Abstract
Metastasis, the spreading of cancer cells from a primary tumor to secondary sites throughout the body, is the primary cause of death for cancer patients. New therapies that prevent invasion and metastasis in combination with current treatments could therefore significantly reduce cancer recurrence and morbidity. Metastasis is driven by altered signaling pathways that induce changes in cell-cell adhesion, the cytoskeleton, integrin function, protease expression, epithelial to mesenchymal transition and cell survival. The RSK family of kinases is a group of ERK/MAPK effectors that can regulate these steps of metastasis by phosphorylating both nuclear and cytoplasmic targets. However, our understanding of RSK function in metastasis remains incomplete and is complicated by the fact that the four RSK isoforms perform non-redundant, sometimes opposing functions. While some isoforms promote cell motility and invasion by altering transcription and integrin activity, others impair cell motility and invasion through effects on the actin cytoskeleton. The mechanism of RSK action depends both on the isoform and the cancer type. However, despite the variance in RSK-mediated outcomes, chemical inhibition of this group of kinases has proven effective in blocking invasion and metastasis of several solid tumors in pre-clinical models. RSKs are therefore a promising drug target for anti-metastatic cancer treatments that could supplement and improve current therapeutic approaches. This review highlights contradiction and agreement in the current data on the function of RSK isoforms in metastasis and suggests ways forward in developing RSK inhibitors as new anti-metastasis drugs.
Keywords: RSK, ERK, metastasis, invasion, migration, motility
The RSK family of protein kinases
RSKs (ribosomal S6 kinases, p90-RSKs) constitute a family of serine/threonine kinases that are activated by the ERK/MAPK (extracellular signal-regulated kinase/mitogen-activated protein kinase) pathway. The four family members, designated RSK1-4, share 73–80% sequence homology and have highly conserved functional motifs. The proteins differ most significantly in their N- and C-terminal sequences and these differences may confer functional specificity to the isoforms (1, 2). Structurally, all four RSK isoforms consist of two independent kinase domains connected by a regulatory linker region. Full activation of the protein requires a series of steps including phosphorylation by ERK and PDK1 (3-phosphoinositide-dependent protein kinase-1), as well as auto-phosphorylation by the C-terminal kinase domain (CTKD) (1). Fully active RSK phosphorylates diverse downstream targets through its N-terminal kinase domain (NTKD). To date a large number of cytoplasmic and nuclear RSK targets have been identified. Through phosphorylation of these targets, RSKs modulate a variety of cellular processes including gene transcription, cell proliferation, and cell growth and differentiation. They can also mediate feedback inhibition of the ERK/MAPK pathway through phosphorylation of SOS (for a complete review of the RSK family see (2)). Three RSK inhibitors have been developed although none is specific to a single isoform. These have been useful both as drug leads and in uncovering many of the functions of RSKs.
Oncogenic EGFR, Ras and BRAF activate the ERK/MAPK/RSK pathway in many tumors. RSKs can also be activated in the absence of oncogenic ERK signaling in response to hypoxic conditions (3). Hyperactive RSK signaling is found in several cancers and supports cell transformation and tumor growth (2). Here we provide an overview of the known RSK-mediated effects on tumor invasion and metastasis with emphasis on migration.
RSK isoforms control tumor cell metastasis
Tumor metastasis occurs as a result of a complex set of changes in the cancer cell. This includes epithelial to mesenchymal transition (EMT) in which cells reduce cell-cell adhesion to the tumor and become mesenchymal and motile through reduced expression or activity of cadherins and other cell-cell adhesion proteins. As a result of EMT cells invade into the surrounding matrix and migrate away from the primary tumor site. Changes in the cytoskeleton, integrin signaling, and protease expression further promote invasion. Once tumor cells move across the vascular endothelium into new tissues, alterations in the cell death pathways protect the cells from apoptosis and permit growth of metastases. RSKs are reported to affect all these processes and are therefore well positioned to play a significant role in metastasis.
