Abstract
The ideal strategy for hair cell regeneration is promoting residual supporting cell proliferation followed by induction of hair cell differentiation. In this study, cultured neonatal mouse organs of Corti were treated with neomycin to eliminate hair cells followed by incubation with recombined adenovirus expressing Pax2 and/or Math1. Results showed that overexpression of Pax2 significantly promoted proliferation of supporting cells. The number of BrdU+/myosin VIIA+ cells increased significantly in hair cell pre-existing region two weeks after adenovirus infection in Ad-Pax2-IRES-Math1 group compared to Ad-Pax2 and Ad-Math1 groups. This indicated that cotransfection of Pax2 and Math1 induced supporting cells to proliferate and differentiate into hair cells in situ. Most new hair cells were labeled by FM1-43 suggesting they acquired certain function. The results also suggest that inducing proliferating cells rather than quiescent cells to differentiate into hair cells by forced expression of Math1 is feasible for mammalian hair cell regeneration.
Hearing loss is one of the most common impairments in humans1. Hair cells in the inner ear play an essential role in converting mechanical sound movement to neural signals for hearing and balance. Unlike lower vertebrates, hair cells in mature mammalian cochlea do not regenerate spontaneously after damaged, although vestibular hair cells retain a limited capacity for regeneration. As a result, loss of hair cells is the major cause of permanent sensorineural hearing loss in adult mammals. Hair cell regeneration is regarded as an ideal solution for complete hearing repair.
In non-mammalian vertebrates, replacement of auditory and vestibular hair cells occurs over a long period after birth2,3. Supporting cells are the source of newly generated hair cells and their proliferation is a crucial step in hair cell regeneration in non-mammalian vertebrates. Under certain conditions, part of supporting cells re-enter the cell cycle and become progenitor cells4,5,6. Theoretically, there are two mechanisms of endogenous hair cell regeneration: direct transdifferentiation in which supporting cells change cell fates to become hair cells, and mitotic regeneration in which supporting cells go through the cell cycle and the resulting daughter cells further differentiate to replace both damaged hair cells and previously differentiated supporting cells7.
Our previous studies show that cochleae in newborn mice contain sphere-forming cells that have the capacity for proliferation in culture and differentiating to cells that express hair cell markers8. However, the origin of these progenitors is not clear. Some reports indicate that Wnt-responsive Lgr5-expressing cells are hair cell progenitors in the cochlea9,10. Hair cells and supporting cells in the inner ear are derived from a common pool of sensory progenitor cells during development11. Their development involves permanent exit from cell cycle, cell fate decision, and differentiation. Differentiated mammalian hair cells remain in a permanent quiescent state throughout life.
Although cochlear supporting cells in newborn mice can be induced to divide and regenerate new hair cells in vitro10,12, spontaneous auditory hair cell regeneration in vivo has not been observed after hair cell loss. Control of cell cycle exit of progenitor cells and maintenance of quiescent status of differentiated hair cells and supporting cells are controlled in part by negative cell growth genes, including P27kip1, p19ink4d, pRb, and p21cip1. It has been reported that down-regulation of P27kip1 or pRb can promote proliferation of supporting cells7,12, and some of these proliferating supporting cells can differentiate into hair cells in vitro12,13. These studies indicate that postnatal mammalian supporting cells might be potential progenitor cells and represent a promising therapeutic avenue for hair cell regeneration in the mammalian cochlea.
The basic helix-loop-helix transcription factor Math1 (also called Atoh1 and Hath1) is both necessary and sufficient for hair cell development in the mammalian cochlea14,15. Embryonic Math1-null mice fail to generate cochlear and vestibular hair cells14. It has been reported that overexpression of Math1 mediated by adenoviral vector induces supporting cells in the lesser epithelial ridge (LER) and greater epithelial ridge (GER) to transdifferentiate into ectopic hair cells in vitro16,17. Overexpression of Math1 in Sox2 positive cells in the organ of Corti only induced ectopic hair cells in newborn and young mice18.
Paired box 2 (Pax2) is a member of the paired-box gene family and is expressed in the developing inner ear and other parts of the embryo, including the hindbrain, the optic vesicle, and the kidney19,20. In the developing chicken and mouse inner ear, Pax2 is expressed in the auditory and vestibular sensory primordia and in the endolymphatic duct20,21,22,23. Mutations in Pax2 gene result in developmental defects of the vestibular and auditory apparatus24,25. Our previous study indicated that Pax2 expression is correlated to proliferation of progenitor cells in the developing inner ear26, suggesting that Pax2 is a good candidate for promoting supporting cell proliferation.
In this study, we aimed to promote cochlear hair cell regeneration by co-expression of Pax2 and Math1 in supporting cells that survived drug-induced damage. Cultured neonatal mouse organs of Corti were treated with neomycin for 24 h to eliminate hair cells, followed by incubation with recombined adenovirus expressing Pax2 and Math1 (Ad-Pax2-IRES-Math1). Other recombined adenoviruses expressing Pax2 or Math1 alone (Ad-Pax2-IRES-EGFP, Ad-Pax2, Ad-Math1-IRES-EGFP or.Ad-Math1) were used in parallel experiments. Results showed that forced overexpression of Pax2 significantly promoted proliferation of supporting cells located beneath the pre-existing hair cells that were killed by neomycin. In the mice treated with Ad-Pax2-IRES-Math1, more new hair cells were observed in the damaged region of the cochlea two weeks after neomycin insult, compared to those treated with Ad-Pax2 or Ad-Math1. This suggests that cotransfection of Pax2 and Math1 promoted supporting cells to proliferate and differentiate into new hair cells.
