Abstract
Two new 9,11-secosteroids, 22α-acetoxy-24-methylene-3β,6α,11-trihydroxy-9,11-seco-cholest-7-en-9-one (1) and 11-acetoxy-24-methylene-1β,3β,6α-trihydroxy-9,11-seco-cholest-7-en-9-one (2), as well as two known norcembranoids, 5-epi-sinuleptolide (3) and sinuleptolide (4), were isolated from the soft coral Sinularia nanolobata. The structures of these metabolites were elucidated on the basis of extensive spectroscopic analysis. The anti-HCMV (human cytomegalovirus) activity of 1–4 and its cytotoxicity against selected cell lines were evaluated.
Keywords: soft coral, Sinularia nanolobata, secosteroid, cytotoxicity
1. Introduction
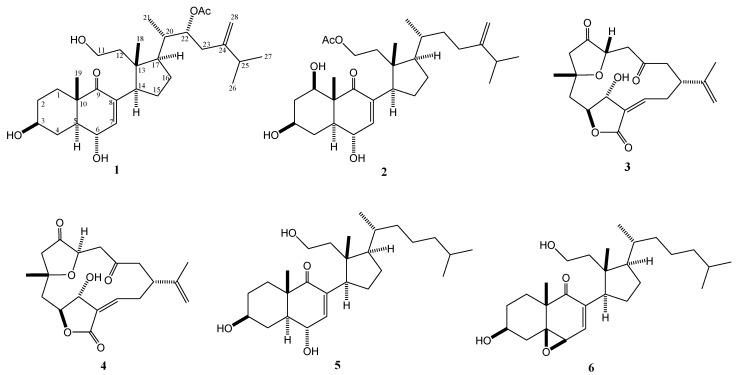
Soft corals have been proven to be a rich source of 9,11-secosterols [1,2,3,4,5,6,7,8,9,10,11,12,13,14] and C-4 norcembranoids [15,16,17,18,19,20,21,22,23,24]. 9,11-Secosteroids were found to possess cytotoxic [2,3,5,6,8,10,11,12,13] and anti-inflammatory activities [13]. C-4 Norcembranoids were found to possess cytotoxic [18,19,20,25], anti-inflammatory [23,24], and antiviral activities [23]. As part of the continuing search for bioactive metabolites from marine invertebrates, we explored the secondary metabolites of the soft coral Sinularia nanolobata (Verseveldt, 1977) which was collected from Xiao-Liuqiu Island, Pingtung County, Taiwan. Chromatographic separation of the EtOAc extract of the soft coral resulted in the isolation of new secosteroids 1 and 2, along with two known norcembranoids, 5-epi-sinuleptolide (3) and sinuleptolide (4) [21] (Figure 1). The structures of these metabolites were established by extensive spectroscopic analysis. The anti-HCMV (human cytomegalovirus) activity of 1–4 and cytotoxicity against P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), and A-549 (human lung carcinoma) cancer cell lines were evaluated in vitro.
Figure 1.
Structures of Metabolites 1–6.