In vivo evidence of RSK function in tumor metastasis was first reported by Kang and colleagues, who showed that RSK2 promotes head and neck squamous cell carcinoma (HNSCC) metastasis (4). The analysis of tissues from patients with this malignancy revealed that higher RSK2 levels correlated with increased metastasis. Knockdown of RSK2 in human HNSCC cells also reduced the metastasis of xenografts in mice. Importantly, these changes are only mediated through RSK2, while RSK1 has no effect on HNSCC metastasis (4). In contrast, RSK1 was later shown to be a negative regulator of non-small cell lung cancer (NSCLC) metastasis (5). In this work a kinome-wide siRNA screen was performed on A549 lung cancer cells to identify proteins that affect lung cancer migration. Silencing of RSK1 enhanced in vitro cell migration and in vivo cell metastasis in zebrafish. Furthermore, human patient samples of metastasizing lung cancer have lower RSK1 expression than sections of the primary tumors. In these experiments only RSK1 had anti-metastatic effects (5). RSK isoforms therefore directly influence cancer metastasis. The simple conclusion from these two studies would be that signaling through RSK1 acts as a negative regulator of metastasis, while activity of the RSK2 isoform promotes it. However, in vitro studies in other cancer models point to a more complex network of RSK-mediated regulation of metastasis, showing that RSK function is not only dependent on the isoform, but also the specific cancer.
For example, in an independent RNAi screen for proteins that control migration in immortalized breast epithelial cells (MCF10A) RSK1 was alternatively identified to be pro-migratory (6). Both chemical inhibition of all RSK isoforms and specific silencing of RSK1 blocked epithelial cell migration. The extent to which RSK1 silencing blocked cell motility was not comparable to the chemical inhibition in this study and the authors concluded that the other RSK isoforms contribute to the regulation of cell motility as well (6). While this is a reasonable suggestion, there are other possibilities including differences in effective silencing of the kinase activity, inhibition of other kinases by the drug, or timing of the inhibition. In addition, inhibitors target only kinase activity and therefore permit RSK mediated protein scaffolding or binding, while siRNA completely removes protein expression. Therefore additional studies are needed to differentiate effects of single RSK isoforms or concomitant mechanisms. In spite of this limitation, these studies taken together support the hypothesis that RSKs act as regulators of cell motility and other processes driving metastasis. Table 1 summarizes identified RSK isoform-specific functions regulating steps in cancer metastasis.
Table 1.
RSKs show isoform- and cancer specific functions that regulate tumor cell motility. Effects on cell migration are shown as (+) “increased migration” and (−) “decreased migration”.
| Tissue | Cell line | RSK Isoform | Effects on migration | Proposed Mechanism | Reference |
|---|---|---|---|---|---|
| Bladder | T24 | RSK1 | + | Phosphorylation of SH3P2 blocks protein's negative effects on cell motility. | Tanimura et al. |
| Breast | MCF10A | RSK1, RSK2 | + | Drives ERK mediated motility through activation of FRA1 and c-JUN dependent transcription to induce EMT. | Doehn et al. |
| Breast | MCF10A | not specified | + | not specified | Smolen et al. |
| Breast | MDA-MB-231 | RSK4 | − | Negative regulation of pro-metastatic factors CXCR4 and CLDN2. | Thakur et al. |
| Cervix | HeLa S3 | RSK1 | + | Phosphorylation of SH3P2 blocks protein's negative effects on cell motility. | Tanimura et al. |
| Cervix | HeLa | RSK2 | + | Phosphorylation of Filamin A increases binding and inactivation of integrins. | Gawecka et al. (2012) |