Results
Gene delivery by the adenovirus is efficient
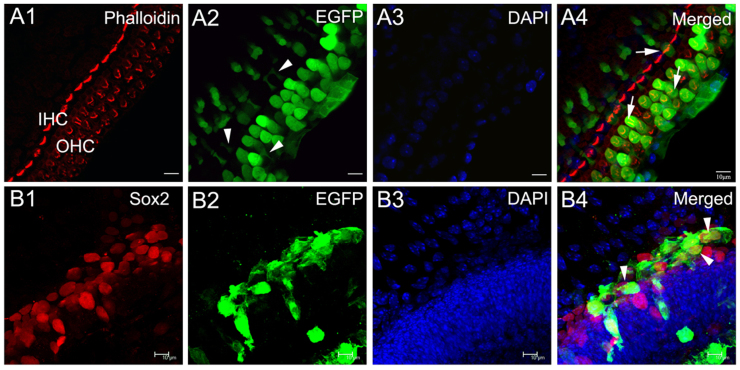
To confirm the efficiency of gene delivery by adenovirus transfection, EGFP fluorescence was examined in cochlear explants 3 days after Ad-EGFP incubation. Phalloidin staining was used to show the distribution of actin in the cochlear tissue. In intact cochlear epithelium, EGFP expressed in hair cells and supporting cells as shown by EGFP fluorescence and phalloidin staining (Fig. 1A). The transfection efficiencies of outer and inner hair cells were 80.1% ± 2.1% and 21.5% ± 1.4%, respectively.
Figure 1. The high efficiency of gene expression mediated by recombinant adenovirus in cultured neonatal cochlear explants.
(A) EGFP fluorescence micrograph of an intact cultured neonatal cochlear explant stained with fluorescent phalloidin. The three rows of outer HCs (OHC) and one row of inner HCs (IHC) can be clearly seen. Arrows in (A4) pointed to transfection positive inner and outer hair cells. Arrowheads in (A2) pointed to transfection positive supporting cells. (B) Image of the positive transfection of supporting cells in the basal turn of a neomycin-damaged cochlear explant. Arrowheads in (B4) pointed to transfection positive supporting cells. Scale bars: 10 μm.
After neomycin damage, a large number of hair cells were killed upon exposure to neomycin. The survival rates of hair cells across various segments of the organ of Corti are detailed in our previous study27. In neomycin-damaged cochlear epithelium, EGFP was mainly detected in the remaining supporting cells as demonstrated by EGFP/Sox2 double labeling (Fig. 1B). The transfection efficiency of supporting cells was 15.17% ± 0.45%. Neomycin treatment had no significant effect on the transfection efficiency of supporting cells.
Pax2 overexpression promoted proliferation of supporting cells in cochlear epithelium
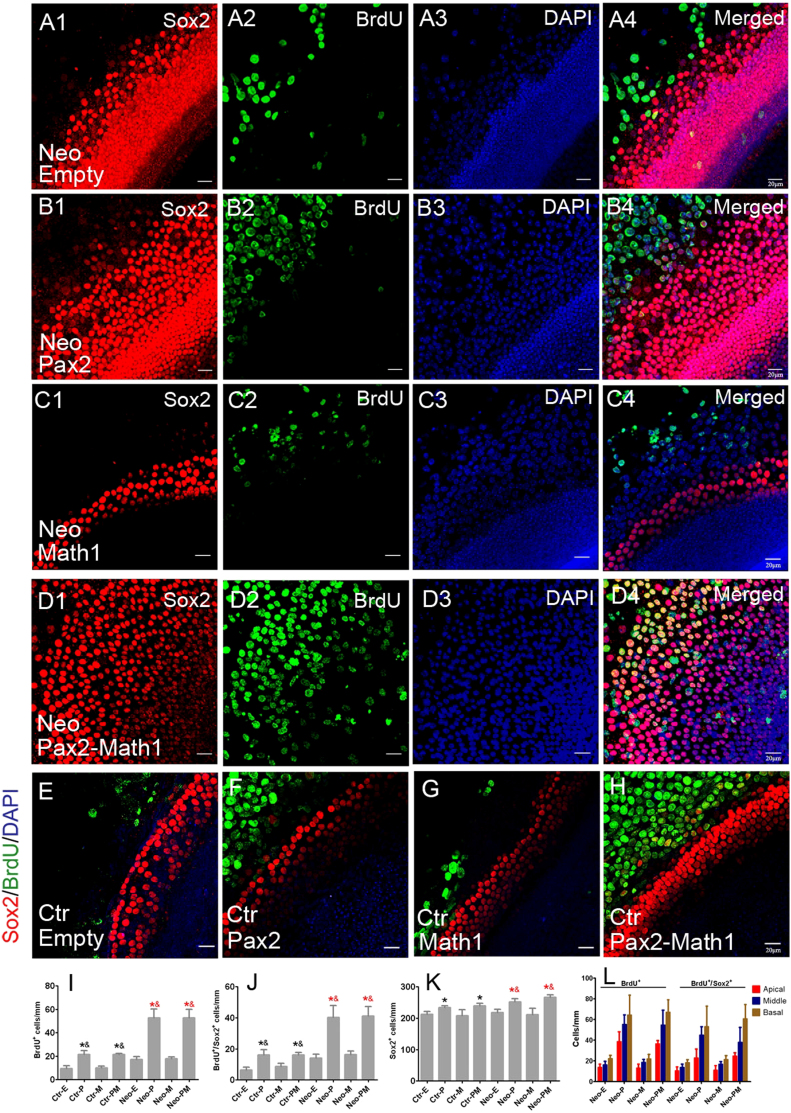
In neomycin-damaged cochlear epithelium, there is a low level proliferation of supporting cells (Fig. 2A). Pax2 is primarily expressed in the developing sensory primordium and early postnatal mouse cochlear hair cells, and disappears within the first week after birth28. In our experiment, Pax2 expression was barely detectable by immunolabeling in postnatal day 7 mice (Fig. 3A). At 1 week after incubation with Ad-Pax2 or Ad-Pax2-IRES-Math1, the number of Pax2+ cells increased significantly in cochlear explants (Fig. 3B), and the number of proliferating supporting cells increased as indicated by BrdU/Sox2 double labeling (Fig. 2). In Ad-empty and Ad-Math1 group, the proliferation rates of supporting cells were 6.9% ± 0.52% and 7.47% ± 0.49%, respectively. Pax2 overexpression significantly promoted proliferation of supporting cells, and in Ad-Pax2 and Ad-Pax2-IRES-Math1 group, the proliferation rates of supporting cells increased to 16.77% ± 1.27% and 16.16% ± 0.97%, respectively. Moreover, 89.11% ± 0.99% of Pax2-positive cells incorporated BrdU in Ad-Pax2 group (Fig. 3B) confirming that Pax2 overexpression promoted cell proliferation. This effect was further confirmed by double labeling with BrdU and EGFP seen in Ad-Pax2-IRES-EGFP group (Fig. 3C and 3D).
