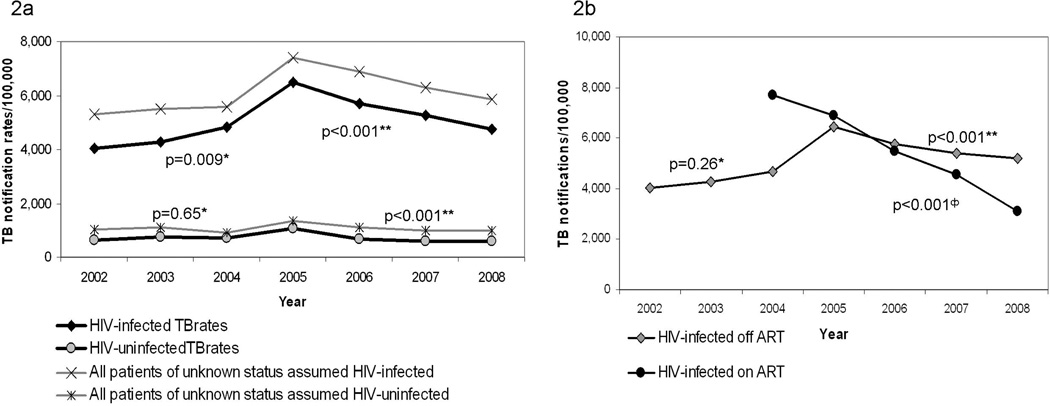
Table 2 shows TB notification data for HIV-uninfected patients, as well as HIV-infected patients not on HAART and those patients on HAART at time of TB diagnosis from 2002 to 2008. Adult TB rates in HIV-infected and HIV-uninfected TB patients are shown in Figure 2a, including extreme case scenarios for HIV-associated and non HIV-associated TB rates assuming 100% of patients with unknown HIV status were HIV-infected and HIV-uninfected respectively. TB rates (per 100,000) in known HIV-uninfected patients did not change substantially from 1997 to 2004 (with an average annual increase of 49 cases; p=0.65), but showed a modest annual decline of 143 cases per year from 2005 (95% CI: −195 to −95; p<0.001). However from 2002 to the end of 2004, overall TB rates in known HIV-infected adults increased by an average of 432 cases per year (95% CI: 109 to 755; p=0.009), after which rates decreased significantly by 578 cases per year (95% CI: −697 to −459; p<0.001). TB treatment completion rates did not change significantly for HIV-infected or HIV-uninfected patients from 2002 to 2008 (p=0.21 and p=0.43 respectively).
Table 2.
TB notification rates among adult HIV-uninfected and HIV-infected patients in the study community, from 1997 to 2008
| Year | Number of Adult TB cases | Adult population HIV prevalence |
Adult HIV- infected Population |
Adult HIV- infected population on HAART |
Adult TB Rates/100,000 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HIV- uninfected |
HIV- infected off HAART |
HIV- infected on HAART |
HIV - uninfected |
HIV- infected off HAART |
HIV- infected on HAART |
||||
| 2002 | 39 | 70 | 0 | 22.5 | 1,737 | 0 | 652 | 4,030 | 0 |
| 2003 | 50 | 85 | 0 | 22.9 | 1,993 | 11 | 744 | 4,289 | 0 |
| 2004 | 52 | 100 | 8 | 23.0 | 2,233 | 113 | 696 | 4,697 | 7,692 |
| 2005 | 81 | 130 | 20 | 23.2 | 2,303 | 308 | 1,061 | 6,458 | 6,897 |
| 2006 | 52 | 121 | 27 | 25.5 | 2,591 | 516 | 687 | 5,762 | 5,499 |
| 2007 | 50 | 126 | 25 | 26.0 | 2,877 | 573 | 611 | 5,408 | 4,570 |
| 2008 | 52 | 129 | 21 | 26.5 | 3,164 | 713 | 591 | 5,193 | 3,088 |
- Patients of unknown HIV status excluded
Figure 2.
TB rates in HIV-uninfected and adult HIV-infected patients (2a) and HIV-infected patients receiving HAART and not receiving HAART (2b) in the study community, from 2002 to 2008
* p-value for autoregression model from 2002 to 2004
** p-value for autoregression model from 2005 to 2008
Φ p-value for autoregression model from 2004 to 2008
TB notification and HAART
Figure 2b shows TB rates (per 100,000) in HIV-infected patients stratified by HAART. TB rates in HIV-infected patients not on HAART increased by an average of 362 cases per year from 2002 to end 2004 (95% CI: -264 to 988; p=0.26). From 2005, there was a significant average annual decrease of 416 TB cases in HIV-infected patients not on HAART (95% CI: −526 to −305; p<0.001). TB rates in HIV-infected patients on HAART decreased significantly by an average of 1,156 cases per year (95% CI: −1191 to −1120; p<0.001). After standardizing for age differences across annual populations HIV-infected patients not on HAART had a lower rate of TB compared HIV-infected patients on HAART early in the HAART program (RR=0.41 in 2004). However, as the period of the HAART program increased, the rate ratio of TB in patients off HAART increased to nearly twice that of the patients receiving HAART (RR=1.98 in 2008).