| CNS | IMR32 | RSK2 | + | RSK2 is the mediator of ERK mediated cell motility regulated by PEA15. | Gawecka et al. (2011) |
| Colon | LIM 1863 | RSK1, RSK2 | + | Drives ERK mediated motility through activation of FRA1 and c-JUN dependent transcription to induce EMT. | Doehn et al. |
| Connective Tissue | HT-1080 | not specified | + | Phosphorylation of proton-exchanger NHE-1 under hypoxic conditions leading to ECM degradation due to extracellular acidification. | Lucien et al. |
| Head and Neck | 212LN, 37A, 37B, 686LN, M4e, Tu212 | RSK2 | + | Induction of CREB-dependent transcription of pro-metastatic genes. Hsp27 and FAK activation regulates actin filaments and focal adhesions. | Kang et al. |
| Kidney | 786-O, RCC10 | RSK1, RSK2 | + | Drives ERK mediated motility through activation of FRA1 and c-JUN dependent transcription to induce EMT. | Doehn et al. |
| Lung | A549 | RSK2, RSK4 | + | not specified | Lara et al. |
| Lung | A549, H23, NME35 | RSK1 | − | VASP phosphorylation impairs protein's function in regulating actin dynamics. | Lara et al. |
| Pancreas | L3.6pl | RSK2 | + | RON signaling induces RSK2 activity to start cellular EMT program. | Ma et al. |
| Prostate | PC3 | RSK1, RSK2 | + | Drives ERK mediated motility through activation of FRA1 and c-JUN dependent transcription to induce EMT. | Doehn et al. |
| Prostate | DU-145 | RSK2 | + | Phosphorylation of Filamin A increases binding and inactivation of integrins. | Gawecka et al. (2012) |
| Skin | A-431 | not specified | + | Phosphorylation of Filamin A increases binding and inactivation of integrins. | Vial et al. |
| Stomach | MKN1 | RSK1 | + | Phosphorylation of SH3P2 blocks protein's negative effects on cell motility. | Tanimura et al. |
RSK isoforms induce a transcriptional program modulating cell motility and invasion
RSK isoforms promote transcription and this can result in changes in cell motility (Figure 1, Inset 1). Doehn and colleagues systematically examined such effects using both knockdown and inhibitor-based approaches (7). The researchers analyzed 2- and 3-dimensional motility in a set of cell lines including non-transformed epithelial cells and metastatic carcinoma cell lines. Treatment with several RSK inhibitors (including FMK, BI-D1870, SL0101) reduced the migration of all analyzed cell lines. FMK also blocked TGFβ/TNFα induced, ERK-dependent EMT in a colon adenocarcinoma cell line. RSKs were therefore proposed to be primary mediators of Ras-ERK activated cell motility. Genome-wide mRNA array analysis designed to determine the RAF-induced gene program that is regulated by ERK and the subprogram regulated by RSK, further revealed that RSKs induce a FRA1- and c-JUN dependent transcriptional program containing genes with known roles in cell motility, many of which act as mediators of EMT. Among the differentially expressed genes involved in invasion were cell adhesion receptors (integrin α6β4), extracellular matrix proteins (laminin), proteases (MMPs) and other signaling proteins (IQGAP1). FMK inhibits RSK1, 2 and 4 isoforms at the same time and so to relate the observed effects to individual RSKs the authors utilized isoform specific RSK knockdowns. This showed that in the in vitro breast epithelial model only RSK1 and RSK2 affected cell motility and that both promoted migration (7). This isoform specificity needs to be further tested in the corresponding invasive cancer cells. For example the involvement of RSK4, whose expression could not be detected in breast epithelial cells (7), might be higher in cancer cells. Indeed, Thakur and colleagues reported that RSK4 suppresses both growth and metastasis of MDA-MB-231 breast cancer cells (8), further highlighting the potential importance of other isoforms.
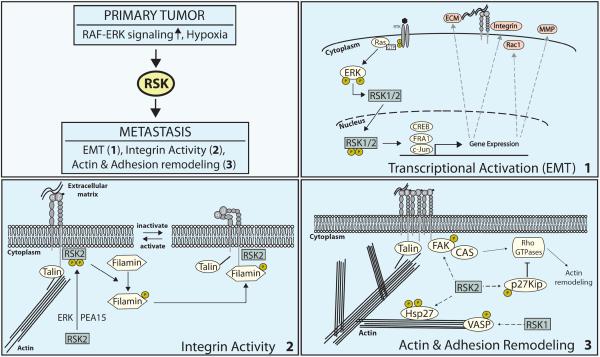
Figure 1.
