Abstract
Conventional major histocompatibility complex (MHC) class I genes encode molecules that present intracellular peptide antigens to T cells. They are ubiquitously expressed and regulated by interferon gamma. Two highly divergent human MHC class I genes, MICA and MICB, are regulated by promoter heat shock elements similar to those of HSP70 genes. MICA encodes a cell surface glycoprotein, which is not associated with beta 2-microglobulin, is conformationally stable independent of conventional class I peptide ligands, and almost exclusively expressed in gastrointestinal epithelium. Thus, this MHC class I molecule may function as an indicator of cell stress and may be recognized by a subset of gut mucosal T cells in an unusual interaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Payne J. A., Shigekawa B., Frelinger J. A., Cresswell P. The transport of class I major histocompatibility complex antigens is determined by sequences in the alpha 1 and alpha 2 protein domains. Immunogenetics. 1990;31(3):169–178. doi: 10.1007/BF00211552. [DOI] [PubMed] [Google Scholar]
- Araki T., Gejyo F., Takagaki K., Haupt H., Schwick H. G., Bürgi W., Marti T., Schaller J., Rickli E., Brossmer R. Complete amino acid sequence of human plasma Zn-alpha 2-glycoprotein and its homology to histocompatibility antigens. Proc Natl Acad Sci U S A. 1988 Feb;85(3):679–683. doi: 10.1073/pnas.85.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Bahram S., Bresnahan M., Geraghty D. E., Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6259–6263. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram S., Spies T. Nucleotide sequence of a human MHC class I MICB cDNA. Immunogenetics. 1996;43(4):230–233. doi: 10.1007/BF00587305. [DOI] [PubMed] [Google Scholar]
- Balk S. P., Burke S., Polischuk J. E., Frantz M. E., Yang L., Porcelli S., Colgan S. P., Blumberg R. S. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994 Jul 8;265(5169):259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- Barbosa J. A., Kamarck M. E., Biro P. A., Weissman S. M., Ruddle F. H. Identification of human genomic clones coding the major histocompatibility antigens HLA-a2 and HLA-B7 by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6327–6331. doi: 10.1073/pnas.79.20.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman E. M., Porcelli S. A., Morita C. T., Behar S. M., Furlong S. T., Brenner M. B. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994 Dec 15;372(6507):691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Cheroutre H., Holcombe H. R., Tangri S., Castaño A. R., Teitell M., Miller J. E., Cardell S., Benoist C., Mathis D., Huse W. D. Antigen-presenting function of the TL antigen and mouse CD1 molecules. Immunol Rev. 1995 Oct;147:31–52. doi: 10.1111/j.1600-065x.1995.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Chowers Y., Holtmeier W., Harwood J., Morzycka-Wroblewska E., Kagnoff M. F. The V delta 1 T cell receptor repertoire in human small intestine and colon. J Exp Med. 1994 Jul 1;180(1):183–190. doi: 10.1084/jem.180.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Deusch K., Lüling F., Reich K., Classen M., Wagner H., Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991 Apr;21(4):1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- Fodil N., Laloux L., Wanner V., Pellet P., Hauptmann G., Mizuki N., Inoko H., Spies T., Theodorou I., Bahram S. Allelic repertoire of the human MHC class I MICA gene. Immunogenetics. 1996;44(5):351–357. doi: 10.1007/BF02602779. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Grandea A. G., 3rd, Androlewicz M. J., Athwal R. S., Geraghty D. E., Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995 Oct 6;270(5233):105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- Gross G. G., Schwartz V. L., Stevens C., Ebert E. C., Blumberg R. S., Balk S. P. Distribution of dominant T cell receptor beta chains in human intestinal mucosa. J Exp Med. 1994 Oct 1;180(4):1337–1344. doi: 10.1084/jem.180.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D., Fricker D., Bieber T., Esposito-Farese M. E., Bausinger H., Cazenave J. P., Donato L., Tongio M. M., de la Salle H. CD1 expression is not affected by human peptide transporter deficiency. Hum Immunol. 1994 Sep;41(1):61–68. doi: 10.1016/0198-8859(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Hershberg R., Eghtesady P., Sydora B., Brorson K., Cheroutre H., Modlin R., Kronenberg M. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe H. R., Castaño A. R., Cheroutre H., Teitell M., Maher J. K., Peterson P. A., Kronenberg M. Nonclassical behavior of the thymus leukemia antigen: peptide transporter-independent expression of a nonclassical class I molecule. J Exp Med. 1995 Apr 1;181(4):1433–1443. doi: 10.1084/jem.181.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Van Kaer L., Bonneville M., Hsu S., Murphy D. B., Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990 Aug 10;62(3):549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. Immunology of the intestinal tract. Gastroenterology. 1993 Nov;105(5):1275–1280. doi: 10.1016/0016-5085(93)90128-y. [DOI] [PubMed] [Google Scholar]