Baseline CD4 count
The number of HIV-infected patients initiating HAART in each year is shown in Table 2.The median baseline CD4 counts in patients commencing HAART increased from 15 cells/µl in 2003, to 86cells/µl in 2004, 129 cells/µl in 2005, peaking at 153 cells/µl in 2006, and then stabilising at 122 cells/µl in 2007 and 141cells/µl in 2008. This is an average increase of 21cells/µl per year (95% CI: −0.71 to 44cells/µl; p=0.06).
Retreatment TB
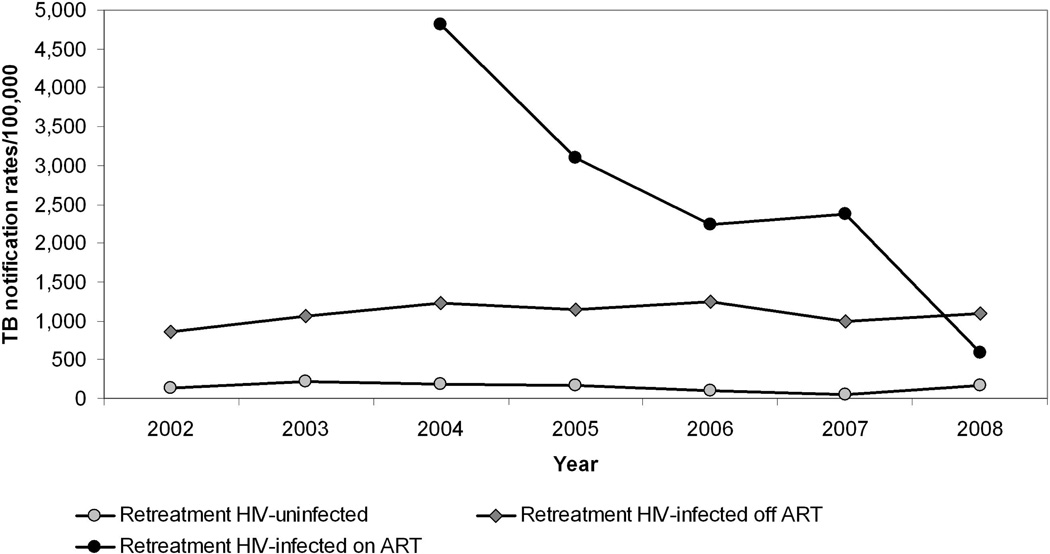
Overall retreatment TB rates (per 100,000) increased significantly from 1997 to 2004, at an annual average increase of 52 cases (95% CI: 35 to 71; p<0.001), after which retreatment rates stabilised (p=0.59). Retreatment TB rates in HIV-uninfected patients remained stable over the study period (p=0.53 from 2002 to end 2004 and p=0.56 from 2005 to end 2008). In HIV-infected patients not receiving HAART, retreatment TB rates increased by an average of 157 cases per year (95% CI: 95 to 219; p<0.001) from 2002 to 2004, and then decreased by an annual average of 67 cases (95% CI: −129 to −5; p=0.03). Annual retreatment TB rates in HIV-infected patients on HAART from 2004 to 2008 decreased significantly by an average of 824 cases/100,000 per year (95% CI: −1492 to −156; p=0.02) (Figure 3).
Figure 3.
