Abstract
Rationale
Sarcoidosis is a chronic systemic granulomatous disease of unknown etiology that disproportionately affects black females. Few studies have specifically addressed causes of death in this population.
Objectives
To assess rates and causes of death among women with sarcoidosis in a prospective cohort study of U.S. black women.
Methods
The Black Women’s Health Study is a follow-up study of 59,000 U.S. black women aged 21–69 (median age 38) at entry in 1995. Data on demographic and lifestyle factors and medical conditions, including sarcoidosis, were obtained through biennial questionnaires. Deaths and causes of death from 1995 through 2009 among study subjects were identified from National Death Index data.
Measurements
We assessed mortality rates among women with and without a history of sarcoidosis. Poisson regression models were used to estimate age-adjusted mortality rates. Cox proportional-hazards models were used to estimate hazard ratios for mortality and 95% confidence intervals (95% CI).
Main Results
A total of 121 deaths occurred among 1,192 women with a history of sarcoidosis and 2813 deaths among women without the diagnosis. Mortality was greater at every age among women with sarcoidosis and the overall multivariable-adjusted hazard ratio was 2.44 (95% CI 2.03–2.93, p<0.0001). Of the deaths among women with sarcoidosis, 24.7% were directly attributable to sarcoidosis.
Conclusions
In the Black Women’s Health Study, women with sarcoidosis were more than twice as likely to die as women without the disease, with many of the deaths directly attributable to sarcoidosis. Sarcoidosis is an important cause of premature death among black women with the disease.
Keywords: granuloma, lung disease, sarcoidosis, African Americans, women, mortality
Introduction
Sarcoidosis is a chronic systemic granulomatous disease of unknown etiology1. The clinical presentation of sarcoidosis is highly variable, with the lungs being the most common organ system affected and progressive pulmonary disease the most common cause of morbidity and mortality2–5. For most patients, sarcoidosis has a benign clinical course, but for a subset of patients it is a chronic, life threatening disease with lifetime risk of death from sarcoidosis estimated between 1 to 7%1,6,7. In the United States, black women have a significantly higher incidence of disease and higher mortality compared to men overall and women of other racial groups3,8–12.
The majority of studies that have assessed mortality rates specifically among black women with sarcoidosis have been small and retrospective or relied largely on administrative data 3,8,14–17. The Black Women’s Health Study (BWHS) is a prospective cohort study of 59,000 U.S. black women which began in 1995. The prevalence of sarcoidosis in this cohort as of the end of 2009 was estimated to be 2%, and the annual incidence was 71/100,00013. The size and prospective nature of the BWHS, including follow-up for death, provide a unique opportunity to investigate all-cause and disease-specific mortality among black women with sarcoidosis.
Methods
The human subjects’ protocol for this study was approved by the Boston University Medical Center Institutional Review Board. The BWHS is a prospective cohort study that enrolled 59,000 African-American women aged 21 through 69 years at baseline in 199513. The cohort is followed biennially by postal health questionnaire. Follow-up has averaged over 80% through seven questionnaire cycles.
The present analyses are based on follow-up from 1995 through 2009. Diagnosis of sarcoidosis was ascertained by self-report13. Women who reported sarcoidosis were asked for permission to contact their physicians for information on diagnosis and treatment. Because of the high level of confirmation (96% of the 148 cases for whom physician data were obtained) 13, all self-reported cases have been included in the present analysis except those disconfirmed by medical records.
Deaths were identified by searching the National Death Index (NDI) database for BWHS participants who did not respond to mailed questionnaires or were deemed lost to follow-up18–20 ; reports of death were also obtained from next of kin, the Social Security Death Master File, and the U.S. Postal Service. Immediate, underlying, and other significant causes of death were obtained from either the NDI or a state-issued death certificate and coded according to the International Classification of Diseases, Ninth Revision (ICD-9). Deaths were classified as directly attributable to sarcoidosis if sarcoidosis was listed as either the immediate or underlying cause of death, and further classified as due to respiratory failure if respiratory failure, respiratory arrest or pulmonary fibrosis was listed as the immediate or underlying cause of death. Information on age, smoking history, alcohol intake, weight, and geographical region was obtained at baseline in 1995 and on each follow-up questionnaire; height was collected in 1995, and attained educational level was assessed in 1995 and in 2003. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Pack-years of smoking were calculated by multiplying the duration of smoking in years by the number of packs of cigarettes smoked per day.