Figure 2. Forced Pax2 overexpression stimulated proliferation of supporting cells.
(A–D) Double immunofluorescence of BrdU and Sox2 in neomycin-damaged cochlear epithelium from the Ad-Empty, Ad-Pax2, Ad-Math1, and Ad-Pax2-IRES-Math1 groups. (E–H) Double immunofluorescence of BrdU and Sox2 in non-damaged cochlear explants from the Ad-Empty, Ad-Pax2, Ad-Math1, and Ad-Pax2-IRES-Math1 groups. Statistical data (I–K) showed that Pax2 overexpression increased the number of BrdU+, Sox2+, and BrdU+/Sox2+ cells. * (red): p < 0.05 vs. Ctr-E; & (red): p < 0.05 vs. Ctr-M; *(black): p < 0.05 vs. Neo-E; & (black): p < 0.05 vs. Neo-M. (L) The distribution of BrdU+ and BrdU+/Sox2+ cells in different regions throughout the damaged cochlea. Scale bars: 20 μm.
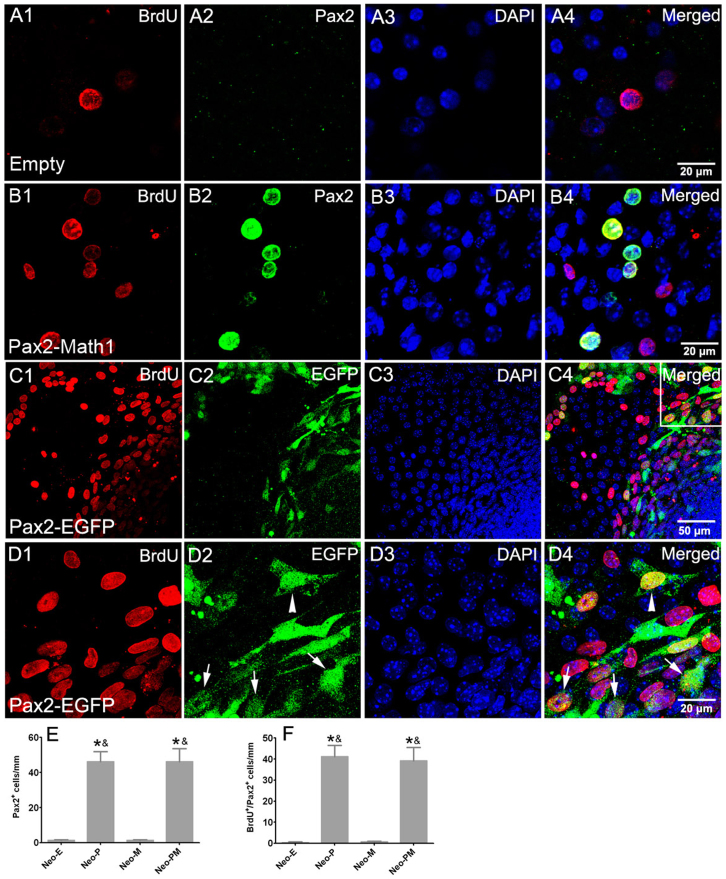
Figure 3. Forced expression of Pax2 stimulated cell proliferation.
(A and B) Double immunofluorescence of Pax2 and BrdU showed that most of Pax2 positive cells were proliferating in the neomycin-damaged cochlear epithelium in Ad-Pax2-IRES-Math1 group. Statistical data (E–F) showed that the number of Pax2+ and BrdU+/Pax2+ cells increased in Ad-Pax2 and Ad-Pax2-IRES-Math1 groups. *: p < 0.05 vs. Neo-E; &: p < 0.05 vs. Neo-M. (C) Double labeling of EGFP and BrdU in damaged cochlear epithelium at 7 days after Ad-Pax2-IRES-EGFP infection. (D) Higher magnification image of (C). Arrows pointed to proliferating supporting cells beneath pre-existing hair cells. The arrowhead pointed to a proliferating fibroblast cell. Scale bars: 20 μm in (A, B, and D); 50 μm in (C).
As a control, we investigated the effect of Pax2 overexpression on supporting cell proliferation in non-damaged cochlear epithelium (Fig. 2E–H). Statistical data (Fig. 2J) showed that the number of BrdU+/Sox2+ cells increased in Ad-Pax2 and Ad-Pax2-IRES-EGFP groups compared with that in Ad-Empty and Ad-Math1 group. This indicated that Pax2 overexpression also stimulated the proliferation of supporting cells in non-damaged cochlear explants. The increase of supporting cell proliferation is greater in neomycin-damaged explants than in non-damaged explants (Fig. 2J), suggesting that Pax2 overexpression and hair cell damage had a synergistic effect on the proliferation of supporting cells.
To investigate the distribution of proliferating cells in different regions of the damaged cochlea, BrdU+ and BrdU+/Sox2+ cells were counted in the apical, middle, and basal turns. In Ad-Pax2 and Ad-Pax2-IRES-Math1 groups, the numbers of BrdU+ and BrdU+/Sox2+ cells appear to be greatest in the basal turn and the least in the apical turn (Fig. 2L). However, there is no statistically significant difference in proliferation between the apical, middle, and basal turns.