Adult retreatment TB rates overall and by HIV and HAART status, in the study community from 2002 to 2008
TB case fatality
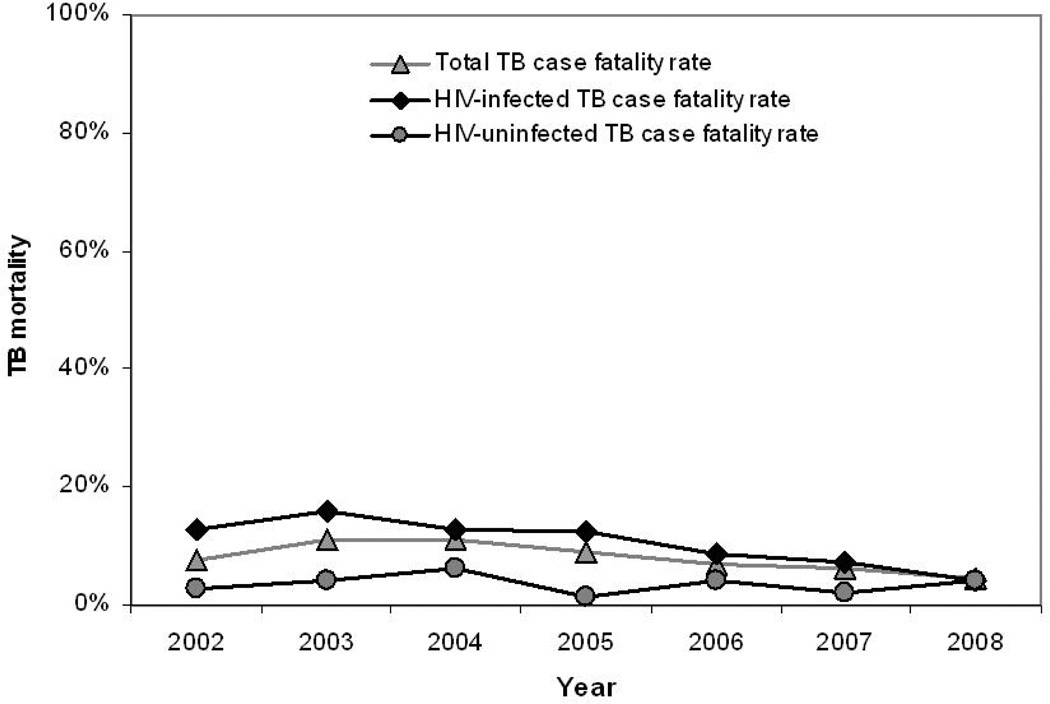
Overall case fatality during TB treatment decreased over the study period (Figure 4). While TB case fatality rates remained relatively constant in HIV-uninfected individuals (p=0.81), TB case fatality dropped significantly in HIV-infected patients from 13% in 2002 to 4% in 2008 (p=0.001).
Figure 4.
Adult TB case fatality rate by HIV-status, in the study community from 2002 to 2008
Discussion
This community-based study from sub-Saharan Africa demonstrated an association between the implementation of an HAART program and TB notification rates. In this community, TB notification and case fatality rates decreased with the rapid and high coverage implementation of an HAART program.
TB is a major cause of mortality in HIV-infected patients in sub-Saharan Africa1;22. A key finding in this study is the significant reduction in TB case fatality rates in HIV-infected patients. The use of HAART has been associated with a reduction in TB-associated mortality among individuals and in treatment cohorts23;24. In our study the implementation of a community HAART program was also associated with a decrease in TB case fatality in HIV-infected patients. In 2002, the case fatality rate in HIV-infected patients was four times greater than that of HIV-uninfected patients, but had decreased to the same rate as HIV-uninfected patients by 2008. This suggests that HAART programs may greatly assist in achieving the Stop TB Partnership goal to halve TB mortality rates by 2015.
The decrease in TB notification rates in the study community occurred against a background of increasing national TB notification rates1. TB rates in HIV-uninfected patients were stable from 1997 to 2004, with HIV-associated TB driving the escalating epidemic in the study population. While HIV-uninfected rates showed a decline from 2005, the reduction in community TB notification rates in this study was predominantly due to a decrease in TB rates in HIV-infected patients, and more specifically in patients receiving HAART.
Although the HIV prevalence in this community was relatively stable from 2002 to 2008, the TB notification rates in HIV-infected patients continued to escalate until 2005. Following the rapid scale up of HAART availability from 2005, the TB rates in HIV-infected patients not on HAART stabilised and then decline moderately. However, there was a dramatic, almost 3 fold decline in TB rates in those HIV-infected patients on HAART. Of note is the high initial retreatment TB rate in patients receiving HAART compared to those patients not on HAART, followed by a substantial reduction in retreatment TB in HIV-infected patients on HAART.
The levelling-off and subsequent decline of TB notification rates in HIV-infected patients not on HAART may be due to the removal of those with the highest TB risk (lowest CD4 counts) from this group into the group on HAART4. This stabilising of notification rates suggests that the rate at which people are removed from the susceptible pool approximates the rate at which HIV-infected patients not on HAART are progressing into a high-risk state of immune compromise. This finding suggested that the rate at which an HAART program is implemented in a community might be an important variable determining the impact of this intervention on overall HIV-associated TB rates.