Person-years were measured from the beginning of follow-up in 1995 until the date of death or the end of follow-up (December 31, 2009), whichever occurred first. We assessed mortality among women with and without a history of sarcoidosis. All analyses were performed using SAS statistical software, version 9.321. Chi-square tests were used to compare proportions between groups. Poisson regression models were used to estimate overall and age-specific mortality rates (per 10,000 person-years) and 95% confidence intervals (95% CI). Overall mortality rates were adjusted for age using the 50–54 year age-group of the BWHS as the reference (modal category for deaths). Cox proportional-hazards models, stratified according to age in 1-year intervals and 2-year questionnaire cycles, were used to estimate mortality ratios (95% CI); our multivariable model included terms for education (≤12, 13–15, ≥16 years), packyears of smoking (none, <5, 5–14, 15–24, ≥25), alcohol consumption (none, <1, 1–6, ≥ 7 drinks/week), geographical region (Northeast, South, Midwest, West), and BMI (<25, 25–29, ≥30). With the exception of education, all covariates were time-varying.
RESULTS
The BWHS cohort was a young cohort at baseline (median age, 38): 22% of participants were 21–29 years of age, 33% were 30–39, 28% were 40–49, and 17% were 50–69. A total of 686 women reported sarcoidosis at the time of enrollment and an additional 506 women reported a diagnosis of sarcoidosis during the follow up period. Of 2,934 deaths ascertained, 121 deaths occurred among women with sarcoidosis and 2,813 deaths among women without sarcoidosis. A total of 7 sarcoidosis cases were identified based on death certificate data alone. As shown in table 1, decedents with sarcoidosis were similar to decedents without sarcoidosis in terms of age, educational level, BMI, alcoholic beverages consumed per week and geographic area of residence. Decedents without sarcoidosis were more likely to be heavy smokers.
Table 1.
Baseline characteristics of deaths among women with and without sarcoidosis in the BWHS
| Deaths | |||
|---|---|---|---|
| Women with sarcoidosis (n=121) | Women without sarcoidosis (n=2813) | P value | |
| Age (years), % | |||
| <30 | 3 | 5 | |
| 30–39 | 19 | 17 | |
| 40–49 | 33 | 29 | |
| 50–59 | 30 | 26 | |
| ≥60 | 15 | 23 | 0.20 |
| Education (years), % | |||
| ≤12 | 25 | 32 | |
| 13–15 | 40 | 35 | |
| ≥16 | 35 | 33 | 0.27 |
| Geographical Region of Residence, % | |||
| Northeast | 22 | 29 | |
| South | 33 | 26 | |
| Midwest | 26 | 26 | |
| West | 19 | 19 | 0.30 |
| Body Mass Index (kg/m2), % | |||
| <25 | 26 | 30 | |
| 25–29 | 30 | 31 | |
| ≥30 | 44 | 39 | 0.56 |
| Packyears of Smoking (years), % | |||
| Never smoker | 42 | 44 | |
| <5 | 21 | 12 | |
| 5–14 | 21 | 20 | |
| 15–24 | 8 | 9 | |
| ≥25 | 8 | 15 | 0.04 |
| Alcohol Consumption (drinks/week), % | |||
| Never, <1 | 76 | 74 | |
| 1–6 | 16 | 18 | |
| ≥7 | 8 | 8 | 0.83 |
The age-adjusted mortality for women with sarcoidosis was 94 deaths per 10,000 person years (95% CI, 77/10,000 – 114/10,000) compared to 43 deaths per 10,000 person years (95% CI, 39/10,000 – 47/10,000) in women without sarcoidosis. Relative to women without sarcoidosis, the age-adjusted mortality ratio was 2.22 (95% CI, 1.85–2.67) for women with sarcoidosis; the multivariable estimate was 2.44 (95% CI, 2.03–2.93).