Cotransfection of Pax2 and Math1 stimulated hair cell regeneration in neomycin-damaged cochlear explants
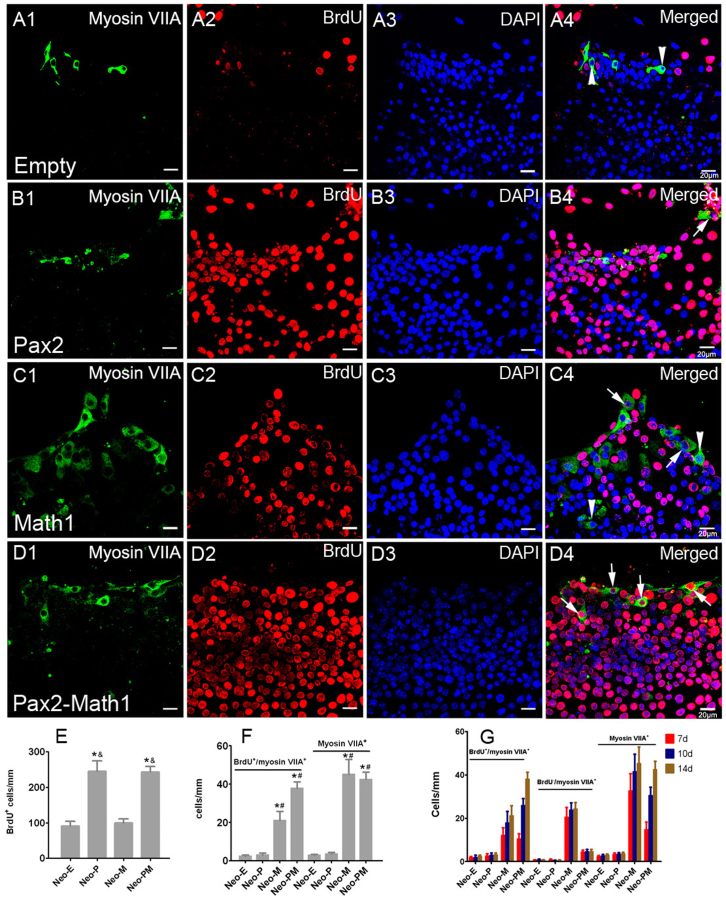
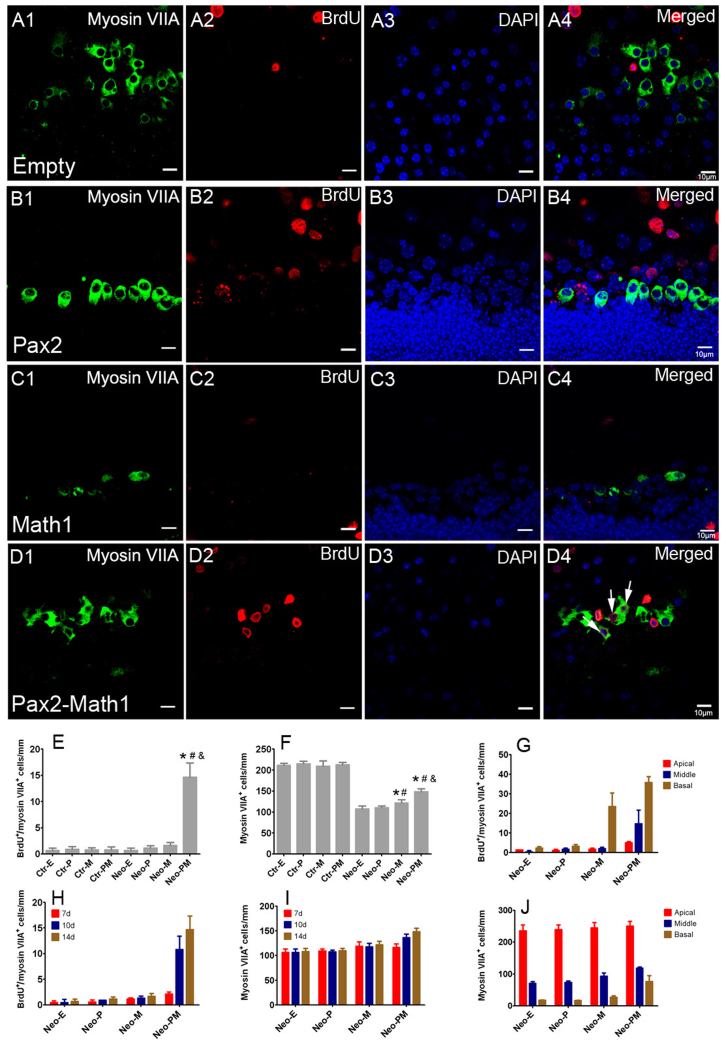
To examine the effect of cotransfection of Pax2 and Math1 on cochlear hair cell regeneration, double labeling of BrdU and myosin VIIA was performed at 7, 10, or 14 days after incubation with recombinant adenovirus. Consistent with a previous report17, forced Math1 overexpression promoted hair cell formation in the LER as indicated by the increased number of myosin VIIA+ cells in Ad-Math1 and Ad-Pax2-IRES-Math1 group (Fig. 4). However, Math1 overexpression alone could not stimulate hair cell formation in the hair cell pre-existing region (HCR), which is referred to as in situ hair cell regeneration (Fig. 5C). While many BrdU+/myosin VIIA+ cells were observed in the HCR in neomycin-damaged cochlear epithelium of Ad-Pax2-IRES-Math1 group (Fig. 5D), which indicated that cotransfection of Pax2 and Math1 stimulated in situ cochlear hair cell regeneration. At 7 days after adenovirus incubation, few new hair cells (BrdU+/myosin VIIA+) were observed in the HCR, but the number of new hair cells increased at 10 days and peaked 14 days after adenovirus incubation. Statistical data (14 days) showed that the number of BrdU+/myosin VIIA+ cells increased significantly in Ad-Pax2-IRES-Math1 group, compared to other three groups (Fig. 5E). In Ad-Pax2 group, only 2.09% ± 0.40% of the BrdU+ cells were co-labeled by myosin VIIA, indicating that most proliferating cells failed to differentiate into hair cells. The ratio of BrdU+/myosin VIIA+ cells to BrdU+ cells increased to 26.08% ± 1.86% in Ad-Pax2-IRES-Math1 group, indicating that cotransfection of Pax2 and Math1 induced proliferating supporting cells to differentiate into hair cells.
