Abstract
We report a 30-year-old male intravenous drug abuser presenting with persistent pacemaker lead thrombosis with superimposed pacemaker lead endocarditis. He underwent urgent surgery, but expired due to refractory sepsis. This case confirms that patients with pacemakers are at risk of developing pacemaker lead thrombosis. In addition, they are at high risk of developing pacemaker lead endocarditis if additional risk factors for endocarditis are present. We believe this case report is unusual on account of pacemaker lead thrombosis as well as endocarditis occurring in a patient with history of intravenous drug abuse. Whether pacemaker patients with multiple leads need to be on long-term antiplatelet or anticoagulation therapy necessitates further studies.
Keywords: Pacemaker lead thrombosis, Pacemaker lead endocarditis, Septic shock, Intravenous drug abuse
1. Case report
A 30-year-old male was admitted with atypical chest pain of one day duration. A dual chamber pacemaker (DDD) had been implanted in the UK via the left subclavian vein because of hypertrophic cardiomyopathy 15 years earlier. He underwent replacement of pacemaker generator electively in 2006 for battery end-of-life. He was regularly followed up in pacemaker clinic. He was known to be intravenous drug abuser (IVDA) and suffer from epilepsy. There was no history of fever, cough, hemoptysis, or shortness of breath. Physical examination was unremarkable. Routine blood investigations were normal. ECG revealed paced rhythm with good capture. The pacemaker checks showed adequate sensing/pacing parameters with satisfactory impedance. His transthoracic echocardiogram (TTE) showed a mobile mass attached to the atrial part of the ventricular pacemaker lead measuring 2.1 × 1.1 cm2 suggestive of thrombus, but not prolapsing into right ventricle (Fig. 1A). The right atrium and ventricle were mildly dilated with mild tricuspid regurgitation and pulmonary artery systolic pressure of 35 mmHg. There was asymmetrical septal hypertrophy with no significant left ventricular outflow tract gradient and good left and right ventricular systolic function. The superior vena cava was free of thrombus. No other intracardiac masses were found. His computed tomography (CT) of chest showed left lower lobe artery pulmonary embolism (Fig. 1B). He was thrombolysed with reteplase followed by anticoagulation therapy with subsequent reduction in size of the thrombus. A repeat echocardiogram done every 4 weeks for the next 3 months showed persistence of thrombus. He was also admitted once with high INR and large left hemorrhagic pleural effusion which subsided after brief discontinuation of warfarin. In view of these findings, he was planned for device removal.
Figure 1.
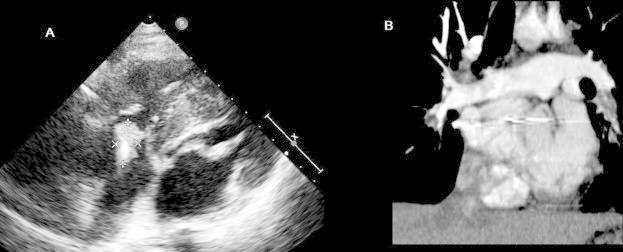
Transthoracic echocardiography (A) showing a thrombus (marked) adherent to a pacemaker lead. Computed tomography pulmonary angiogram (B) showing a large left lower pulmonary artery embolism in a patient with pacemaker lead thrombosis.
While waiting for device removal he was re-admitted with fever, rigors, and night sweats of one week duration with three blood cultures growing methicillin-susceptible Staphylococcus aureus. He gave history of recent use of intravenous heroin. Clinically there was no evidence of pacemaker pocket infection. His TTE done showed a large mass measuring 4.3 × 2.5 cm2 attached to pacemaker lead in the right atrium prolapsing through the tricuspid valve during diastole, suggestive of vegetation superimposed on previous thrombus (Fig. 2). There were no tricuspid valve vegetations. CT chest done showed bilateral multiple septic emboli. He was treated with intravenous flucloxacillin and advised urgent surgery, but he and his family refused any intervention. He was in persistent septic shock on multiple inotropes. When patient agreed for surgery, after written consent he was taken for urgent surgery, but unfortunately peri-operatively he expired due to severe sepsis. The explanted lead with vegetation was sent to the laboratory for gram stain and culture. A Staphylococcus was identified as the pathologic organism.
