SUMMARY
We previously demonstrated that infants with a history of bronchopulmonary dysplasia (BPD) exhibit airflow obstruction and air trapping. The purpose of this study was to assess longitudinal changes in pulmonary function in infants with a history of BPD over the first three years of life, and the relationship to somatic growth. Spirometry was measured using the raised volume rapid thoracoabdominal compression technique, and lung volumes measured by plethysmography. Eighteen infants (mean gestational age±SD 27.3±2.2 weeks, birthweight 971±259 g) underwent two lung function studies. Average age at first test was 58.8 wks. Spirometry demonstrated significant reductions in forced expiratory volume in 0.5 sec (FEV0.5, 76.0±15.9% predicted, Z score −2.13±1.69), forced expiratory flow at 75% of expired forced vital capacity (FEF75, 54.8±31.1%, −3.58±2.73) and FEF25–75 (67.8±33.3%, −1.79±1.76). Group mean total lung capacity (TLC) was in the low normal range (82.9±13.5% predicted) and residual volume (RV)/TLC was mildly elevated (122.4±38.2% predicted). Repeat testing was performed an average of 32.7 weeks after initial testing. At re-evaluation, group mean lung volumes and flows tracked at or near their previous values; thus, in general, there was a lack of catch-up growth. However, compared to infants with below average or average somatic growth (as represented by gm/day), infants with above average growth showed significantly greater improvements in percent predicted FVC, FEV0.5, TLC and RV/TLC (all p<0.05, ANOVA). We conclude that longitudinal measures of pulmonary function in infants and young children with BPD demonstrate significant airflow obstruction and modest restriction which tends to persists with time. On the other hand, infants with above average somatic growth showed greater lung growth than their peers. Additional studies examining the effects of various nutritional regimens on lung function are warranted.
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is currently defined as a lung disease resulting from mechanical ventilation and oxygen therapy in infants born prematurely who require oxygen at 36 weeks postmenstrual age 1. The epidemiology and natural history of BPD have evolved in recent years. With widespread use of prenatal steroids, exogenous surfactant, high frequency oscillatory mechanical ventilation and other modalities, survival of smaller, more premature infants has become a frequent occurrence 2. Reductions in the intensity of mechanical ventilation and oxygen supplementation, superimposed on a more immature lung, have led to changes in the clinical and pathologic features of BPD 3. In contrast to the airway inflammation, fibrosis, and smooth muscle hypertrophy observed in earlier cohorts, “new” BPD is primarily a disease of an arrest of alveolarization.
Advent of the raised volume rapid thoracoabdominal compression (RVRTC) technique has allowed a more comprehensive description of airway function and lung volumes in infants with a history of BPD. Normal values for forced expiratory flow in infants using this technique have been established 4. We have shown that infants with BPD continue to have significant reductions in forced expiratory flows and increased lung volumes indicative of hyperinflation and air trapping, and that infants with the most severe obstruction also demonstrate increased bronchodilator responsiveness 5. However, few studies have examined longitudinal lung function in infants with BPD. Tepper and colleagues 6 found that both functional residual capacity (FRC) and VmaxFRC, the expiratory flow rate obtained during a partial forced expiratory maneuever, were significantly decreased in BPD. FRC subsequently increased to normal, but VmaxFRC remained about half normal, perhaps indicative of poor growth of the airways. Gerhardt and coworkers 7 found that lung compliance and FRC were decreased in the first six months of life but improved thereafter, presumably due to catch-up growth of the lung. Mallory and colleagues8, using the forced deflation technique, found that, in patients with moderate-to-severe BPD requiring tracheostomy, forced vital capacity (FVC) caught up to normal levels by 36 months of age, but that severe lower airway obstruction, as assessed by maximum expiratory flow at 25% FVC, persisted in all infants. Subsequent studies of babies with “new” BPD also show persistently low VmaxFRC values 9,10. However, longitudinal measurements of lung function in infants using the RVRTC technique, which measures airway function throughout the full range of lung volumes and is independent of dynamically-maintained FRC 11, have not been performed.