Figure 4. Forced Math1 expression promoted hair cell formation in the lesser epithelial ridge (LER).
(A–D) Double immunofluorescence of BrdU and myosin VIIA in the LER of neomycin-damaged cochlear epithelium at 2 weeks after adenovirus incubation. Myosin VIIA was used as a marker of hair cells. The arrows pointed to BrdU+/myosin VIIA+ hair cells that means hair cells formed by mitotic regeneration. The arrowheads pointed to BrdU−/myosin VIIA+ cells that mean hair cells formed by direct transdifferentiation in the LER. Statistical data (E–F) showed that Math1 expression promoted hair cell formation in the LER in both Ad-Math1 and Ad-Pax2-IRES-Math1 groups. *: p < 0.05 vs. Neo-E; #: p < 0.05 vs. Neo-P; &: p < 0.05 vs. Neo-M. Data in (G) showed the number of BrdU+/myosin VIIA+, BrdU−/myosin VIIA+, and total myosin VIIA+ cells in the LER at different times after adenovirus incubation. Scale bars: 20 μm.
Figure 5. Cotransfection of Pax2 and Math1 promoted in situ hair cell regeneration in neomycin-damaged cochleae.
(A–D) Double immunofluorescence of BrdU and myosin VIIA in hair cell pre-existing region (HCR) of neomycin-damaged cochlear epithelium at 2 weeks after adenovirus incubation. New hair cells were occasionally observed in Ad-empty (A, apical-middle turn), Ad-Pax2 (B, middle turn), and Ad-Math1 (C, basal turn) groups. However, in Ad-Pax2-IRES-Math1 group (D, basal turn), many BrdU+/myosin VIIA+ cells (arrows) were observed in HCR. Because hair cells in the cochlear basal turn are more sensitive to aminoglycoside antibiotics than those in the apical turn, more residual hair cells were observed in the apical turn than the basal turn after neomycin treatment. Arrows pointed to BrdU+/myosin VIIA+ cells. Statistical data (E–F) showed that the numbers of BrdU+/myosin VIIA+ and myosin VIIA+ cells significantly increased in Ad-Pax2-IRES-Math1 group, compared with other groups. *: p < 0.05 vs. Neo-E; #: p < 0.05 vs. Neo-P; &: p < 0.05 vs. Neo-M. Data in (G and J) showed the distribution of BrdU+/myosin VIIA+ and myosin VIIA+ cells in different regions throughout the damaged cochlea. Data in (H and I) showed the number of BrdU+/myosin VIIA+ and total myosin VIIA+ cells in the HCR at different times after adenovirus incubation. Scale bars: 10 μm.
Most of the newly generated hair cells were located in the basal turn (Fig. 5G), where fewer pre-existing hair cells survived (Fig. 5J). This is because hair cells in the basal turn are more sensitive to aminoglycoside antibiotics such as neomycin than those in the apical turn27. BrdU+/myosin VIIA+ cells were occasionally observed in non-damaged cochlear explants in Ad-Pax2-IRES-Math1 group (Fig. 5E), suggesting that the generation of new hair cells might be related to the removal of lateral inhibition mediated by Notch signaling.
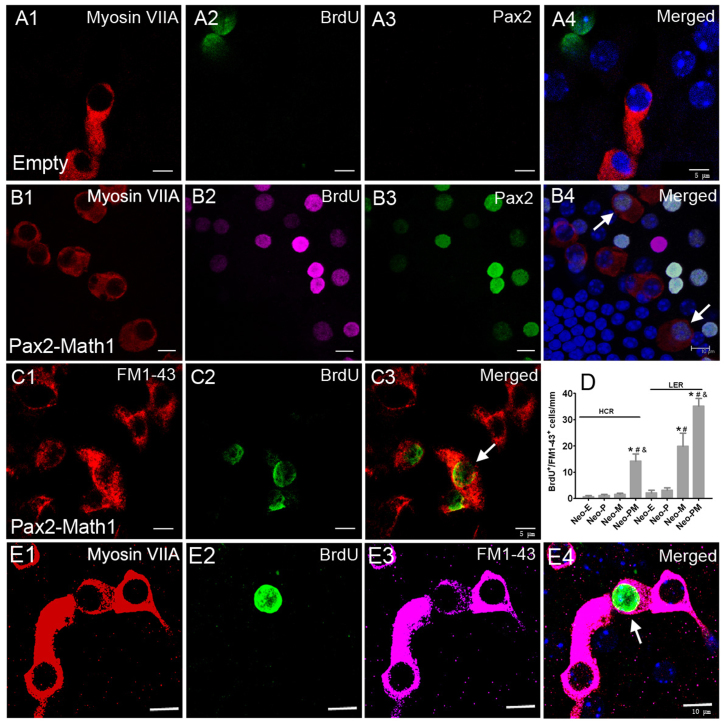
Because endogenous Pax2 expression had nearly ceased in the mouse cochlea on postnatal day 7 (Fig. 3A), nearly all Pax2 expression was exogenous at 2 weeks after recombinant adenovirus incubation and all Pax2+ cells could be regarded as transfection positive cells. To confirm the origin of newly generated hair cells in Ad-Pax2-IRES-Math1 group, we performed triple immunofluorescence of BrdU, Pax2, and myosin VIIA (Fig. 6). These results showed that newly generated hair cells expressed Pax2 and suggested that these new hair cells originated from transfected cells.