RSK isoforms control motility and other aspects of tumor cell invasion and metastasis. In response to hypoxia or hyperactivation of the RAF/ERK pathway through oncogenes, RSKs modulate motility and invasion through effects on transcription (1), integrin activity (2), and the remodeling of the actin cytoskeleton (3). (1) RSK1 and RSK2 drive Ras-ERK activated cell motility by inducing a transcriptional program leading to EMT. (2) RSK2 controls Ras mediated inactivation of integrins by phosphorylating Filamin A (FLNa), an adapter protein that binds integrin cytoplasmic tails, suppresses integrin activation and cell adhesion. (3) RSKs modulate cellular adhesions and the remodeling of the actin cytoskeleton either directly through phosphorylation of actin binding proteins (VASP or Hsp27) or indirectly through interference with RhoGTPase signaling (FAK or p27Kip).
RSK2 also drives EMT in response to macrophage-stimulating protein (MSP) activation in both Madin-Darbin Canine Kidney epithelial (MDCK) and pancreatic cancer cells. Upon stimulation with MSP, the RON receptor tyrosine kinase signals through the Ras-ERK pathway to activate RSK2, induce EMT and promote cell motility (9). However, many of the experimental findings in this study are based on the use of the RSK inhibitor SL0101, a compound that inhibits all RSK isoforms. While RSK1 does not seem to be required for the induction of EMT in MDCK cells, potential regulatory effects and contributions from the other RSK isoforms can not be excluded. Nevertheless, the current data supports that RSK dependent transcription is one important mechanism that regulates EMT and can promote cell migration and invasion.
RSK isoforms regulate cell adhesion
Dynamic changes in cell adhesion to the extracellular matrix (ECM) and neighboring cells drive cell migration. In addition to changes in transcription, RSKs can regulate cell migration by controlling cell adhesion through effects on cell adhesion molecules like integrins, cadherins, and the immunoglobulin superfamily (10, 11). In particular RSK2 can control cell adhesion by phosphorylating the scaffold protein Filamin A (FLNa) (Figure 1, Inset 2). FLNa can bind to integrin cytoplasmic tails and induce a conformational change that reduces integrin affinity for ECM ligand (12). Woo and colleagues found that RSKs phosphorylate FLNa at Ser2125 in response to activation of the Ras-ERK cascade. They also showed that FLNa overexpression enhances migration of human melanoma cells in vitro in an ERK dependent manner (13). Gawecka and colleagues reported that in CHOK1 and HeLa cells RSK2 signals through FLNa to modulate cell adhesion and promote migration (14). In this study, active RSK2 in the focal adhesion phosphorylated FLNa at Ser2125. This promoted FLNa binding to β1 and β7 integrin cytoplasmic tails and suppressed integrin activation, fibronectin matrix assembly, and cell adhesion. FLNa appeared to be the only protein in the integrin complex phosphorylated by RSK2. RSK inhibitors, shRNA knockdown, and dominant-negative constructs of RSK2 all blocked Ras effects on integrin activation, showing that RSK2 phosphorylation of FLNa is a primary mediator of Ras-ERK dependent regulation of integrin-mediated cell adhesion and motility. Interestingly, RSK2 was also activated by integrin ligation to fibronectin, suggesting a possible feedback loop that allows cells to bind and release fibronectin during migration (14). While shRNA studies revealed RSK2 functions in this regulatory process, contributions of other RSK isoforms are conceivable and need to be specifically addressed. Whether RSK2 prevents formation of the focal adhesion complex or promotes its dissolution or both was left unresolved. An independent study similarly found that RSK inhibition blocked integrin-mediated cell adhesion and proposed that RSKs regulate α5β1 integrin activation in colon cancer and human squamous carcinoma cells through FLNa phosphorylation (15). The study did not differentiate between different RSK isoforms.
RSK isoforms regulate the cytoskeleton
In HNSCC cell lines the degree of invasiveness correlates with RSK2 expression and RSK2, but not RSK1, controls adhesion assembly and the rearrangement of the actin cytoskeleton to affect motility (Figure 1, Inset 3) (4). Mechanistically, knockdown of RSK2 results in decreased phosphorylation of the RSK2 targets CREB and Hsp27, as well as c-MET and FAK, two protein kinases that regulate cell migration (4). CREB-dependent gene transcription and c-MET mediated signaling have widespread effects that can alter cell motility (16, 17). FAK and Hsp27 have been directly implicated in the regulation of cell migration through their effects on actin remodeling and the assembly of focal adhesions (18, 19). The authors of this study used siRNA knockdown to demonstrate that the observed effects are mediated by RSK2, but not RSK1. Contributions of RSK3 and RSK4 were not analyzed. These findings exemplify how RSK isoforms in a single cancer type can have diverse effects on transcription and the cytoskeleton that together promote cell motility.