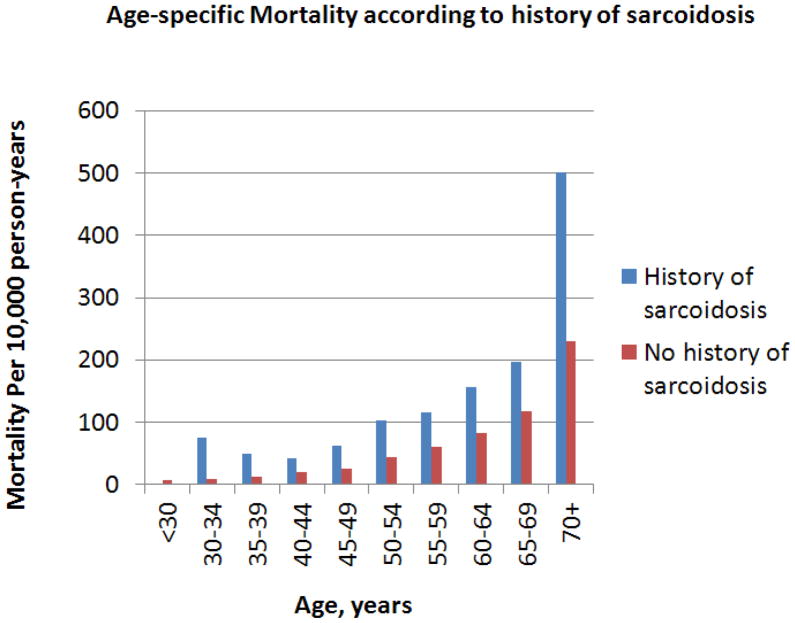
Figure 1 shows age-specific mortality according to sarcoidosis status. Women with sarcoidosis had higher mortality compared to women without sarcoidosis in every age group.
Figure 1.
Age-specific Mortality according to history of sarcoidosis
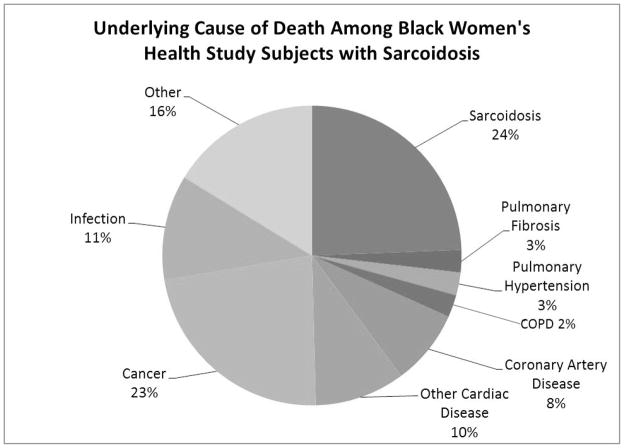
Figure 2 shows the underlying causes of death among decedents with sarcoidosis. Twenty-five percent (n=30) of the deaths were directly attributable to the disease; that is, sarcoidosis was listed as either the immediate or underlying cause of death on the death certificate. For an additional 13% (n=16) of decedents, sarcoidosis was listed as a significant cause of death on the death certificate but not as the immediate or underlying cause of death. The remaining 62% of decedents with sarcoidosis had no mention of the disease on the death certificate.
Figure 2.
Underlying Cause of Death Among Black Women’s Health Study Subjects with Sarcoidosis
Of the decedents with sarcoidosis whose deaths were not directly attributable to the disease, 5% (n=6) had pulmonary hypertension or pulmonary fibrosis listed as the underlying cause of death. Of deaths considered directly attributable to sarcoidosis, 43% (13/30) were classified as being secondary to respiratory failure.