Figure 6. Newly generated hair cells induced by co-expression of Pax2 and Math1 acquired certain function.
(A and B) Triple immunofluorescence of BrdU, Pax2, and myosin VIIA showed that the newly generated hair cells originated from transfection positive cells. Arrows pointed to BrdU+/myosin VIIA+/Pax2+ cells. (C) Double immunofluorescence of BrdU and FM1-43 suggested that newly generated hair cells developed mechanotransduction channels and acquired certain function. Arrow pointed to BrdU+/FM1-43+ cell. Data in (D) showed the number of BrdU+/FM1-43+ cells in the HCR and LER at 2 weeks after adenovirus incubation. (E) Triple immunofluorescence of BrdU, myosin VIIA, and FM1-43 confirmed that the newly generated hair cells acquired certain function. Arrow pointed to BrdU+/myosin VIIA+/FM1-43+ cell. Scale bars: 5 μm in (A and C); 10 μm in (B and E).
In the LER, BrdU−/myosin VIIA+ cells could be regarded as hair cells that were generated by transdifferentiation of supporting cells. We counted the number of BrdU−/myosin VIIA+ and BrdU+/myosin VIIA+ cells in the LER at different times after adenovirus incubation. As shown in Fig. 4G, most of the BrdU−/myosin VIIA+ cells formed at 7 days after adenovirus incubation. Only a few BrdU+/myosin VIIA+ cells had formed at 7 days, but the number of BrdU+/myosin VIIA+ cells increased significantly at 10 days and peaked at 14 days. These data suggest that mitotic hair cell regeneration took more time than hair cell regeneration by transdifferentiation.
New hair cells induced by cotransfection of Pax2 and Math1 acquired certain function
FM1-43, a marker of functional mechanotransduction channels in hair cells29,30, was used to detect the function of the newly generated hair cells. In Ad-Pax2-IRES-Math1 group, many BrdU+ cells were labeled by FM1-43 (Fig. 6), indicating that these new hair cells had developed mechanotransduction channels and were functionally active. The number of BrdU+/FM1-43+ cells and BrdU−/FM1-43+ cells was counted. In the HCR, the ratios of BrdU+/FM1-43+ cells to BrdU+/myosin VIIA+ cells were 95% ± 5% and 96.89% ± 0.28% in Ad-Math1 and Ad-Pax2-IRES-Math1 groups, respectively. In the LER, the ratios of BrdU+/FM1-43+ cells to BrdU+/myosin VIIA+ cells were 95.2% ± 45.28% and 94.38% ± 3.29% in Ad-Math1 and Ad-Pax2-IRES-Math1 groups, respectively. The ratios of BrdU−/FM1-43+ cells to BrdU−/myosin VIIA+ cells were 97.53% ± 1.02% and 94.14% ± 0.99% in Ad-Math1 and Ad-Pax2-IRES-Math1 groups, respectively. These data suggested that the majority of newly generated hair cells acquired certain function.
Discussion
Stimulating hair cell regeneration in situ should be one of the main approaches to restore hearing that is lost due to hair cell death and damage. A growing body of evidence shows that postnatal cochlear supporting cells have the potential to divide and differentiate, or transdifferentiate, into functional hair cells12,31,32. Not all supporting cells have the capacity to proliferate, and only a subset of cells in the postnatal cochlea can serve as hair cell progenitors33. Heller et al. purified four different non-sensory cell populations from the neonatal mouse cochlea and found that only cochlear supporting cells and cells of the LER have robust intrinsic potential to de-differentiate into prosensory cells34. Recent reports show that Wnt-responsive Lgr5-expressing supporting cells, sorted by flow cytometry and cultured in a single-cell suspension, displayed strong capacity for self-renewing neurosphere formation and hair cell formation9,10. These cells were highly proliferative and capable of differentiating into hair cells when isolated and cultured in vitro. However, these cells remain quiescent in vivo after loss of hair cells.
Transgenic overexpression of Math1 in Sox2 positive cells induced numerous ectopic hair cells in the LER and GER of the cochlea in postnatal and young mice18. Inhibition of Notch signaling can induce supporting cells to transdifferentiate into new hair cells in adult mouse utricles after neomycin damage35. Cochlear hair cell regeneration induced by LY411575, a potent Notch signaling inhibitor, results in a partial recovery of hearing after acoustic trauma36. However, the transdifferentiation of supporting cells into hair cells induced by forced Math1 expression or Notch signaling inhibition leads to the loss of supporting cells. In consideration of the limited number of supporting cells in the organ of Corti long after hair cell death, it is necessary to promote proliferation of supporting cells before induction of hair cell differentiation.
Previous studies suggest that Pax2 expression is essential for the proliferation of progenitor cells in the developing inner ear26,28. In this study, our results demonstrated that forced Pax2 overexpression promoted supporting cells to re-enter cell cycle and proliferate in both undamaged controls and neomycin-damaged cochlear explants (Fig. 2 and Fig. 3). However, only a small number of the proliferating cells differentiated into new hair cells. This indicated that overexpression of Pax2 could stimulate the proliferation of supporting cells but was not enough to regenerate cochlear hair cells. Similarly, another study37 reported that co-infection of utricles with adenoviral vectors encoding the degradation-resistant T58A mutant of c-Myc (c-MycT58A) triggered significant levels of supporting cell S-phase entry in adult vestibular sensory epithelium, but only a small number of the proliferating cells differentiated into hair cells. These results suggest that inducing hair cell differentiation in addition to promoting residual supporting cell proliferation is necessary for hair cell regeneration.