- Kaliyaperumal A., Falchetto R., Cox A., Dick R., 2nd, Shabanowitz J., Chien Y. H., Matis L., Hunt D. F., Bluestone J. A. Functional expression and recognition of nonclassical MHC class I T10b is not peptide-dependent. J Immunol. 1995 Sep 1;155(5):2379–2386. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kurlander R. J., Shawar S. M., Brown M. L., Rich R. R. Specialized role for a murine class I-b MHC molecule in prokaryotic host defenses. Science. 1992 Jul 31;257(5070):678–679. doi: 10.1126/science.1496381. [DOI] [PubMed] [Google Scholar]
- Lehner P. J., Cresswell P. Processing and delivery of peptides presented by MHC class I molecules. Curr Opin Immunol. 1996 Feb;8(1):59–67. doi: 10.1016/s0952-7915(96)80106-3. [DOI] [PubMed] [Google Scholar]
- Mellins E., Kempin S., Smith L., Monji T., Pious D. A gene required for class II-restricted antigen presentation maps to the major histocompatibility complex. J Exp Med. 1991 Dec 1;174(6):1607–1615. doi: 10.1084/jem.174.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
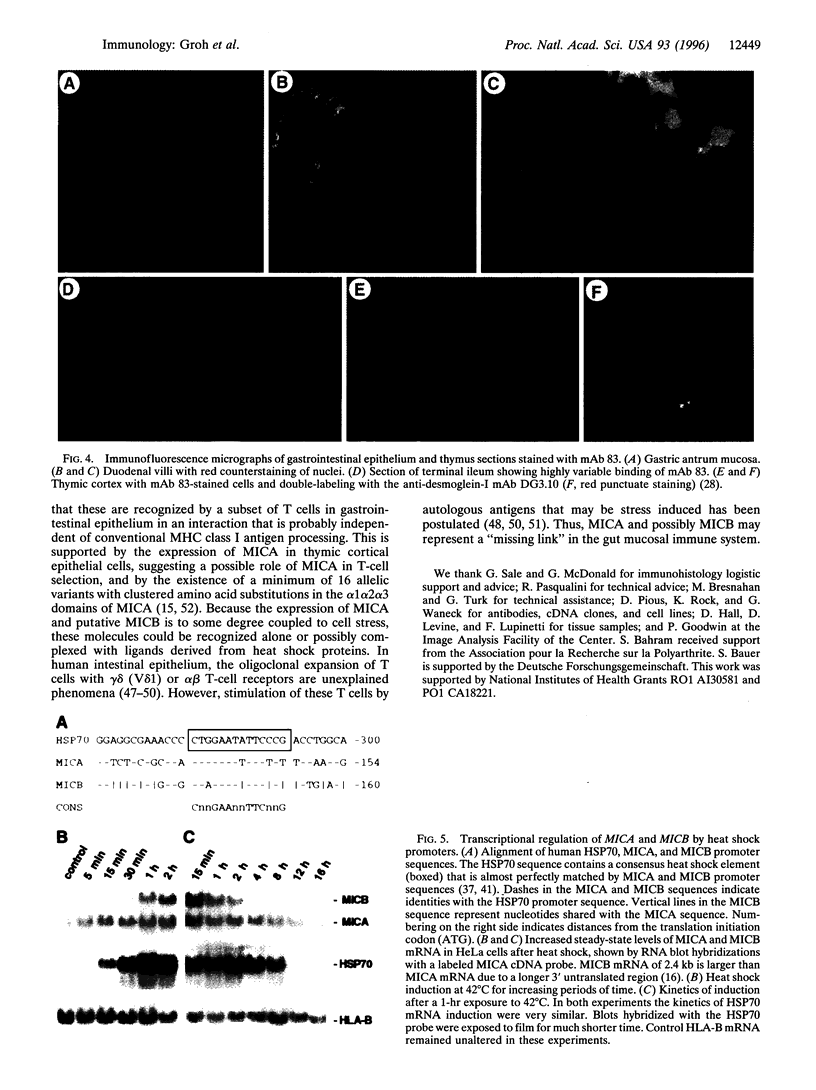
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Delarbre C., Kourilsky P., Gachelin G. Major histocompatibility complex class I molecules and resistance against intracellular pathogens. Crit Rev Immunol. 1994;14(3-4):193–220. doi: 10.1615/critrevimmunol.v14.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pamer E. G., Wang C. R., Flaherty L., Lindahl K. F., Bevan M. J. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992 Jul 24;70(2):215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- Parham P., Androlewicz M. J., Holmes N. J., Rothenberg B. E. Arginine 45 is a major part of the antigenic determinant of human beta 2-microglobulin recognized by mouse monoclonal antibody BBM.1. J Biol Chem. 1983 May 25;258(10):6179–6186. [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Trowsdale J., Campbell R. D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H., Mavaddat N., Litzenberger C., Ehrich E. W., Davis M. M., Bluestone J. A., Matis L., Draper R. K., Chien Y. H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994 Jan 14;76(1):29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Heid H. W., Schäfer S., Nuber U. A., Zimbelmann R., Franke W. W. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994 Dec;65(2):229–245. [PubMed] [Google Scholar]
- Sieling P. A., Chatterjee D., Porcelli S. A., Prigozy T. I., Mazzaccaro R. J., Soriano T., Bloom B. R., Brenner M. B., Kronenberg M., Brennan P. J. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995 Jul 14;269(5221):227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Blanck G., Bresnahan M., Sands J., Strominger J. L. A new cluster of genes within the human major histocompatibility complex. Science. 1989 Jan 13;243(4888):214–217. doi: 10.1126/science.2911734. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Strominger J. L. Human major histocompatibility complex contains a minimum of 19 genes between the complement cluster and HLA-B. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8955–8958. doi: 10.1073/pnas.86.22.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerckhove C., Russell G. J., Deusch K., Reich K., Bhan A. K., DerSimonian H., Brenner M. B. Oligoclonality of human intestinal intraepithelial T cells. J Exp Med. 1992 Jan 1;175(1):57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Sherman D. H., Calvin S., Allen H., Flavell R. A. Tissue-specific expression of cell-surface Qa-2 antigen from a transfected Q7b gene of C57BL/10 mice. J Exp Med. 1987 May 1;165(5):1358–1370. doi: 10.1084/jem.165.5.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub B. C., Jackson M. R., Hedrick S. M. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994 Oct 1;153(7):3051–3058. [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., van Kaer L., Itohara S., Tonegawa S. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J Exp Med. 1991 Jul 1;174(1):213–218. doi: 10.1084/jem.174.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]