2. Results and Discussion
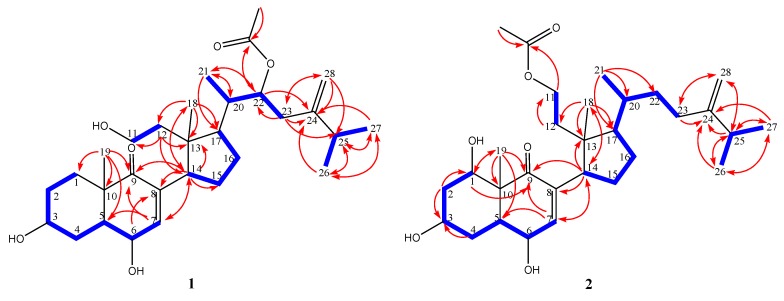
Metabolite 1 was isolated as colorless needles. Its molecular formula, C30H48O6, was established by HRESIMS (m/z 527.3351, [M + Na]+), implying seven degrees of unsaturation. The presence of hydroxyl groups was suggested by a strong absorption band at 3459 cm−1 in the IR spectrum. The 13C NMR and DEPT (Table 1) spectroscopic data showed signals of six methyls, eight sp3 methylenes (including one oxymethylene), one sp2 methylene, eight sp3 methines (including three oxymethines), one sp2 methine, two sp3 and four sp2 quaternary carbons. The quaternary carbon signal at δC 205.4 combined with the chemical shifts of H3-18 (δ 0.67, s), H3-19 (δ 1.14, s), H3-21 (δ 1.02, d, J = 6.8 Hz), H3-26 (δ 1.03, d, J = 6.8 Hz), H3-27 (δ 1.03, d, J = 6.8 Hz), H-3 (δ 3.61, m), H-6 (δ 4.30, dd, J = 10.0, 1.6 Hz), H-7 (δ 6.61, d, J = 1.6 Hz), and H2-11 (each 1H, δ 3.70, m; δ 3.93, m) were found to be similar to those of 3β,6α,11-trihydroxy-9,11-seco-5α-cholest-7-en-9-one (5) [4]. Moreover, the signals appearing at δC 170.7 (qC), 152.2 (qC), 108.8 (CH2), 74.7 (CH), and 21.2 (CH3) indicated the presence of an acetoxy and an exomethylene in 1. This was further supported by the 1H NMR signals of acetoxy and exomethylene protons at δH 1.99 (3H, s) and 4.71 and 4.80 (each 1H, s), respectively. From the COSY spectrum measured in CDCl3, it was possible to establish five proton sequences from H-1 to H-7, H2-11 to H2-12, H-14 to H-17, H-21 to H2-23 through H-20, and H-25 to H3-26 and H3-27 (Figure 2). Key HMBC correlations of H2-4 to C-3 and C-5; H-7 to C-5, C-9 and C-14; H2-12 to C-13 and C-14; H-14 to C-7, C-8, C-9, C-13, C-15 and C-18; H3-18 to C-12, C-13, C-14, and C-17; H3-19 to C-1, C-5, C-9, and C-10; H-20 to C-21; H3-21 to C-17, C-20 and C-22; H-22 to C-24; H2-23 to C-25; H3-26 to C-24, C-25, and C-27; H3-27 to C-24, C-25, and C-26; H2-28 to C-23, C-24 and C-25; H3-OAc to OAc-22 permitted the establishment of the secosterol-type skeleton of 1.
Table 1.
1H and 13C NMR Spectroscopic Data for compounds 1 and 2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH a | δC b | δH a | δC b | |
| 1 | 1.48 m; 1.90 m | 31.8, CH2 d | 3.87 dd (12.0, 4.4) | 70.7, CH |