Growth failure and malnutrition are common in infants with BPD. The precise causes of poor growth are unknown, but likely relate to dysfunction of other organ systems, decreased nutrient intake and increased energy requirements. Optimizing growth has remained a principal goal of nutritional support in infants with BPD, although the relationship between somatic growth and lung growth is not well defined. Historically, an increased growth rate to achieve “catch-up” growth has been the goal of nutritional support. On the other hand, a recent study of term infants found that higher rates of weight gain in early infancy are associated with impaired lung development 12. We therefore assessed longitudinal changes in pulmonary function using the RVRTC technique and the relationship between lung function and somatic growth in infants with a history of BPD.
MATERIALS AND METHODS
Subjects
Infants with BPD who were undergoing infant lung function testing for routine clinical assessment were recruited to participate in this study. All subjects are followed and were referred for testing by the physicians in the Section of Pediatric Pulmonology at the University of Michigan. Eighteen infants with a history of BPD underwent at least two studies of lung function at University of Michigan between October, 2003 and May, 2007. Subject characteristics are shown in Table 1. All infants were less than three years of age at the time of the baseline study, and were at their baseline state of health, with no signs of acute respiratory symptoms for at least two weeks prior to testing. All infants were breathing room air at the time of study. The study was approved by the University of Michigan Institutional Review Board. Informed consent was obtained from one parent or legal guardian.
Table 1.
Subject Characteristics.
| Mean ±SD (range), n=18 | |
|---|---|
| Gestational Age, weeks | 27.3 ±2.2 (24.0–31.6) |
| Birthweight, grams | 971.3 ±259.3 (435–1387) |
| Gender, M/F | 8/10 |
| Race, %Caucasian | 83% |
| Mechanical Ventilation, total days | 37.2 ±33.5 (1–89) |
| Supplemental Oxygen, total days | 235 ±131.2 (42–495) |
Lung function measurements
Infants were sedated with 75–100 mg/kg of chloral hydrate given orally. Heart rate and oxygen saturation were monitored continuously. Maximal expiratory flow volume (MEFV) curves were obtained using the RVRTC technique, as previously described (Collins IPL, nSpire Health, Longmont, CO) 4,13. FVC, forced expiratory volume in 0.5 sec (FEV0.5), forced expiratory flow at 25% of expired forced vital capacity (FEF25), FEF50, FEF75, FEF85 and FEF25–75 were measured. Analysis was based on the best loop, defined as the maneuver with the highest sum of FVC and FEF25–75. All curves used for analysis had FVC measurements within 10% of the highest baseline FVC for that set of maneuvers.
FRC was measured using a 90.5L Plexiglas whole body plethysmograph 14. Measurements of FRC preceded MEFV maneuvers both at baseline and following albuterol treatment. Fractional lung volumes were calculated from FRC, FVC and expiratory reserve volume as described. Expiratory forced flows and fractional lung volumes are expressed as percent of predicted for length 4,14.
Measurement of bronchodilator responsiveness
Infants had not received a bronchodilator for at least six hours prior to testing. Albuterol was administered as described previously 15. Briefly, albuterol was given using a metered dose inhaler with a spacer in a dose of two to eight puffs (180–720 μg), as needed to produce a 10% increase in heart rate. Each puff was followed by an inflation of the lungs to 25 cm H2O. MEFV curves and lung volume measures were repeated beginning at 10 minutes following the first dose of albuterol.
Growth calculation
Growth rate was calculated based on weight gain (grams/day) between studies, and subjects were compared to expected rates 16.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Spirometric measurements of lung function are expressed as both percent predicted and Z-score4,14. For spirometric measurements, the loop with the highest sum of FVC and FEF25–75 was recorded for analysis. Plethysmographic lung volumes are expressed as percent predicted 14.
Body weights and lengths are expressed as percentile and Z-score. Changes in pulmonary function measures from baseline to follow-up and changes per unit length were compared using paired t-tests. Group means for somatic growth data were compared using one-way analysis of variance with Bonferroni correction for multiple comparisons where indicated. Bivariate correlations were performed using the Pearson correlation coefficient.