DISCUSSION
The present study, to the best of our knowledge, is the largest prospective epidemiologic study to date to evaluate sarcoidosis-related mortality in African American women. In this relatively young cohort, mortality among women with sarcoidosis was higher than that in women without sarcoidosis across all age groups. The overall adjusted mortality ratio among African American women with sarcoidosis was 2.44, and 25% of the deaths were directly attributable to sarcoidosis. Thus, sarcoidosis appears to be an important cause of premature mortality in this population.
A recent large population-based study used data from the National Center for Health Statistics to evaluate trends in sarcoidosis-related mortality8. Based on death certificates of U.S. decedents with sarcoidosis listed as an associated cause of death, Swigris et al. found that age-adjusted sarcoidosis-related mortality increased by 50.5% in women from 1988 to 2008, with the largest absolute increase in sarcoidosis-related mortality occurring in non-Hispanic black females (from 18.85 to 27.58 deaths per million). Whether this increase is real or an artifact due to increased detection is difficult to determine as incidence rates across this time period are not readily available.
In the BWHS cohort, the most common cause of death among decedents with sarcoidosis was the disease itself, accounting for 25% of deaths. This is a lower estimate of sarcoidosis-attributable mortality than that published by Swigris et al., who reported that 58.8% of decedents with sarcoidosis died as a result of the disease8. Swigris and colleagues did not have access to decedents’ clinical records and relied exclusively on death certificate data to identify individuals with a history of sarcoidosis. In contrast, the 121 BWHS decedents with a history of sarcoidosis were identified using a combination of questionnaire, medical records, and death certificate data; only 7 sarcoidosis cases were identified exclusively from death certificate data. When our analysis was limited to the 45 BWHS decedents for whom sarcoidosis was listed on the death certificate, our estimate of sarcoidosis-attributable mortality increased to 65%, similar to the estimate reported by Swigris et al8.
Prior studies suggest that respiratory system involvement of sarcoidosis is the most common cause of sarcoidosis-specific mortality in the United States3,4. Among the 25% of BWHS decedents whose deaths were considered to be directly attributable to sarcoidosis, 43% of deaths were classified as due to respiratory failure. An additional 13% of death certificates of BWHS decedents whose deaths were directly attributed to sarcoidosis mentioned cardiomyopathy or congestive heart failure as a significant cause of death. An association between sarcoidosis and pulmonary embolism has recently been suggested22,23; only two death certificates in BWHS decedents with sarcoidosis mentioned pulmonary embolism.
Strengths of the current study include its large size, prospective design, and high follow-up rates. Important potential confounding factors including age, geographic region, educational attainment, smoking, alcohol consumption, and body mass index, were taken into account in the analysis. Study participants are representative of most US black women and are residents of all regions of the United States. However, literacy is required for participation in studies using detailed, mailed questionnaires. Thus women participating in the BWHS underrepresent the 15% of black women in the United States who did not graduate from high school, which may limit the generalizability of the results24.
Examination of subjects in studies of the size of the BWHS is infeasible and prohibitively expensive. Therefore, a diagnosis of sarcoidosis for the purpose of the present study relied on participant self-report. As previously described, an examination of a subset of these self-diagnoses confirmed the diagnosis in 96% of cases13. However, it is possible that some study participants had undiagnosed sarcoidosis and that others were mis-diagnosed, which could have affected our mortality estimates. It is also possible that BWHS cohort under-represents the most severe levels of sarcoidosis disease activity. Specifically, there is very low documentation of cardiac sarcoidosis within this cohort.
Another limitation is the use of death certificates to determine cause of death among BWHS decedents. Death certificates are well-known to provide limited clinical information and there are inaccuracies in the diagnoses provided. Cause of deaths from death certificates may in general underrepresent deaths from sarcoidosis.
In summary, in the BWHS cohort of 59,000 black women in the United States, the relative risk of death among participants with sarcoidosis was twice that of participants without sarcoidosis, with 25% of deaths directly attributable to sarcoidosis. These findings highlight the importance of sarcoidosis as a cause of premature death among black women with the disease.