Figure 2.
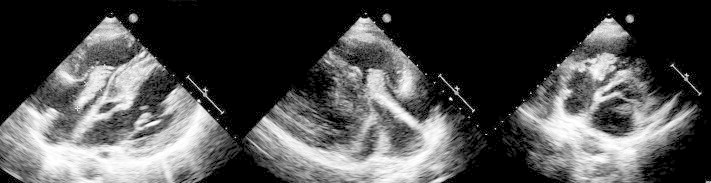
Multiple transthoracic echocardiographic views demonstrating large thrombotic vegetation attached to a pacemaker lead prolapsing through tricuspid valve.
2. Discussion
Thrombosis and infections of pacemaker system are rare, but potentially lethal complications. They cause significant morbidity and mortality (Alizadeh et al., 2006; Baddour et al., 2010; Carda et al., 2008; Korkeila et al., 2006; van Rooden et al., 2004). Recent echocardiographic studies have reported that pacemaker lead thrombosis is fairly common and they can be potentially life-threatening due to the high risk of pulmonary embolism (Korkeila et al., 2006). The incidence of pacemaker lead thrombosis (usually asymptomatic) detected by transesophageal echocardiography (TEE) has ranged from 9% to 32% (Korkeila et al., 2006; Alizadeh et al., 2006). Right atrial pacemaker lead thrombosis can present either as an incidental echocardiographic finding or with right-sided heart failure symptoms or pulmonary embolism. Asymptomatic pulmonary embolism is known (Korkeila et al., 2006). van Rooden et al. (2004) reported that, established risk factors for venous thrombosis and the presence of multiple leads contribute substantially to occurrence of venous thrombosis associated with permanent pacemaker leads. Even though, TEE detects smaller thrombi (<10 mm); TTE is possibly the first option due to its immediate availability, as noted in our patient (Carda et al., 2008). The treatment of pacemaker lead thrombosis is controversial (Carda et al., 2008). The site, size, mobility of the thrombus and the duration and type of symptoms are the main determinants of the treatment strategy. The options are medical therapy (antiplatelet, anticoagulation, and/or thrombolysis), surgical or percutaneous extraction (Carda et al., 2008). In our patient, the thrombus was attached to the right atrial lead, mobile, but not obstructing the tricuspid valve and there was evidence of acute pulmonary embolism by CT. Hence, he was thrombolysed.
Pacemaker system infections are not uncommon with reported rates ranging from 0.13% and 19.9% with most infections limited to the pocket. Frank pacemaker endocarditis accounts for approximately 10% of permanent pacemaker infections (Baddour et al., 2010). Cabell et al. (2004) have reported a rate of cardiac device infections at 2.11 per 1000 beneficiaries and the rate of frank endocarditis at 0.39 cases/1000 beneficiaries. Various risk factors have been implicated for cardiac device infections like diabetes, heart failure, renal impairment, oral anticoagulant use, steroid use, hematoma formation, and more than 2 pacing leads. Procedural factors like fever within 24 h before implantation, use of preprocedural temporary pacing, device revision/replacement, underuse of periprocedural antibiotic prophylaxis, implantation sites other than pectoral, procedure performed by low-volume physicians, and Staphylococcus bacteremia (Baddour et al., 2010). Staphylococcal species cause the bulk of pacemaker infections and account for 60–80% of cases in most reported series. In our patient S. aureus was identified in blood as well as from the thrombotic vegetation adhered to the explanted pacemaker lead. Lead infection generally occurs due to hematogenous seeding of the organism in the absence of pocket infection. In addition, this patient had a sterile thrombus which was at high risk of secondarily infected due to his IVDA. Right-sided endocarditis is well-known in IVDAs. In a recent study among IVDAs with endocarditis, the indications for admission to intensive care unit were severe sepsis or septic shock (36%), respiratory failure (33%), and neurologic deterioration (18%) (Saydain et al., 2010). S. aureus was found in 94% of patients and 15% had polymicrobial infection. Forty-five percent of patients had septic emboli to 1 or more organs. In-hospital mortality was high at 27%.