RESULTS
Patient characteristics
Eighteen infants with a history of bronchopulmonary dysplasia underwent two tests of pulmonary function. Babies tended to be extremely low birth weight (average gestational age, 27.3 weeks, range, 24.0–31.6 weeks; average birth weight, 971 g, range, 435–1387 g). Infants required an average of 235 days of supplemental O2 (range, 42–495 days), indicating a diagnosis of “moderate” to “severe” BPD 1. Average age at the time of first study was 58.8±17.6 weeks. Repeat testing was performed an average of 32.7 weeks after initial testing.
Forced expiratory maneuvers
Forced expiratory maneuvers were performed using the RVRTC technique. When adjusted for body size, FVC was low normal at baseline and mildly reduced at follow-up compared to a cohort of normal infants4 (Table 2). Average FEF75, a measure of flow at low lung volumes, was severely reduced at both baseline and follow-up. Longitudinal changes in individual values of FEF75 are plotted against length in Figure 1. On average, changes in FEF75 tracked at the predicted slope but substantially below predicted values, indicating a lack of catch-up growth. On a percent predicted basis, there was no difference in FVC, FEV0.5, FEF75 or FEF25–75 between the first and second lung function tests.
Table 2.
Subject Demographics and Pulmonary Function
| Baseline, mean (SD) | Z score (SD) | Follow-up, mean (SD) | Z score (SD) | |
|---|---|---|---|---|
| Age at Study, weeks | 58.79 (17.55) | 91.44 (31.11)* | ||
| Weight, kg | 8.64 (1.71) | −1.34 (1.06) | 10.80 (1.85)* | −0.97 (1.12) |
| Length, cm | 70.04 (6.54) | −2.30 (1.46) | 80.77 (6.39)* | −1.36 (1.40) |
| FVC, % predicted | 81.4 (16.6) | −1.53 (1.57) | 79.8 (16.2) | −1.63 (1.33) |
| FEV0.5, % predicted | 76.0 (15.9) | −2.13 (1.69) | 77.6 (14.6) | −1.94 (1.42) |
| FEF75, % predicted | 54.8 (31.1) | −3.58 (2.73) | 54.9 (28.0) | −3.54 (2.76) |
| FEF25–75, % predicted | 67.8 (33.3) | −1.79 (1.76) | 70.5 (28.1) | −1.56 (1.63) |
| FRC, % predicted | 98.5 (23.6) | 97.3 (18.2) | ||
| TLC, % predicted | 82.9 (13.5) | 80.2 (10.0) | ||
| RV, % predicted | 134.1 (32.4) | 126.8 (31.4) | ||
| RV/TLC, % predicted | 145.5 (33.7) | 146.4 (35.4) | ||
| Bronchodilator Responsive | 6/15 | 4/14 |
p<0.001 Baseline vs. Follow-up.
Figure 1.
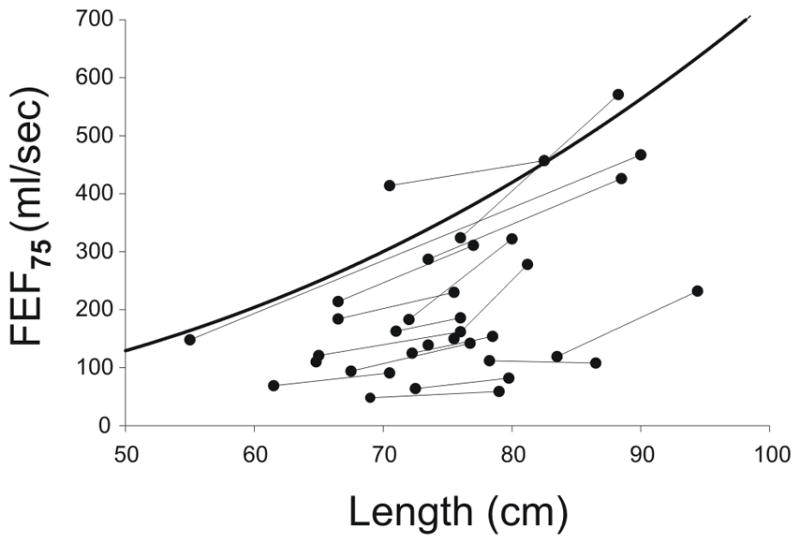
Longitudinal measures of FEF75 vs. length. On average, changes in FEF75 per unit length track at the predicted slope but substantially below predicted values (heavy line), indicating a lack of catch-up growth.