| 2 | 1.48 m; 1.94 m | 30.5, CH2 | 1.52 m; 2.14 m | 37.8, CH2 |
| 3 | 3.61 m | 69.9, CH | 3.71 m | 67.4, CH |
| 4 | 1.45 m; 2.31 m | 32.8, CH2 | 1.43 m; 2.29 m | 32.2, CH2 |
| 5 | 1.80 m | 48.5, CH | 1.70 m | 46.4, CH |
| 6 | 4.30 dd (10.0, 1.6) c | 69.4, CH | 4.38 dd (9.2, 2.0) | 69.1, CH |
| 7 | 6.61 d (1.6) | 147.8, CH | 6.58 d (1.6) | 147.4, CH |
| 8 | 136.5, qC | 136.8, qC | ||
| 9 | 205.4, qC | 206.5, qC | ||
| 10 | 44.9, qC | 49.0, qC | ||
| 11 | 3.70 m; 3.93 m | 59.3, CH2 | 4.16 td (10.4, 6.4); 4.23 td (10.4, 5.2) | 61.2, CH2 |
| 12 | 1.04 m; 1.64 m | 40.7, CH2 | 1.15 m; 1.70 m | 36.8, CH2 |
| 13 | 46.3, qC | 46.0, qC | ||
| 14 | 3.47 dd (9.6, 8.8) | 42.2, CH | 3.25 dd (10.8, 9.6) | 42.2, CH |
| 15 | 1.62 m | 26.4, CH2 | 1.62 m | 26.4, CH2 |
| 16 | 1.65 m; 1.91 m | 27.0, CH2 | 1.45 m; 1.87 m | 26.2, CH2 |
| 17 | 1.75 m | 45.9, CH | 1.67 m | 50.1, CH |
| 18 | 0.67 s | 16.9, CH3 | 0.69 s | 17.0, CH3 |
| 19 | 1.14 s | 16.2, CH3 | 1.17 s | 9.8, CH3 |
| 20 | 1.78 m | 39.8, CH | 1.46 m | 34.7, CH |
| 21 | 1.02 d (6.8) | 11.8, CH3 | 1.02 d (6.4) | 18.8, CH3 |
| 22 | 5.12 m | 74.7, CH | 1.18 m; 1.56 m | 34.0, CH2 |
| 23 | 2.17 m | 32.6, CH2 | 1.89 m; 2.11 m | 31.6, CH2 |
| 24 | 152.2, qC | 156.5, qC | ||
| 25 | 2.20 m | 33.4, CH | 2.21 m | 33.8, CH |
| 26 | 1.03 d (6.8) | 21.6, CH3 | 1.02 d (6.8) | 21.8, CH3 |
| 27 | 1.03 d (6.8) | 22.1, CH3 | 1.02 d (6.8) | 22.0, CH3 |
| 28 | 4.71 s; 4.80 s | 108.8, CH2 | 4.66 s; 4.73 s | 106.2, CH2 |
| OAc | 1.99 s
|
21.2, CH3
170.7, qC |
2.01 s | 21.1 CH3
171.2, qC |
a Spectra recorded at 400 MHz in CDCl3; b Spectra recorded at 100 MHz in CDCl3; c J values (in Hz) are in parentheses; d Carbon types are deduced by HSQC and DEPT experiments.
Figure 2.
Selected 1H−1H COSY (▬) and HMBC (→) correlations of 1 and 2.
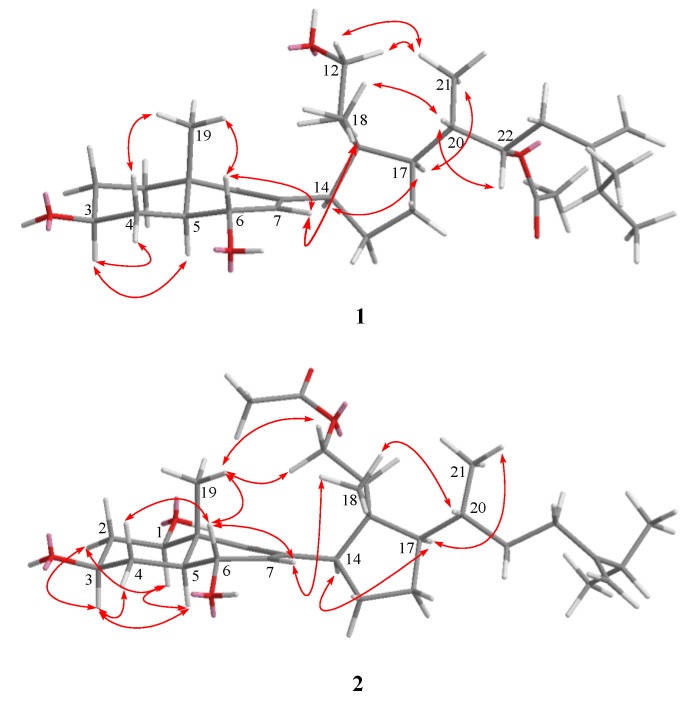
The relative configurations of eight chiral centers at C-3, C-5, C-6, C-10, C-13, C-14, C-17, and C-20 in 1 were found to be the same as those of 3β,6α,11-trihydroxy-9,11-seco-5α-cholest-7-ene-9-one [4] (Figure 2). Key NOE correlations for 1 showed interactions between H-20/H-22. Thus, OAc-22 should be α-oriented. The above data established the structure of compound 1 as 22R-acetoxy-3β,6α,11-trihydroxy-24-methylene-9,11-secocholestan-9-one.