Although TB does occur at all CD4 strata, the highest risk of TB in HIV-infected patients occurs at low CD4 counts (<200cells/µl)25;26. The South African National Antiretroviral program recommended initiating patients on HAART at CD4 count <200cells/µl, or WHO clinical stage IV27. Patients initiating HAART have a high risk of TB in the first months of treatment compared to later in treatment due to the risks associated with low baseline CD4 counts and with possible unmasking of sub-clinical TB28–30. The overall increase in median baseline CD4 count in our study reflects that patients with more advanced disease were initiated onto treatment in the early stages of the HAART program. With increasing duration of the HAART program, the pool of severely immune-compromised patients off treatment decreased, and patients were started on HAART at higher baseline CD4 counts. However, despite the overall increase in median baseline CD4 count, by 2008 the median CD4 count at initiation of HAART was still well below 200cells/µl and TB risk prior to HAART initiation remained high, as evidenced by the nearly 2 fold higher standardised rate ratio in HIV-infected patients off HAART compared to those patients on HAART in 2008. HIV-infected patients off HAART accounted for 64% of TB disease in 2008, and thus contribute a large portion of the TB burden. By 2008 the HAART coverage in this community was high, at approximately 90% of the estimated community need as defined by the national HAART treatment guidelines27;31. If similar coverage rates and impacts were achieved nationally, a >20% reduction in TB rates might be attained. But this impact could be more substantial if treatment was initiated earlier in the HIV-disease process, thus also reducing TB burden prior to HAART initiation and during the early months on HAART.
Prevalence of TB is a function of TB incidence and period of infectivity in the community. Period of infectivity can be decreased by earlier diagnosis and treatment of cases. The key change to case-finding activities has been the introduction of active-case finding for TB in patients initiating HAART. This practise may have resulted in a decrease in TB prevalence, thus indirectly contributing to the decrease in notifications over time. In addition, in 2005 a community-based cross-sectional survey was performed in 10% of the study community, investigating participants for active TB disease. A sensitivity analysis, excluding those participants diagnosed in that survey from the notification data, showed no change in the study results, including the peak noted in 2005 (data not shown). Therefore this survey does not appear to have had a direct effect on the notification rates in this community. The increased TB screening of HAART-eligible patients associated with the scale-up of the HAART program in 2005 may explain the 2005 peak in notification rates. This increased screening together with the survey may have increased community awareness of TB, potentially contributing to the moderate increase in TB notifications also noted in HIV-uninfected patients.
While there was a strong temporal association between the decline in TB notification rates and the implementation of an HAART program and there is substantive biological plausibility to support this association, it is worth considering alternative explanations for the decrease noted in TB notification rates. Improved TB treatment completion rates may, for example, result in decreased TB transmission which could reduce TB incidence. However TB completion rates remained stable over the study period, and this was consistent for both HIV-infected and HIV-uninfected patients. Period of infectivity, and thus prevalence could be decreased by increased TB-associated mortality; however, TB case fatality has decreased. There were no changes in the infection control policies within the clinic over this period, and therefore decreased nosocomial TB transmission is unlikely to explain the study findings. Although changing social conditions may also impact TB transmission in communities, this community has remained one of extremely poor socio-economic status since its establishment in 1994.
HIV testing uptake was not complete among TB patients in this community. However in order to explore the potential biasing influence of those patients with unknown status we performed an extreme case scenario analysis. The result of this analysis showed that our findings were robust and did not alter the findings of the study. The population denominators were derived from community census data with the assumption of linear growth between censuses, and HIV–infected denominators were obtained from a mathematical model fitted to local HIV data. Sensitivity analyses were run for models assuming different trends of population growth and different assumptions for HIV prevalence. These analyses also did not show substantive changes in study inferences. This community is typical of many recently urbanised populations in South Africa, but further investigations are needed to confirm the generalisability of our findings in other high prevalence settings.
This study was performed in a well-demarcated community, with population data derived from frequent community censuses. All residents receive their TB treatment at a single clinic, and therefore TB notification data is likely to be a complete representation of TB notifications in the community. Similarly residents obtained HAART from the clinic or the local referral hospital and access to both these databases ensured an accurate description of the HAART program in this community. These analyses are dependent on the fidelity of the relevant data records, and therefore the databases underwent a 10% quality control assessment.
In conclusion, against a background of increasing TB notifications nationally, we have shown that a rapidly implemented, high coverage HAART program can reduce the TB notification rates and TB case fatality within a community heavily affected by both HIV and TB epidemics. This reduction in TB was due predominantly to the decrease in TB rates in HIV-infected patients receiving HAART, and may be the result of both active TB screening and improved immune function in these patients.