When Math1 was overexpressed in the neomycin-damaged organ of Corti, most new hair cells were formed in the LER (Fig. 5) but nearly no new hair cells were generated in HCR (Fig. 4). In subsequent experiments, forced Math1 overexpression was used to induce hair cell differentiation at the same time to promote residual supporting cell proliferation with Pax2. Results showed that forced co-expression of Pax2 and Math1 induced these Pax2 positive proliferating supporting cells to differentiate into new hair cells (Fig. 5 and Fig. 6). Interestingly, by cotransfection of Pax2 and Math1, many newly regenerated hair cells were observed in the HCR (Fig. 5) in addition to the LER (Fig. 4), suggesting that Pax2 responsive proliferating supporting cells are crucial source for mammalian hair cell regeneration. Statistical data showed that co-expression of Pax2 and Math1 could more effectively promote hair cell regeneration compared to Math1 expression alone (Fig. 4 and Fig. 5), suggesting that strong proliferative potential could impact the effectiveness of hair cell regeneration. Moreover, most of newly generated hair cell developed mechanotransduction channels and acquired certain function (Fig. 6). Co-expression of Pax2 and Math1 might be involved in a novel mechanism or in switching particular cell signals on or off in response to cell proliferation and regeneration, and this is a subject that deserves further detailed investigation.
In conclusion, our findings indicate that cotransfection of Pax2 and Math1 strongly stimulates in situ hair cell regeneration in neomycin-damaged cochlear explants. These results also suggest that inducing proliferating cells rather than quiescent cells to differentiate into hair cells by forced expression of Math1 is a feasible method for hair cell regeneration in mammalian sensory epithelium.
Methods
Ethics statement
This study was carried out in strict accordance with the “Guiding Directive for Humane treatment of Laboratory Animals” issued by the Chinese National Ministry of Science and Technology on September, 2006. All experiments were approved by the Shanghai Medical Experimental Animal Administrative Committee (Permit Number: 2009-0082). All efforts were made to minimize suffering and reduce the number of animals used.
Tissue culture and hair cell damage
Cochlear sensory epithelium was dissected from anaesthetized postnatal day 2 mice in PBS at pH 7.4. The stria vascularis and surrounding epithelial tissue and the remains of nerve fibers were carefully removed. Sensory epithelia were transferred onto poly-L-lysine treated (Sigma-Aldrich, St Louis, Missouri, USA) cover slides in a 35 mm dish filled with 200 μL DMEM/high glucose medium with 10% fetal calf serum and allowed to attach for 24 hours. After attachment, tissues were washed twice with PBS and then cultured in 2 mL serum-free DMEM/high glucose and F12 medium (mixed 1∶1) supplemented with N2 and B27 solutions (media and supplements were obtained from Invitrogen, Carlsbad, California, USA). Neomycin (1 mM) was added for 24 hours to kill hair cells. After neomycin was removed, the tissues were washed twice with PBS and cultured in serum-free medium. Half of the media was replaced every second day.
Adenovirus incubation
Recombinant adenoviruses were prepared with pAd/CMV/V5-DEST Gateway® Vector, pAd/PL-DEST Gateway® Vector, and 293 cell line (Invitrogen, Carlsbad, California, USA). Mouse cDNA for Pax2 (NM_011037.4) and Math1 (NM_007500.4) were inserted into the adenoviral vectors. After removal of neomycin, recombinant adenovirus (Ad-EGFP, Ad-empty, Ad-Pax2-IRES-EGFP, Ad-Pax2, Ad-Math1-IRES-EGFP, Ad-Math1 or Ad-Pax2-IRES-Math1) was added to the medium for 6 hours. The final concentration of recombinant virus was 1 × 109 ifu/mL. Tissues were fixed and prepared for immunohistochemical labeling of proliferation and differentiation at 3, 7, 10, or 14 days after adenovirus incubation.
Cell proliferation
To label proliferating cells, the thymidine analog 5-bromo-2′-deoxyuridine (BrdU, the final concentration is 6 μg/mL) was added to the medium. Incorporated BrdU was immunohistochemically detected by labeling with a specific monoclonal anti-BrdU antibody.
Immunofluorescence analysis
For immunolabeling, we used monoclonal anti-BrdU (Santa Cruz Biotechnology, Santa Cruz, California, USA), rabbit polyclonal anti-EGFP (Invitrogen), polyclonal anti-myosin VIIA (Myo7a) (Proteus Biosciences, Ramona, California, USA), polyclonal anti-Pax2 (Covance, Princeton, New Jersey, USA), and polyclonal anti-SRY (sex-determining region Y)-box 2 (Sox2) (Santa Cruz Biotechnology). Briefly, nonspecific binding sites were blocked for 1 hour in 0.1% Triton X-100, 1% BSA (w/v), and 5% (v/v) heat-inactivated normal serum in PBS (PBT1). Tissues were then incubated overnight at 4°C in PBT1 with 1∶500 dilutions of anti-BrdU, anti-EGFP, anti-Myo7a, or anti-Pax2 or a 1∶1000 dilution of anti-Sox2. For BrdU labeling, epithelia were placed in 2 N HCl to denature DNA before incubation with antibodies. After unbound antibodies were removed, tissues were incubated with the corresponding secondary antibodies conjugated with tetramethyl rhodamine (TRITC), fluorescein isothiocyanate (FITC), or Cy5 (Jackson ImmunoResearch, West Grove, Pennsylvania, USA). Counterstaining with low-wavelength nuclear staining agent DAPI (Sigma-Aldrich) allowed visualization of the cell nuclei. Specimens were examined by confocal fluorescence microscopy (Leica SP5, Heidelberg, Germany). Negative control experiments were performed as above by omitting the primary antibodies.
Functional mechanotransducing hair cells were labeled with the vital dye FM1-43FX (Molecular Probes, Eugene, Oregon, USA), a fixable version of FM1-43 [N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)-styryl) pyridinium dibromide], that enters mature hair cells through mechanotransduction-dependent activity. Samples were placed in medium containing 3 mM FM1-43FX for 45 s and rinsed three times in PBS (pH 7.2). Samples were then fixed in 4% paraformaldehyde overnight at 4°C and washed several times in PBS with 0.1% Triton X-100 before being processed for immunohistochemistry.