Metabolite 2 possessed the same molecular formula (C30H48O6) as that of 1, as revealed from HRESIMS. The 13C NMR spectral data of 2 were found to be close to those of 1, except for the signals due to C-1, C-11, and C-22. COSY correlations from H-1 to H-2, H2-11 to H2-12, and H-20 to H2-22. as well as HMBC correlations from H-1 to C-10, C-9, from H2-11 to 11-OAc, and from Me-21 to C-17, C-20, C-22 confirmed the presence of a hydroxyl at C-1, an acetoxyl at C-11, and the absence of the acetoxyl at C-22 (Figure 2). Furthermore, the configuration of C-1 was deduced from the NOE correlations of H-1/H-5 and H-3/H-5 (Figure 3). Thus, the structure of compound 2 was established as 11-acetoxy-24-methylene-1β,3β,6α-trihydroxy-9,11-seco-cholest-7en-9-one.
Figure 3.
Key NOESY Correlations for 1 and 2.
Cytotoxicity of compounds 1–4 against the proliferation of a limited panel of cancer cell lines, including P-388, A549, and HT-29, were evaluated (Table 2). Metabolites 1–3 displayed weak cytotoxicity against P-388, with IC50 of 10.2, 27.8, and 15.7 μg/mL, respectively. Compounds 1–4 was also examined for antiviral activity against human cytomegalovirus (HCMV) using a human embryonic lung (HEL) cell line. Compounds 4 showed anti-HCMV activity with ED50 of 1.92 μg/mL (Ganciclovir showed anti-HCMV activity with ED50 of 0.12 μg/mL).
Table 2.
Cytotoxicity and anti- human cytomegalovirus (HCMV) activity of 1–4.
| Compounds | IC50 (μg/mL) | Anti-HCMV | |||
|---|---|---|---|---|---|
| A549 | HT-29 | P-388 | HEL | ||
| 1 | >50 | >50 | 10.2 | >50 | >50 |
| 2 | >50 | >50 | 27.8 | >50 | >50 |
| 3 | >50 | >50 | 15.7 | >50 | >50 |
| 4 | >50 | >50 | >50 | >50 | 1.92 |
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a JASCO P1020 digital polarimeter. IR spectra were recorded on a JASCO FT/IR4100 infrared spectrophotometer. The NMR spectra were recorded on a Varian MR 400 FT-NMR at 400 MHz for 1H and 100 MHz for 13C, in CDCl3 using solvent peak as internal standard. LRMS and HRMS were obtained by ESI on a Bruker APEX ΙΙ mass spectrometer. Silica gel 60 (Merck, Darmstadt, Germany, 230–400 mesh) and LiChroprep RP-18 (Merck, 40–63 μm) were used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254s plates (Merck) were used for TLC analysis. High-performance liquid chromatography was carried out using a Hitachi L-7100 pump equipped with a Hitachi L-7400 UV detector at 220 nm together with a semi-preparative reversed-phase column (Merck, Hibar LiChrospher RP-18e, 5 μm, 250 × 25 mm).
3.2. Animal Material
The octocoral S. nanolobata was collected by hand using scuba at the Xiao Liuqiu, Pingtung County, Taiwan, in November 2011, at a depth of 6 m. This soft coral was identified by Prof. Chang-Fong Dai, Institute of Oceanography, National Taiwan University. A voucher specimen (SL-10) was deposited in the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The frozen soft coral (1.5 kg) was chopped into small pieces and extracted with EtOAc (3L × 3) in a percolator at room temperature. The EtOAc extract of S. nanolobata was concentrated to a dark brown gum (12.3 g). The EtOAc residue was subjected to Si 60 CC using n-hexane-EtOAc-MeOH mixtures of increasing polarity for elution. Fraction 15~21, eluted with n-hexane-EtOAc (1:10) to EtOAc-MeOH (2:1), was further subjected to Si 60 CC EtOAc-MeOH (5:1) to give four subfractions. A subfraction 15~21-1, was purified by reverse-phase HPLC (MeOH-H2O, 55:45) to afford 3 (50.0 mg) and 4 (35.0 mg). A subfraction 15~21-4, was purified by reverse-phase HPLC (MeOH-H2O, 85:15) to afford 1 (2.5 mg) and 2 (3.1 mg).