Fractional lung volumes
Consistent with the FVC data, average TLC was low normal at baseline when adjusted for body size14, and there was no change at follow-up (Table 1). When individual values of TLC were plotted against length, changes in TLC tracked at the predicted slope 4,14, again indicating a lack of catch-up growth.(Figure 2). Consistent with the severe reduction in flow rates at low lung volumes noted above, RV/TLC ratio was increased at baseline and follow-up, indicative of air trapping. On a percent predicted basis, there was no difference in FRC, TLC, RV or RV/TLC between tests.
Figure 2.
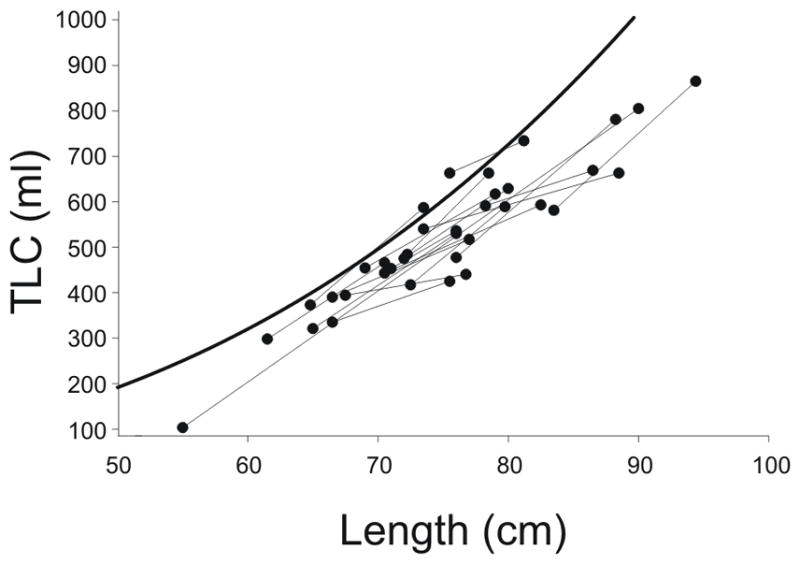
Longitudinal measures of TLC vs. length. TLC measures for individual subjects (black lines) track at or below the predicted values per unit length, as determined by Castile et al 14 (heavy line). Indeed, the majority of our data points fell below Castile’s lower 95% confidence limit, consistent with the notion that TLC was significantly reduced in infants with BPD.
We also examined whether there was a relationship between expiratory flow, plethysmographic lung volumes and the severity of prematurity or neonatal lung disease. There was no statistical correlation between any measures of pulmonary function and birthweight, gestational age, days of mechanical ventilation, or days of oxygen therapy.
Bronchodilator responsiveness
At baseline, 6 out of 15 (40%) infants showed significant bronchodilator responsiveness, as defined by a change in FEF75 greater than 24.3%, which is two standard deviations from the mean change for previously published normal infants who received placebo 4. At follow-up, 4 out of 14 (29%) infants showed bronchodilator responsiveness.
Growth measurements
Infants were weighed at baseline and follow-up and subsequently divided into two groups on the basis of weight gain (grams/day) in comparison to the expected rate 16. The pulmonary function parameters of infants growing at below average (n=3) or average (n=6) rates were compared to infants growing at above average rates (n=9). There was no difference in group mean weight, length or age between the two groups (not shown). Baseline FVC was higher in infants who grew at below average or average growth (Table 3). On the other hand, compared to infants with below average or average growth, infants with above average growth showed proportionally greater improvements in FVC, FEV0.5, TLC and RV/TLC (all p<0.05, ANOVA). When all infants were considered together, somatic growth significantly correlated with changes in percent predicted FVC, FEV0.5, and RV/TLC (Figure 3). On the other hand, parameters of airflow (FEF75, FEF25–75) were not significantly different between groups.