Quantification of hair cell regeneration
Cells labeled with BrdU or cell markers were counted at minimum five 700 μm over the whole length of an entire cochlea. Data are presented as the mean ± S.E.M. Student's t-test was used to analyze the differences, and differences between groups were considered significant when p < 0.05.
Author Contributions
Conceived and designed the experiments: H.L. Z.W. and Y.C. Performed the experiments: Y.C. H.Y. Y.Z. W.L. S.S. N.L. W.N. J.L. Y.H. Analyzed the data: Y.C. H.L. Wrote the paper: H.L. Y.C.
Acknowledgments
We thank Tianfeng Wang, Yunzhen Shen for their excellent technique support; Yalin Huang, Xia Sun (Institutes of Biomedical Sciences of Fudan University) for help with the confocal microscope. This project was supported by research grants from National Basic Research Program of China (2011CB504506), National Natural Science Foundation of China (81230019), National Nature Science Found of China (30772399), Program for Changjiang Scholars and Innovative Research Team in Universities (IRT1010), and Program of Outstanding Shanghai Academic Leaders (11XD1401300) to Huawei Li,National Nature Science Found of China (81100709) and Shanghai Nature Science Found (11ZR1406000) to Yan Chen, National Nature Science Found of China (81300825 and 81070793) and Shanghai Rising-Star Program (12QA1400500).
References
- Lalwani A. K. & Castelein C. M. Cracking the auditory genetic code: nonsyndromic hereditary hearing impairment. Am. J. Otol. 20, 115–132 (1999). [PubMed] [Google Scholar]
- Corwin J. T. & Cotanche D. A. Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774 (1988). [DOI] [PubMed] [Google Scholar]
- Ryals B. M. & Rubel E. W. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240, 1774–1776 (1988). [DOI] [PubMed] [Google Scholar]
- Tsue T. T., Watling D. L., Weisleder P., Coltrera M. D. & Rubel E. W. Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J. Neurosci. 14, 140–152 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. S. & Cotanche D. A. Hair cell regeneration in the avian auditory epithelium. Int. J. Dev. Biol. 51, 633–647 (2007). [DOI] [PubMed] [Google Scholar]
- Behra M. et al. Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line. PLoS Genet. 5, e1000455 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. et al. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J. Neurosci. 30, 5927–5936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport 17, 767–771 (2006). [DOI] [PubMed] [Google Scholar]
- Shi F., Kempfle J. S. & Edge A. S. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 32, 9639–9648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R. et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. U S A 109, 8167–8172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben R. J. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. Suppl 220, 1–44 (1967). [PubMed] [Google Scholar]
- White P. M., Doetzlhofer A., Lee Y. S., Groves A. K. & Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987 (2006). [DOI] [PubMed] [Google Scholar]
- Lu N. et al. Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein. Biochem. Biophys. Res. Commun. 430, 700–705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999). [DOI] [PubMed] [Google Scholar]
- Raft S. et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134, 4405–4415 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng J. L. & Gao W.-Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580–586 (2000). [DOI] [PubMed] [Google Scholar]
- Shou J., Zheng J. L. & Gao W. Q. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci. 23, 169–179 (2003). [DOI] [PubMed] [Google Scholar]
- Kelly M. C., Chang Q., Pan A., Lin X. & Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci. 32, 6699–6710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nornes H. O., Dressler G. R., Knapik E. W., Deutsch U. & Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development 109, 797–809 (1990). [DOI] [PubMed] [Google Scholar]
- Rinkwitz-Brandt S., Arnold H. H. & Bober E. Regionalized expression of Nkx5-1, Nkx5-2, Pax2 and sek genes during mouse inner ear development. Hear. Res. 99, 129–138 (1996). [DOI] [PubMed] [Google Scholar]
- Hutson M. R., Lewis J. E., Nguyen-Luu D., Lindberg K. H. & Barald K. F. Expression of Pax2 and patterning of the chick inner ear. J. Neurocytol. 28, 795–807 (1999). [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G., Rivolta M. N. & Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J. Comp. Neurol. 442, 378–391 (2002). [DOI] [PubMed] [Google Scholar]
- Sanchez-Calderon H., Martin-Partido G. & Hidalgo-Sanchez M. Differential expression of Otx2, Gbx2, Pax2, and Fgf8 in the developing vestibular and auditory sensory organs. Brain Res. Bull. 57, 321–323 (2002). [DOI] [PubMed] [Google Scholar]
- Favor J. et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc. Natl. Acad. Sci. U S A 93, 13870–13875 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Gomez-Pardo E. & Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122, 3381–3391 (1996). [DOI] [PubMed] [Google Scholar]
- Li H. W., Liu H., Corrales C. E., Mutai H. & Heller S. Correlation of Pax-2 expression with cell proliferation in the developing chicken inner ear. J. of Neurobiol. 60, 61–70 (2004). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. Inhibition of H3K9 methyltransferases G9a/GLP prevents ototoxicity and ongoing hair cell death. Cell. Death Dis. 4, e506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Q., Cole L. K., Mulheisen M., Chang W. & Wu D. K. The role of Pax2 in mouse inner ear development. Dev. Biol. 272, 161–175 (2004). [DOI] [PubMed] [Google Scholar]
- Tanimoto M., Ota Y., Inoue M. & Oda Y. Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. J. Neurosci. 31, 3784–3794 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J. E., Marcotti W., Kennedy H. J., Kros C. J. & Richardson G. P. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 21, 7013–7025 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S. et al. Isolation and culture of hair cell progenitors from postnatal rat cochleae. J. Neurobiol. 65, 282–293 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J. Neurosci. Methods 164, 271–279 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z. M. et al. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport 17, 767–771 (2006). [DOI] [PubMed] [Google Scholar]
- Sinkkonen S. T. et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci. Rep. 1, 26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V. et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J. Neurosci. 31, 15329–15339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K. et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58–69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Yoo J. J., Atala A. & Jackson J. D. MYC gene delivery to adult mouse utricles stimulates proliferation of postmitotic supporting cells in vitro. PLoS One 7, e48704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]