The data on the RSK2 isoform has consistently revealed that it promotes migration and invasion. In contrast, the data on RSK1 is less consistent as this isoform is reported to have both pro- and anti-migratory effects depending on the cancer cell. In melanoma cells RSK1 promotes motility by increasing the phosphorylation of p27Kip1 at Thr198 (20). This induces a re-localization of p27Kip1 to the cytoplasm where it binds RhoA and blocks signaling through the RhoA/ROCK pathway. This leads to changes in actomyosin stability causing increased cell migration. Here purified RSK1 was examined in vitro and the work also incorporated RSK1 specific knockdown studies in cell lines. The findings therefore strongly support that RSK1 promotes motility in these cells. In contrast, RSK1 impairs lung cancer cell metastasis and that has been attributed to changes in the actin cytoskeleton as well (5). In these cells RSK1 phosphorylates the actin binding protein VASP at Thr278, a modification that impairs actin polymerization and results in decreased cell motility. Phosphorylation at this site was not mediated by any other RSK isoform. Only overexpression of constitutively active RSK1 or inhibition with the RSK inhibitor SL0101 were used to assess RSK1 function in this study. The work would benefit from re-examination with other approaches such as knockdown or genetic deletion of RSK1 and the use of alternative RSK inhibitors such as FMK. Therefore RSK1 may affect motility in opposite directions in melanoma versus lung cancer cells. It is unclear what accounts for the observed differences; although it may be that overexpression of active RSK1 is inducing artificial effects that mimic another RSK isoform or other kinase. If RSK1 indeed has the reported opposing effects in these cancer cell lines, this would provide a potential context in which to investigate how the same RSK isoform might have different effects on cell motility that are dependent on the cancer type.
One of the few studies examining RSK4 function in migration indicates that it can act as a negative regulator of breast cancer cell invasion through effects on claudin-2 and CXCR4 expression (8). However, the authors did not take potential effects of other RSK isoforms expressed in this cancer model into account. Regarding the effects on migration, the study relied entirely on the overexpression of RSK4, a method that can lead to significant artifacts, as overexpression of one isoform might cause it to perform a function normally reserved for another isoform. As mentioned before, methods like isoform specific silencing and inhibitor-based approaches will help clarify the RSK-specific regulatory mechanisms in this tumor model.
Targeting invasion and metastasis by inhibiting RSK isoforms
Cancers kill when they become invasive or metastatic and spread. Since some RSK isoforms promote invasion and tumor metastasis, they are promising targets for the development of new drugs. Three inhibitors have been reported to preferentially affect RSK isoforms with IC50 values in the low nanomolar range. SL0101, the synthetic version of a natural kaempferol-glycoside, acts by competing for the ATP-binding pocket within the NTKD of RSK (IC50 = 89nM). This reversible inhibitor has been shown to block the activity of all four RSK isoforms (21), but also affects Aurora B, PIM1 and PIM3 kinases at higher concentrations (22). FMK is a pyrrolopyrimidine compound that potently (IC50 = 15nM) inhibits RSK's CTKD. Through formation of a covalent bond it acts as an irreversible inhibitor. Due to the mechanism of inhibition, FMK is specific for the isoforms RSK1, RSK2 and RSK4 (23). Although FMK preferentially affects RSKs, at higher concentrations in vitro it can also inhibit purified S6K1, Yes, Eph-A2 and Src and Lck (22). In addition, if the N-terminal kinase domain is activated by other means than the C-terminal kinase domain, FMK will have no effect. BI-D1870 is a dihydropteridinone that reversibly binds the ATP pocket of the NTKD and inhibits its function (IC50 = 10–30nM). Like SL0101, BI-D1870 shows selectivity for RSK over other kinases, but does not distinguish between the four RSK isoforms (24). The only other kinase affected with similar potency is PLK1. At 10–100 fold higher concentrations BI-D1870 can affect Aurora B, PIM3 MST2, and MELK kinases (22). The high potency of these three compounds favors their use as RSK-targeting drugs. However, none of these drug-leads show specificity for only one of the RSK isoforms and they can also inhibit additional kinases depending on the inhibitor and concentration. Mechanistic studies with RSK inhibitors therefore require the independent use of two or more of these compounds to exclude off target effects. The inhibitors' target overlap also opens the question of how useful compounds like this could be for anti-metastasis therapy. As discussed earlier, RSK's regulation of cell motility can go both ways and blocking the activity of multiple RSK isoforms simultaneously might not greatly affect tumor progression or worse yet might promote metastasis depending on the cancer. However, despite their lack of isoform specificity all three compounds have shown promising results in pre-clinical studies.