22α-acetoxy-24-methylene-3β,6α,11-trihydroxy-9,11-seco-cholest-7-en-9-one (1): White powder; [α]25D = +54 (c 0.6, CHCl3); IR (KBr) νmax 3459, 2956, 1733, 1031, 1009 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 527 [M + Na]+; HRESIMS m/z 527.3351 (calcd for C30H48O6Na, 527.3348).
11-acetoxy-24-methylene-1β,3β,6α-trihydroxy-9,11-seco-cholest-7-en-9-one (2): White powder; [α]25D = +16 (c 0.8, CHCl3); IR (KBr) νmax 3444, 2925, 1741, 1260, 1036 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 527 [M + Na]+; HRESIMS m/z 527.3345 (calcd for C30H48O6Na, 527.3348).
3.4. Cytotoxicity Testing
Cytotoxicity was determined on P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), and A-549 (human lung epithelial carcinoma) tumor cells using a modification of the MTT colorimetric method according to a previously described procedure [26,27]. The provision of the P-388 cell line was supported by J.M. Pezzuto, formerly of the Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago. HT-29 and A-549 cell lines were purchased from the American Type Culture Collection. To measure the cytotoxic activities of tested compounds, five concentrations with three replications were performed on each cell line. Mithramycin was used as a positive control.
3.5. Anti-HCMV Assay
To determine the effects of natural products upon HCMV cytopathic effect (CPE), confluent human embryonic lung (HEL) cells grown in 24-well plates were incubated for 1 h in the presence or absence of various concentrations of tested natural products with three replications. Ganciclovir was used as a positive control. Then, cells were infected with HCMV at an input of 1000 pfu (plaque forming units) per well of a 24-well dish. Antiviral activity was expressed as IC50 (50% inhibitory concentration), or compound concentration required to reduce virus induced CPE by 50% after 7 days as compared with the untreated control. To monitor the cell growth upon treating with natural products, an MTT-colorimetric assay was employed [28].
4. Conclusions
In the previous studies, 5-epi-sinuleptolide (3) and sinuleptolide (4) both showed inhibition of iNOS protein by LPS stimulation [23], TNF-α production in a dose-dependent manner [24], and nitric oxide (NO) production [24]. 5-Epi-sinuleptolide (3) inhibited human skin cancer cells growth [25]. Sinuleptolide (4) exerted antiviral activity against HCMV (human cytomegalovirus) cells [23]. 9,11-Secosteroid 6 with epoxide at C-5 and C-6 exhibited cytotoxicity against HT-29 cells [29]. This investigation of soft coral S. nanolobata collected at Xiao-Liuqiu Island (Pingtung County, Taiwan) has led to the isolation of two new 9,11-secosteroids (1 and 2) and two known compounds, 5-epi-sinuleptolide (3) and sinuleptolide (4). Metabolites 1–3 displayed weak cytotoxicity and selectivity against P-388, with IC50 of 10.2, 27.8, and 15.7 μg/mL, respectively. Metabolite 4 exhibited antiviral activity against HCMV with ED50 of 1.92 μg/mL.
Acknowledgements
This research was financially supported by grants from the National Science Council (NSC102-2320-B-110-003-MY3) and NSYSUKMU Joint Project (NSYSUKMU 2013-P018) awarded to C.-Y. Duh.