Table 3.
Comparison of lung function changes in infants with below average or average somatic growth (n=9) to infants with above average somatic growth (n=9).
| Growth parameter | Somatic growth | P value* | |
|---|---|---|---|
| Below average or average | Above average | ||
| Somatic growth grams/day, mean (SD) | 4.8 (2.1) | 10.0 (2.2) | <0.001 |
| Weight for age, Z score, mean (SD) | |||
| First test | −1.46 (1.18) | −1.23 (0.98) | 0.659 |
| Second test | −1.59 (1.18) | −0.35 (0.65) | 0.013 |
| Change | −0.14 (0.42) | 0.88 (0.90) | 0.007 |
| Height for age, Z score, mean (SD) | |||
| First test | −2.51 (1.43) | −2.10 (1.55) | 0.561 |
| Second test | −1.92 (1.31) | −0.81 (1.33) | 0.093 |
| Change | 0.60 (0.70) | 1.29 (1.42) | 0.208 |
| FVC, Z score, mean (SD) | |||
| First test | −0.67 (0.41) | −2.40 (1.83) | 0.014 |
| Second test | −1.36 (0.77) | −1.90 (1.72) | 0.405 |
| Change | −0.69 (.65) | 0.50 (1.29) | 0.025 |
| FEV0.5, Z score, mean (SD) | |||
| First test | −1.54 (0.99) | −2.72 (2.07) | 0.142 |
| Second test | −1.90 (1.31) | −1.99 (1.61) | 0.900 |
| Change | −0.36 (0.54) | 0.73 (1.12) | 0.018 |
| FEF75, Z score, mean (SD) | |||
| First test | −3.92 (2.30) | −3.24 (3.21) | 0.613 |
| Second test | −3.87 (2.80) | −3.21 (2.86) | 0.627 |
| Change | 0.05 (1.06) | 0.03 (0.9) | 0.969 |
| FEF25–75, Z score, mean (SD) | |||
| First test | −2.03 (1.50) | −1.55 (2.04) | 0.579 |
| Second test | −1.86 (1.69) | −1.25 (1.60) | 0.446 |
| Change | 0.17 (0.55) | 0.30 (0.69) | 0.669 |
| TLC, % predicted, mean (SD) | |||
| First test | 87.7 (8.3) | 78.2 (16.4) | 0.143 |
| Second test | 82.1 (9.1) | 78.3 (11.1) | 0.441 |
| Change | −7.5% (7.1%) | 1.7% (10.4%) | 0.044 |
| RV, % predicted, mean (SD) | |||
| First test | 101.4 (23.8) | 108.6 (44.3) | 0.677 |
| Second test | 105.3 (28.3) | 103.6 (33.0) | 0.904 |
| Change | −0.33% (11.4%) | −0.33% (30.8%) | 1.000 |
| RV/TLC, mean (SD) | |||
| First test | 109.8 (16.9) | 135.1 (49.6) | 0.166 |
| Second test | 123.8 (25.8) | 131.8 (45.4) | 0.652 |
| Change | 7.8% (9.7%) | −2.8% (10.1%) | 0.037 |
P value calculated by ANOVA
Figure 3.
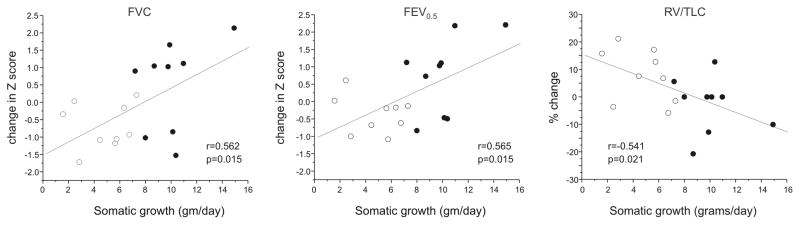
Correlations between somatic growth (gm/day) and changes in FVC (Z score, left panel), FEV0.5 (Z score, middle panel) and RV/TLC (% predicted, right panel). Pearson correlation coefficients and P values are shown. Open circles represent infants with below average or average somatic growth. Closed circles represent infants with above average somatic growth.