The utility of TEE in the diagnosis of pacemaker lead endocarditis is well established (Baddour et al., 2010). On echocardiography, a mass adherent to the lead is usually a thrombus or infected vegetation. Since it is impossible to distinguish between the two with echocardiography, it is suggested that overall picture need to be considered along with results of blood cultures. Masses that are detected in patients without positive blood cultures or other suggestive features for infection are likely to represent thrombus and by themselves do not require lead removal or antibiotic treatment (Baddour et al., 2010). In this patient a TTE initially demonstrated a pacemaker lead thrombus without evidence of infection, but later it had increased in size with positive blood cultures suggesting pacemaker lead thrombosis with superimposed endocarditis.
Management of pacemaker lead endocarditis includes generator removal along with complete lead extraction (via either percutaneous extraction or open surgical removal using cardiopulmonary bypass), and pathogen-specific intravenous antimicrobial therapy. In high-volume centers, percutaneous lead removal can be performed relatively safely with a high rate of success (Baddour et al., 2010). Surgical approach is indicated in patients who have significant retained hardware after attempts at percutaneous removal or in patients with lead vegetations >2 cm in diameter (Baddour et al., 2010). Even though, pacemaker related endocarditis is relatively rare, recent studies have reported in-hospital mortality rates ranging between 3.7% and 14% despite generator removal, complete lead excision, and pathogen-specific intravenous antimicrobial therapy (Sohail et al., 2007, 2008). We believe this case report is unusual on account of pacemaker lead thrombosis as well as endocarditis occurring in same pacemaker lead at same site in a patient with history of IVDA. Previously, Mellert et al. (2007) have reported a similar case, but the vegetations were seen in atrial part of lead and the thrombus on the ventricular part.
3. Conclusions
This case confirms that patients with pacemakers are at risk of developing pacemaker lead thrombosis. In addition, they are at high risk of developing pacemaker lead endocarditis if additional risk factors for endocarditis are present. We believe this case report is unusual on account of pacemaker lead thrombosis as well as endocarditis occurring in a patient with history of intravenous drug abuse. Whether pacemaker patients with multiple leads need to be on long-term antiplatelet or anticoagulation therapy necessitates further studies.
Conflict of interest
There is no conflict of interest or financial disclosure for our article.
References
- Alizadeh A., Maleki M., Bassiri H. Evaluation of atrial thrombus formation and atrial appendage function in patients with pacemaker by transesophageal echocardiography. Pacing Clin. Electrophysiol. 2006;29:1251–1254. doi: 10.1111/j.1540-8159.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Baddour LM., Epstein AE., Erickson CC. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- Cabell CH., Heidenreich PA., Chu VH. Increasing rates of cardiac device infections among medicare beneficiaries: 1990–1999. Am. Heart J. 2004;147:582–586. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Carda R., Almería C., Lennie V. What to do with an atrial thrombus? Eur. J. Echocardiogr. 2008;9:204–205. doi: 10.1093/ejechocard/jem072. [DOI] [PubMed] [Google Scholar]
- Korkeila PJ., Saraste MK., Nyman KM. Transesophageal echocardiography in the diagnosis of thrombosis associated with permanent transvenous pacemaker electrodes. Pacing Clin. Electrophysiol. 2006;29:1245–1250. doi: 10.1111/j.1540-8159.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- Mellert F., Bimmel D., Preusse CJ. Abnormal coiling of a pacemaker lead into the pulmonary artery with massive infectious thrombosis. Europace. 2007;9:904–905. doi: 10.1093/europace/eum139. [DOI] [PubMed] [Google Scholar]
- Saydain G., Singh J., Dalal B. Outcome of patients with injection drug use-associated endocarditis admitted to an intensive care unit. J. Crit. Care. 2010;25:248–253. doi: 10.1016/j.jcrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sohail MR., Uslan DZ., Khan AH. Management and outcome of permanent and implantable cardioverter-defibrillator infections. J. Am. Coll. Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- Sohail MR., Uslan DZ., Khan AH. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin. Proc. 2008;83:46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- van Rooden CJ., Molhoek SG., Rosendaal FR. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J. Cardiovasc. Electrophysiol. 2004;15:1258–1262. doi: 10.1046/j.1540-8167.2004.04081.x. [DOI] [PubMed] [Google Scholar]