Acknowledgements
We wish to acknowledge Drs Catherine Orrell and Jennifer Zeinecker who oversaw the HAART program in the study community and who, together with Drs Katharina Kranzer and Nosindiso Kalawe provided the HAART databases for this study.
Financial Support
National Institutes of Health (Comprehensive Integrated Programme of Research on AIDS) grant 1U19AI053217 to K.M., L.G.B. and R.W), and NIH CIPRA grant 1U19AI05321 and NIH RO1 grant AI058736-02 to R.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abstract presented at: 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town 2009. Abstract CDB041
Conflict of Interest Statement
All authors have no conflict of interest.
Reference List
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing. 2009 http://www.who.int/tb/publications/global_report/2009/en/index.html.
- 2.World Health Organization. WHO Three I's Meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for people living with HIV. 2008 http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf.
- 3.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation. The Global Plan to Stop TB, 2006–2015 Stop TB Partnership. Geneva, Switzerland: WHO; 2006. http://www.stoptb.org/globalplan/plan_p2main.asp?p=2http://www.stoptb.org/globalplan/plan_p2main.asp?p=2. [Google Scholar]
- 6.World Health Organisation. Treating 3 million people by 2005: Making it Happen. The WHO Strategy. 2003 http://www.who.int/3by5/publications/documents/en/3by5StrategyMakingItHappen.pdf.
- 7.The United States President's Emergency Plan For AIDS Relief: Fact Sheet. 2009 http://www.pepfar.gov/documents/organization/115411.pdf.
- 8.The Global Fund to Fight AIDS, Tuberculosis and Malaria: Annual Report. 2008 http://www.theglobalfund.org/documents/publications/annualreports/2008/AnnualReport2008.pdf.
- 9.World Health Organisation, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. 2008 http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf.
- 10.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19(18):2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 12.Girardi E, Sabin CA, d'Arminio MA, Hogg B, Phillips AN, Gill MJ, Dabis F, Reiss P, Kirk O, Bernasconi E, Grabar S, Justice A, Staszewski S, Fatkenheuer G, Sterne JA. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41(12):1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 13.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301(5639):1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 14.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South African Department of Health. Practical Guidelines. South Africa: Pretoria; 2004. The South African Tuberculosis Control Programme. http://www.capegateway.gov.za/Text/2003/tb_guidelines2000.pdf. [Google Scholar]
- 16.Health Systems Trust. Cape Town TB Control. Progress report 1997–2003. 2004 www.hst.org.za./publications/618.
- 17.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42(7):1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 18.Johnson LF, Dorrington RE. Modelling the demographic impact of HIV/AIDS in South Africa and the likely impact of interventions. Demographic Research. 2006;14:541–574. [Google Scholar]
- 19.Actuarial Society of South Africa. ASSA2003 AIDS and Demographic model. 2005 http://aids.actuarialsociety.org.za/ASSA2003-Model-3165.htm.
- 20.Middelkoop K, Bekker LG, Myer L, Whitelaw A, Grant AD, Kaplan G, McIntyre J, Wood R. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201004-0598OC. Epub ahead of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochran WG. Some methods for strengthening the common X2 test. Biometrics. 1954;10(4):417–451. [Google Scholar]
- 22.De Cock KM, Soro B, Coulibaly IM, Lucas SB. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA. 1992;268(12):1581–1587. doi: 10.1001/jama.268.12.1581. [DOI] [PubMed] [Google Scholar]
- 23.Akksilp S, Karnkawinpong O, Wattanaamornkiat W, Viriyakitja D, Monkongdee P, Sitti W, Rienthong D, Siraprapasiri T, Wells CD, Tappero JW, Varma JK. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13(7):1001–1007. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varma JK, Nateniyom S, Akksilp S, Mankatittham W, Sirinak C, Sattayawuthipong W, Burapat C, Kittikraisak W, Monkongdee P, Cain KP, Wells CD, Tappero JW. HIV care and treatment factors associated with improved survival during TB treatment in Thailand: an observational study. BMC Infect Dis. 2009;9:42. doi: 10.1186/1471-2334-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant AD, Djomand G, De Cock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11(Suppl B):S43–S54. [PubMed] [Google Scholar]
- 26.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, Zheng H, Lu Z, Freedberg KA, Losina E. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 27.South African Department of Health. National Antiretroviral Treatment Guidelines. 2004 http://www.hst.org.za/uploads/files/sa_ART_Guidelines1.pdf.
- 28.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177(7):680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 31.Zeinecker J, Morrow C, van Soelen N, Orrell C, Wood R. Missing the target despite high population levels of anti-retroviral therapy coverage in a South African community. 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention. 2009 MOPED031 http://www.ias2009.org/mainpage.aspx?pageId=334.