Supplementary Files
Supplementary Materials (PDF, 610 KB)
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kazlauskas R., Murphy P.T., Ravi B.N., Sanders R.L., Wells R. Spermidine derivatives and 9,11-secosteroids from a soft coral (Sinularia sp.) Aust. J. Chem. 1982;35:69–75. doi: 10.1071/CH9820069. [DOI] [Google Scholar]
- 2.Koljak R., Pehk T., Järving I., Liiv M., Lopp A., Varvas K., Vahemets A., Lille Ű., Samel N. New antiproliferative 9,11-secosterol from soft coral Gersemia fruticosa. Tetrahedron Lett. 1993;34:1985–1986. doi: 10.1016/S0040-4039(00)91981-6. [DOI] [Google Scholar]
- 3.Lopp A., Pihlak A., Paves H., Samuel K., Koljak R., Samel N. The effect of 9,11-secosterol, a newly discovered compound from the soft coral Gersemia-fruticosa, on the growth and cell-cycle progression of various tumor-cells in culture. Steroids. 1994;59:274–281. doi: 10.1016/0039-128X(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 4.Aknin M., Costantino V., Mangoni A., Fattorusso E., Gaydou E.M. New 9,11-secosterols from gorgonia Subergorgia suberosa of the Indian Ocean. Steroids. 1998;63:575–578. doi: 10.1016/S0039-128X(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.Koljak R., Lopp A., Pehk T., Varvas K., Műűrisepp A.-M., Järving I., Samel N. New cytotoxic sterols from the soft coral Gersemia fruticosa. Tetrahedron. 1998;54:179–186. doi: 10.1016/S0040-4020(97)10268-X. [DOI] [Google Scholar]
- 6.Morris L.A., Christie E.M., Jaspars M., van Ofwegen L.P. A bioactive secosterol with an unusual A- and B-ring oxygenation pattern isolated from an Indonesian soft coral Lobophytum sp. J. Nat. Prod. 1998;61:538–541. doi: 10.1021/np9705118. [DOI] [PubMed] [Google Scholar]
- 7.van Altena I.A., Butler A.J., Dunne S.J. A new cyclized 9,11-secosterol enol-ether from the Australian sponge Euryspongia arenaria. J. Nat. Prod. 1999;62:1154–1157. doi: 10.1021/np9805591. [DOI] [PubMed] [Google Scholar]
- 8.Naz S., Kerr R.G., Narayanan R. New antiproliferative epoxysecosterols from Pseudopterogorgia americana. Tetrahedron Lett. 2000;41:6035–6040. doi: 10.1016/S0040-4039(00)01015-7. [DOI] [Google Scholar]
- 9.Anta C., González N., Rodríguez J., Jiménez C. A new secosterol from the Indonesian octocoral Pachyclavularia vioacea. J. Nat. Prod. 2002;65:1357–1359. doi: 10.1021/np010592e. [DOI] [PubMed] [Google Scholar]
- 10.Su J.-H., Tseng Y.-J., Huang H.-H., Ahmed A.F., Lu C.-K., Wu Y.-C., Sheu J.-H. 9,11-Secosterols from the soft corals Sinularia lochmodes and Sinularia leptoclados. J. Nat. Prod. 2006;69:850–852. doi: 10.1021/np060031t. [DOI] [PubMed] [Google Scholar]
- 11.Cheng S.-Y., Chen H.-P., Wang S.-K., Duh C.-Y. Three new 9,11-secosterols from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2011;84:648–652. doi: 10.1246/bcsj.20110046. [DOI] [Google Scholar]
- 12.Chen B.-W., Chang S.-M., Huang C.-Y., Su J.-H., Wen Z.-H., Wu Y.-C., Sheu J.-H. Hirsutosterols A–G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsute. Org. Biomol. Chem. 2011;9:3272–3278. doi: 10.1039/c1ob05106g. [DOI] [PubMed] [Google Scholar]
- 13.Huang C.-Y., Su J.-H., Duh C.-Y., Chen B.-W., Wen Z.-H., Kuo Y.-H., Sheu J.-H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012;22:4373–4376. doi: 10.1016/j.bmcl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Sica D., Musumeci D. Secosteroids of marine origin. Steroids. 2004;69:743–756. doi: 10.1016/j.steroids.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bowden B.F., Coll J.C., Mitchell S.J., Mulder J., Stokie G.J. Studies of Australian soft corals. IX a novel nor-diterpene from the soft coral Sinularia leptoclados. Aust. J. Chem. 1978;31:2049–2056. doi: 10.1071/CH9782049. [DOI] [Google Scholar]