DISCUSSION
The precise biological mechanisms responsible for alveolar arrest in the “new BPD” are unclear. Data suggest that BPD results from a complex interplay between inflammation and alveolar/vascular development, with interleukin (IL)-1β 17,18, vascular endothelial growth factor 19–21, transforming growth factor-β 22,23 and the myofibroblast 24,25 playing key roles. In the present study, we found that infants with a history of moderate-to-severe BPD demonstrate a severe reduction in flows at low lung volumes, as evidenced by reductions in FEF75. Accordingly, babies also demonstrated air trapping, as shown by increased RV/TLC values. Average FVC and TLC values were in the low normal or mildly reduced range, indicating modest restriction. Despite abnormal airway function, bronchodilator responsiveness was present in only a minority of infants.
Infants with a history of BPD were re-studied an average of 32 weeks after initial testing, at a mean postnatal age of almost two years. Severe reductions in flow at low lung volumes, consistent with peripheral airways obstruction and resultant air trapping, persisted. In addition, FVC decreased into the mildly reduced range compared to a cohort of normal infants 4. Average TLC remained in the low normal range. Thus, longitudinal measures of lung function showed little change with growth, with abnormalities in forced expiratory flow, lung volume and compliance persisting over time. These data confirm and extend previous work showing no improvement in VmaxFRC with time in infants with a history of BPD 6,9,10. However, since VmaxFRC measurements are obtained at dynamically-maintained end-expiratory volume 11, our data provide a more accurate measure of the level of airflow obstruction. These data are consistent with recent studies in school age children showing persistent abnormalities in small airways function in infants with a history of moderate-to-severe BPD, in spite of surfactant treatment26,27. Together, these results add new, more accurate information on lung function in infants with a history of BPD, at a time period in development when previous data were lacking.
It has been argued that changes in lung function in infants with BPD fundamentally reflect premature birth, rather than lung injury. However, when compared to results in healthy premature infants 28,29, infants with BPD showed further diminished levels of airflow, suggesting that lung function changes in BPD cannot be fully explained by prematurity alone.
The prevalence of bronchodilator responsiveness in infants with a history of BPD was less than 30% at follow-up. In our previous study, 35% of infants with a history of BPD were bronchodilator responsive 5, and only half of the infants with a history of recurrent wheezing demonstrated a significant change in airflow with bronchodilator administration. Together, these results suggest that airflow obstruction in these infants is not fully explained by airway smooth muscle dysfunction, and that an alternative mechanism is possible. Consistent with this, preschool children with a history of BPD do not show bronchial responsiveness to adenosine 5-monophosphate 30, indicating the lack of an airway inflammatory response characteristic of asthmatic subjects. One potential mechanism for airway narrowing is hypoalveolarization, which would reduce the number of alveolar attachments per airway, leading to reduced airway-parenchymal coupling. Airway parenchymal coupling in BPD may also be reduced by thickening of the outer airway wall 31. Airway narrowing could also relate to smaller-sized airways, decreased pulmonary elastic recoil, more collapsible airways and increased bronchial tone. Finally, it is conceivable that airway edema, fibrosis and airway smooth muscle hypertrophy, structural changes in the “old BPD,” continue to play a role.
Consistent with animal studies demonstrating the deleterious effects of malnutrition on lung development 32, we demonstrated higher longitudinal changes in pulmonary function (specifically FVC, FEV0.5, TLC and RV/TLC) in infants with above average somatic growth. On the other hand, measurements of airflow such as FEF75 and FEF25–75 were not different between groups. These data suggest an association between improved nutrition and lung (but perhaps not airway) growth. Few reports have examined the effect of nutrition on pulmonary function in infants with a history of BPD. A previous study showed a significant correlation between undernutrition at two years of age and lung hyperinflation, as defined by a helium dilution FRC of greater than 120% predicted, at 4–8 years of age 33. It should be noted that specific nutrients such as vitamins, amino acids, trace elements, inositol and glutathione also play an important role in lung development and repair, in addition to caloric intake34. Also, measurement of body weight does not distinguish between the accumulation of fat or muscle, a determination which could be made by measurements of body composition.