Acknowledgments
This work was supported by grant K01HL088709 from the National Heart, Lung, and Blood Institute, and grant CA058420 from the Division of Cancer Control and Population Science, National Cancer Institute (http://www.cancercontrol.cancer.gov).
The authors thank Dr. Lauren Wise for her helpful comments.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]; Bresnitz EA, Strom BL. Epidemiology of sarcoidosis. Epidemiol Rev. 1983;5:124–156. doi: 10.1093/oxfordjournals.epirev.a036255. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 3.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100:423–427. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 4.Huang CT, Heurich AE, Sutton AL, Lyons HA. Mortality in sarcoidosis. A changing pattern of the causes of death. Eur J Respir Dis. 1981;62:231–238. [PubMed] [Google Scholar]
- 5.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:154–158. [PubMed] [Google Scholar]
- 6.Reich JM. Mortality of intrathoracic sarcoidosis in referral vs population-based settings: influence of stage, ethnicity, and corticosteroid therapy. Chest. 2002;121:32–39. doi: 10.1378/chest.121.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 10.Rybicki BA, Maliarik MJ, Major M, Popovich J, Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect. 1998;13:166. [PubMed] [Google Scholar]
- 11.Israel HL, Gottlieb JE, Peters SP. The importance of ethnicity in the diagnosis and prognosis of sarcoidosis. Chest. 1997;111:838–840. doi: 10.1378/chest.111.4.838. [DOI] [PubMed] [Google Scholar]
- 12.Israel HL, Karlin P, Menduke H, DeLisser OG. Factors affecting outcome of sarcoidosis. Influence of race, extrathoracic involvement, and initial radiologic lung lesions. Ann N Y Acad Sci. 1986;465:609–618. doi: 10.1111/j.1749-6632.1986.tb18537.x. [DOI] [PubMed] [Google Scholar]
- 13.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139:144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reich JM, Johnson RE. Course and prognosis of sarcoidosis in a nonreferral setting. Analysis of 86 patients observed for 10 years. Am J Med. 1985;78:61–67. doi: 10.1016/0002-9343(85)90463-2. [DOI] [PubMed] [Google Scholar]
- 15.Reich JM. Mortality of intrathoracic sarcoidosis in referral vs population-based settings: influence of stage, ethnicity, and corticosteroid therapy. Chest. 2002;121:32–39. doi: 10.1378/chest.121.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. 2003;124:922–928. [PubMed] [Google Scholar]
- 17.Shorr AF, Helman DL, Davies DB, Nathan SD. Sarcoidosis, race, and short-term outcomes following lung transplantation. Chest. 2004;125:990–996. doi: 10.1378/chest.125.3.990. [DOI] [PubMed] [Google Scholar]
- 18.Albert MA, Cozier Y, Ridker PM, Palmer JR, Glynn RJ, Rose L, Halevy N, Rosenberg L. Perceptions of race/ethnic discrimination in relation to mortality among Black women: results from the Black Women’s Health Study. Arch Intern Med. 2010;170:896–904. doi: 10.1001/archinternmed.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boggs DA, Rosenberg L, Cozier YC, Wise LA, Coogan PF, Ruiz-Narvaez EA, Palmer JR. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365:901–908. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. National Death Index user’s manual. Hyattsville: 2000. [Google Scholar]
- 21.SAS Institute, Inc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute, Inc; 2008. [Google Scholar]
- 22.Crawshaw AP, Wotton CJ, Yeates DG, Goldacre MJ, Ho LP. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax. 2011;66:447–448. doi: 10.1136/thx.2010.134429. [DOI] [PubMed] [Google Scholar]
- 23.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon JJ, Sprunger D, Brown KK. Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007. Chest. 2011;140:1261–1266. doi: 10.1378/chest.11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Commerce. Educational Attainment in the United States. Washington, DC: Department of Commerce; 1998. [Google Scholar]