- 16.Sato A., Fenical W., Zheng Q.-T., Clardy J. Norcembrene diterpenoids from Pacific soft-corals of the genus sinularia (Alcyonacea; octocorallia) Tetrahedron. 1985;41:4303–4308. doi: 10.1016/S0040-4020(01)97201-1. [DOI] [Google Scholar]
- 17.Shoji N., Umeyama A., Arihara S. A novel norditerpenoid from the Okinawan soft coral Sinularia sp. J. Nat. Prod. 1993;56:1651–1653. doi: 10.1021/np50099a035. [DOI] [Google Scholar]
- 18.Sheu J.-H., Ahmed A.F., Shiue R.-T., Dai C.-F., Kuo Y.-H. Scabrolides A–D, four new norditerpenoids isolation from the soft coral Sinularia scabra. J. Nat. Prod. 2002;65:1904–1908. doi: 10.1021/np020280r. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A.F., Su J.-H., Kuo Y.-H., Sheu J.-H. Scabrolides E–G, three new norditerpenoids from the soft coral Sinularia scabra. J. Nat. Prod. 2004;67:2079–2082. doi: 10.1021/np040112u. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed A.F., Shiue R.-T., Wang G.-H., Dai C.-F., Kuo Y.-H., Sheu J.-H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron. 2003;59:7337–7344. doi: 10.1016/S0040-4020(03)01138-4. [DOI] [Google Scholar]
- 21.Tseng Y.-J., Ahmed A.F., Dai C.-F., Chiang M.Y., Sheu J.-H. Sinulochmodins A–C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005;7:3813–3816. doi: 10.1021/ol051513j. [DOI] [PubMed] [Google Scholar]
- 22.Tseng Y.-J., Ahmed A.F., Hsu C.-H., Su J.-H., Dai C.-F., Sheu J.-H. New norcembranoids from the soft coral Sinularia lochmodes. J. Chin. Chem. Soc. 2007;54:1041–1044. [Google Scholar]
- 23.Cheng S.-Y., Chuang C.-T., Wen Z.-H., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Duh C.-Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010;18:3379–3386. doi: 10.1016/j.bmc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Takaki H., Koganemaru R., Iwakawa Y., Higuchi R., Miyamoto T. Inhibitory effect of norditerpenes on LPS-Induced TNF-α production from the Okinawan soft coral, Sinularia sp. Biol. Pharm. Bull. 2003;26:380–382. doi: 10.1248/bpb.26.380. [DOI] [PubMed] [Google Scholar]
- 25.Liang C.-H., Wang G.-H., Chou T.-H., Wang S.-H., Lin R.-J., Chan L.-P., So E.-C., Sheu J.-H. 5-epi-Sinuleptolide induces cell cycle arrest and apoptosis through tumor necrosis factor/mitochondria-mediated caspase signaling pathway in human skin cancer cells. Biochim. Biophys. Acta. 2012;1820:1149–1157. doi: 10.1016/j.bbagen.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Hou R.-S., Duh C.-Y., Chiang M.Y., Lin C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995;58:1126–1130. doi: 10.1021/np50121a026. [DOI] [PubMed] [Google Scholar]
- 27.Geran R.I., Greenberg N.H., MacDonald M.M., Schumacher A.M., Abbott B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972;3:1–91. [Google Scholar]
- 28.Stevens M., Balzarini J., Tabarrini O., Andrei G., Snoeck R., Cecchetti V., Fravolini A., de Clercq E., Pannecouque C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005;56:847–855. doi: 10.1093/jac/dki328. [DOI] [PubMed] [Google Scholar]
- 29.Duh C.-Y., Li C.-H., Wang S.-K., Dai C.-F. Diterpenoids, norditerpenoids, and secosteroids from the Formosan soft coral Cespitularia hypotentaculata. J. Nat. Prod. 2006;69:1188–1192. doi: 10.1021/np0505465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials (PDF, 610 KB)