SL0101 blocks cell migration of neuroblastoma cells. Interestingly, in this malignancy RSK2 activity is tightly regulated by PEA-15, a protein scaffold that supports ERK activation of RSK2. PEA-15 negatively affects cell motility and high PEA-15 expression correlates to reduced RSK2 activity and reduced metastasis in neuroblastoma patients (25, 26). RSK inhibition through SL0101 also inhibits migration of some prostate cancer lines, but does not affect mesothelioma cells (14). FMK has been reported to inhibit cell migration in neuroblastoma and cervical cancer models, and prevent invasion in a series of human cancer cell lines including HNSCC, colon carcinoma, renal cell carcinoma and prostate cancer (4, 7, 14, 26). These studies also show similar effects of BI-D1870. In addition, BI-D1870 decreased invadopodia formation of fibrosarcoma cells under hypoxic conditions (3). Inhibitor treatment increased the level of cell adhesion after EGF stimulation in carcinoma cells and blocked cell migration in gastric and bladder cancers (15, 27). These studies show that a drug lead does not have to be specific for only one RSK isoform in order to be effective in blocking cancer cell motility. Importantly, RSK inhibitors have not yet been reported to promote migration, invasion or metastasis in preclinical models. While the development of isoform selective RSK inhibitors would help to further clarify each isoform's cellular function and would allow for a more selective targeting of pro-metastatic RSKs, the use of the three less specific RSK inhibitors could still prove to be a beneficial therapeutic approach for some cancers. It is expected that the expression ratio of pro- and anti-metastatic RSK isoforms will contribute to the success or failure of RSK-targeted therapy. A more rigorous profiling of cancers in respect to their RSK isoform expression is therefore required to determine the benefit of RSK inhibition. To date no clinical trials of RSK inhibitors have been published.
Conclusions and Future Directions
By affecting processes like EMT, cytoskeletal rearrangement and integrin activity RSKs act as a nexus in the control of cell motility. However, the overall outcome of this regulation varies among different malignancies and appears to depend on the RSK isoform. Future studies must therefore focus on a more comprehensive experimental approach to analyze the contributions of all RSK isoforms to the observed outcome. Multiple lines and primary tumor cells derived from the same cancer type should be examined using RSK specific inhibitor-based approaches that utilize two or more known RSK inhibitors to determine if the effects are RSK specific. In addition the analysis should include isoform-specific gene silencing using multiple shRNA/siRNAs that target different sequences or genetic deletion techniques, such as TALEN, ZFN or CRISPR/Cas9, to determine which RSK isoforms function in migration, invasion and metastasis in a specific cancer. Moreover, it remains to be determined if RSK isoforms may function to regulate each other in the dynamic process of migration and invasion. The in vivo relevance of the in vitro findings, while clearly shown in some cancer models, remains to be verified for others in mouse models or patients. Nevertheless, it is now established that RSKs are versatile regulators that control migration and invasion in response to hyperactivation of the ERK/MAPK pathway or hypoxia. This family of kinases is therefore a highly promising set of drug targets and inhibitors of one or multiple RSK isoforms could serve as powerful new drugs to control invasion and metastasis and thereby augment current cancer therapies.