There are a number of limitations to our study. There is a paucity of normative, control data with which to compare our results, especially the lung volume measurements. Because the RVRTC technique requires sedation, it is particularly difficult to obtain lung function data from normal infants. We were therefore forced to compare our longitudinal results with cross-sectional reference data. Also, on average, our first measurements were made at approximately one year of age, making it difficult to comment on the relationship between growth and lung function in the first year of life, when lung development and somatic growth are most rapid. However, since our two longitudinal measurements showed remarkably consistent reductions in airflow and lung volume, it is unlikely that earlier measurements would have revealed a different pattern. Finally, the observed correlation between weight gain and selected parameters of pulmonary function does not establish a cause and effect relationship between somatic growth and lung growth.
We conclude that, based on longitudinal measurements of pulmonary function using the RVRTC technique, infants with a history of moderate-to-severe BPD show persistent, severe reductions in airflow at low lung volumes. Such reductions may be due to persistent smooth muscle dysfunction and/or hypertrophy, airway fibrosis, impaired airway growth or hypoalveolarization. On the other hand, infants with above average somatic growth showed greater lung growth than their peers. Controlled randomized studies examining the effects of various nutritional regimens on lung function are warranted.
Acknowledgments
These studies were supported by National Institutes of Health grant HL90134 (M.B.H.)
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile L-A, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e141–147.e148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 5.Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, Castile RG, Solway J, Hershenson MB, Goldstein-Filbrun A. Pulmonary function in infants with a history of bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37:236–242. doi: 10.1002/ppul.10424. [DOI] [PubMed] [Google Scholar]
- 6.Tepper RS, Morgan WJ, Cota K, Taussig LM. Expiratory flow limitation in infants with bronchopulmonary dysplasia. J Pediatr. 1986;109:1040–1046. doi: 10.1016/s0022-3476(86)80296-7. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt T, Hehre D, Feller R, Reifenberg L, Bancalari E. Serial determination of pulmonary function in infants with chronic lung disease. J Pediatr. 1987;110:448–456. doi: 10.1016/s0022-3476(87)80516-4. [DOI] [PubMed] [Google Scholar]
- 8.Mallory GB, Jr, Chaney H, Mutich RL, Motoyama EK. Longitudinal changes in lung function during the first three years of premature infants with moderate to severe bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;11:8–14. doi: 10.1002/ppul.1950110103. [DOI] [PubMed] [Google Scholar]
- 9.Iles R, Edmunds AT. Assessment of pulmonary function in resolving chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1997;76:F113–117. doi: 10.1136/fn.76.2.f113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofhuis W, Huysman MWA, van der Wiel EC, Holland WPJ, Hop WCJ, Brinkhorst G, de Jongste JC, Merkus PJFM. Worsening of V’maxFRC in infants with chronic lung disease in the first year of life: A more favorable outcome after high-frequency oscillation ventilation. Am J Respir Crit Care Med. 2002;166:1539–1543. doi: 10.1164/rccm.2202046. [DOI] [PubMed] [Google Scholar]
- 11.Kosch PC, Stark AR. Dynamic maintenance of end-expiratory lung volume in full-term infants. J Appl Physiol. 1984;57:1126–1133. doi: 10.1152/jappl.1984.57.4.1126. [DOI] [PubMed] [Google Scholar]
- 12.Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, Clough JB. Small size at birth and greater postnatal weight gain: Relationships to diminished infant lung function. Am J Respir Crit Care Med. 2004;170:534–540. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- 13.Feher A, Castile R, Kisling J, Angelicchio C, Filbrun D, Flucke R, Tepper R. Flow limitation in normal infants: a new method for forced expiratory maneuvers from raised lung volumes. J Appl Physiol. 1996;80:2019–2025. doi: 10.1152/jappl.1996.80.6.2019. [DOI] [PubMed] [Google Scholar]