Acknowledgements
The authors thank Steen Hansen (Harvard Medical School), Michelle Matter (University of Hawaii Cancer Center), and members of the Ramos group for critical review of the manuscript and important suggestions. They also acknowledge the generous support of the Victoria S. and Bradley L. Geist Foundation (J.W.R) and the National Institutes of Health (R01-GM088266 to J.W.R.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have no potential conflicts of interest.
References
- 1.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 2.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441:553–69. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 3.Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK) PLoS One. 2011;6:e28851. doi: 10.1371/journal.pone.0028851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W, et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest. 2010;120:1165–77. doi: 10.1172/JCI40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lara R, Mauri FA, Taylor H, Derua R, Shia A, Gray C, et al. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene. 2011;30:3513–21. doi: 10.1038/onc.2011.61. [DOI] [PubMed] [Google Scholar]
- 6.Smolen GA, Zhang J, Zubrowski MJ, Edelman EJ, Luo B, Yu M, et al. A genome-wide RNAi screen identifies multiple RSK-dependent regulators of cell migration. Genes Dev. 2010;24:2654–65. doi: 10.1101/gad.1989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doehn U, Hauge C, Frank SR, Jensen CJ, Duda K, Nielsen JV, et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell. 2009;35:511–22. doi: 10.1016/j.molcel.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur A, Sun Y, Bollig A, Wu J, Biliran H, Banerjee S, et al. Anti-invasive and antimetastatic activities of ribosomal protein S6 kinase 4 in breast cancer cells. Clin Cancer Res. 2008;14:4427–36. doi: 10.1158/1078-0432.CCR-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Q, Guin S, Padhye SS, Zhou YQ, Zhang RW, Wang MH. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol Cancer. 2011;10:66. doi: 10.1186/1476-4598-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moh MC, Shen S. The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adh Migr. 2009;3:334–6. doi: 10.4161/cam.3.4.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–35. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawecka JE, Young-Robbins SS, Sulzmaier FJ, Caliva MJ, Heikkila MM, Matter ML, et al. RSK2 Suppresses Integrin Activation and Fibronectin Matrix Assembly and Promotes Cell Migration. J Biol Chem. 2012 doi: 10.1074/jbc.M112.423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vial D, McKeown-Longo PJ. Epidermal Growth Factor (EGF) Regulates alpha5beta1 Integrin Activation State in Human Cancer Cell Lines through the p90RSK-dependent Phosphorylation of Filamin A. J Biol Chem. 2012;287:40371–80. doi: 10.1074/jbc.M112.389577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conkright MD, Montminy M. CREB: the unindicted cancer co-conspirator. Trends Cell Biol. 2005;15:457–9. doi: 10.1016/j.tcb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 1997;94:701–6. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, et al. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–74. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Golubovskaya VM. Focal adhesion kinase as a cancer therapy target. Anticancer Agents Med Chem. 2010;10:735–41. doi: 10.2174/187152010794728648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larrea MD, Hong F, Wander SA, da Silva TG, Helfman D, Lannigan D, et al. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci U S A. 2009;106:9268–73. doi: 10.1073/pnas.0805057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65:1027–34. [PubMed] [Google Scholar]
- 22.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–21. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidyanathan H, Opoku-Ansah J, Pastorino S, Renganathan H, Matter ML, Ramos JW. ERK MAP kinase is targeted to RSK2 by the phosphoprotein PEA-15. Proc Natl Acad Sci U S A. 2007;104:19837–42. doi: 10.1073/pnas.0704514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawecka JE, Geerts D, Koster J, Caliva MJ, Sulzmaier FJ, Opoku-Ansah J, et al. PEA15 impairs cell migration and correlates with clinical features predicting good prognosis in neuroblastoma. Int J Cancer. 2011 doi: 10.1002/ijc.27415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimura S, Hashizume J, Kurosaki Y, Sei K, Gotoh A, Ohtake R, et al. SH3P2 is a negative regulator of cell motility whose function is inhibited by ribosomal S6 kinase-mediated phosphorylation. Genes Cells. 2011;16:514–26. doi: 10.1111/j.1365-2443.2011.01503.x. [DOI] [PubMed] [Google Scholar]