- 14.Castile R, Filbrun D, Flucke R, Franklin W, McCoy K. Adult-type pulmonary function tests in infants without respiratory disease. Pediatr Pulmonol. 2000;30:215–227. doi: 10.1002/1099-0496(200009)30:3<215::aid-ppul6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein AB, Castile RG, Davis SD, Filbrun DA, Flucke RL, McCoy KS, Tepper RS. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med. 2001;164:447–454. doi: 10.1164/ajrccm.164.3.2005080. [DOI] [PubMed] [Google Scholar]
- 16.Groh-Wargo S, Thompson M, Cox J, editors. Nutritional care for high risk newborns. Chicago: Precept Press; 2000. [Google Scholar]
- 17.Shimotake TK, Izhar FM, Rumilla K, Jing L, Tan A, Page K, Brasier AR, Schreiber MD, Hershenson MB. IL-1β in tracheal aspirates from premature infants induces airway epithelial cell IL-8 expression via an NF-κB dependent pathway. Pediatr Res. 2004;56:907–913. doi: 10.1203/01.PDR.0000145274.47221.10. [DOI] [PubMed] [Google Scholar]
- 18.Bry K, Whitsett JA, Lappalainen U. IL-1β Disrupts Postnatal Lung Morphogenesis in the Mouse. Am J Respir Cell Mol Biol. 2006;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 20.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: Evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 21.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jonsson B, Li YH, Noack G, Brauner A, Tullus K. Downregulatory cytokines in tracheobronchial aspirate fluid from infants with chronic lung disease of prematurity. Acta Paediatr. 2000;89:1375–1380. doi: 10.1080/080352500300002606. [DOI] [PubMed] [Google Scholar]
- 23.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol. 2004;31:650–656. doi: 10.1165/rcmb.2004-0092OC. [DOI] [PubMed] [Google Scholar]
- 24.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol. 1997;24:22–28. doi: 10.1002/(sici)1099-0496(199707)24:1<22::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB. Autocrine production of TGF-β1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–43. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle LW, Anderson P, Callanan C, Carse E, Casalaz D, Charlton MP, Davis N, Duff J, Ford G, Fraser S, Freezer N, Hayes M, Kaimakamis M, Kelly E, Opie G, Watkins A, Woods H, Yu V Group tVICS. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41:570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 27.Broström EB, Thunqvist P, Adenfelt G, Borling E, Katz-Salamon M. Obstructive lung disease in children with mild to severe BPD. Respir Med. 2010;104:362–70. doi: 10.1016/j.rmed.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173:442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 29.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165:83–87. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 30.Kim DK, Choi SH, Yu J, Yoo Y, Kim BI, Koh YY. Bronchial responsiveness to methacholine and adenosine 5′-monophosphate in preschool children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2006;41:538–543. doi: 10.1002/ppul.20402. [DOI] [PubMed] [Google Scholar]
- 31.Tiddens HAWM, Hofhuis W, Casotti V, Hop WC, Hulsmann AR, Jongste JC. Airway dimensions in bronchopulmonary dysplasia: Implications for airflow obstruction. Pediatr Pulmonol. 2008;43:1206–1213. doi: 10.1002/ppul.20928. [DOI] [PubMed] [Google Scholar]
- 32.Maritz GS, Cock ML, Louey S, Joyce BJ, Albuquerque CA, Harding R. Effects of fetal growth restriction on lung development before and after birth: A morphometric analysis. Pediatr Pulmonol. 2001;32:201–210. doi: 10.1002/ppul.1109. [DOI] [PubMed] [Google Scholar]
- 33.Bott L, Beghin L, Devos P, Pierrat V, Matran R, Gottrand F. Nutritional status at 2 years in former infants with bronchopulmonary dysplasia influences nutrition and pulmonary outcomes during childhood. Pediatr Res. 2006;60:340–344. doi: 10.1203/01.pdr.0000232793.90186.ca. [DOI] [PubMed] [Google Scholar]
- 34.Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:200–208. doi: 10.1053/j.semperi.2006.05.007. [DOI] [PubMed] [Google Scholar]


