Summary
Although it is widely believed that non-segmental vitiligo (NSV) results from the autoimmune destruction of melanocytes, a clear understanding of defects in immune tolerance, which mediate this uncontrolled self-reactivity, is still lacking. In the present study, we systemically evaluated circulating regulatory T (Treg) cells, including CD4+CD25+FoxP3+ Treg cells and invariant natural killer T (iNKT) cells, as well as naïve and memory CD4+ and CD8+ T cells and their cytokine production, in a cohort of 43 progressive NSV patients with race-, gender-, and age-matched healthy controls. We found that the general immunophenotypes of CD4+ and CD8+ T cells and the percentage of CD4+CD25+FoxP3+ Tregs were comparable between NSV and healthy controls. However, percentages of peripheral iNKT cells were significantly decreased in NSV patients compared to that in healthy controls. Our data confirm the previous notion that the percentage of peripheral CD4+CD25+FoxP3+ Tregs remains unaltered in NSV and suggests the involvement of defective iNKT cells in the pathogenesis of NSV.
Keywords: invariant natural killer T cells, non-segmental vitiligo, regulatory T cells, immunophenotypes
Introduction
Vitiligo is a chronic skin disorder, characterized by progressive skin depigmentation because of the loss of melanocytes. There are two broad types of vitiligo: segmental vitiligo (SV) and NSV, with the latter comprising several topographic and extent variants. At the Vitiligo Global Issues Consensus Conference held during the 2011 International Pigment Cell Conference, it was suggested that SV should be classified separately from all other forms of vitiligo (V). Although not fully satisfactory, the term NSV is currently used as an umbrella term for different clinical subtypes of vitiligo, which are all clearly different from SV, including acrofacial, generalized, mucosal (multifocal), and universal (Ezzedine et al., 2012). The prevalence of NSV in general population is approximately 0.5–1%; it accounts for up to 90% of total vitiligo cases (Spritz, 2010; Taieb and Picardo, 2009). The pathogenesis of NSV is still not completely understood, although many hypotheses have been proposed, including autoimmune, cytotoxic (an intrinsic defect of melanocytes), oxidative damage, and neural mechanisms. The destruction of melanocytes by autoimmune mechanism is the major hypothesis toward explaining the pathogenesis of NSV. Results from recent genome-wide association studies identified NSV susceptibility genes that are almost exclusively involved in biologic pathways related to immune regulation and immune targeting of melanocytes, further supporting the hypothesis of NSV as a primary autoimmune disease (Jin et al., 2007, 2010; Quan et al., 2010; Spritz, 2012). Recently, three additional susceptibility loci, namely thymic stromal lymphopoietin, FOXP3 (forkhead box P3), and CTLA-4 loci were found to be associated with NSV (Birlea et al., 2011). These three loci have been also clearly shown to have a critical role in T-cell differentiation and proliferation, further suggesting a relationship between immune disregulation and NSV development. Similar to other autoimmune diseases, the current dogma proposes that several genetic susceptibility genes in certain environments trigger the immune system to attack ‘self-antigens’, in this case the pigmentary system, resulting in the development of NSV. It should be noted that the exact mechanism of melanocyte loss by autoimmunity is still unclear, even though many studies have pointed out that cellular immunity plays an important role in the pathogenesis of NSV.
Previous studies have focused on determining the different types of immune cells in the local and peripheral compartment, which are involved in this process. Immunohistochemical studies indicated that both CD4+ and CD8+ T cells were detected in the perilesional skin of NSV, with decreased CD4/CD8 ratio (Ongenae et al., 2003). CD8+ T cells isolated from peripheral blood and perilesional skins of NSV patients were reactive to melanocytes antigen-specific stimulation and were cytotoxic to melanocytes (Van Den Boorn et al., 2009; Wankowicz-Kalinska et al., 2003). Furthermore, analysis of the broad spectrum of cytokines produced by perilesion-derived CD4+ and CD8+ T cells confirmed polarization toward a type-1-like phenotype in both CD4+ and CD8+ compartments, which paralleled the depigmentation process observed clinically. T-cell infiltrates are commonly observed in progressive NSV; NSV patients have been reported to have increased numbers of peripheral T cells, which were reactive to melanocyte differentiation antigens, including tyrosinase, gp100, and melanoma antigen recognized by T cells (MART-1), compared to healthy donors (Mandelcorn-Monson et al., 2003; Palermo et al., 2001). Disappearing melanocytes were found to be co-localized with CD8+ T cells, and they were demonstrated to express the skin homing and T-cell activation markers, perforin, and granzyme B (Le Poole et al., 1996; Van Den Wijngaard et al., 2000). A recent study further substantiated the role of T cells in NSV by showing melanocyte-specific cytotoxic activity of perilesional T cells in NSV patients (Van Den Boorn et al., 2009). However, melanocyte-reactive CD8+ T cells are also found in healthy controls, suggesting that autoimmune reactivity is kept in check in healthy controls, and this modulation is defective in NSV (Visseren et al., 1995).
The mechanisms underlying the induction of autoreactive T cells and the loss of tolerance to melanocyte antigens have not been fully elucidated. Regulatory T (Treg) cells are known to inhibit autoreactivity and keep autoimmune responses in check. Different populations of Treg cells have been described, including thymically derived CD4+CD25+FoxP3+ Treg cells and natural killer T (NKT) cells. Accumulating data indicate that a deficiency or dysfunction of CD4+CD25+FoxP3+ Tregs may cause either systemic or organ-specific autoimmune diseases. Recently, Klarquist et al. (2010) reported that the number and function of circulating Tregs in NSV were comparable to that of healthy controls, but NSV patients had reduced skin homing functional Treg. Interestingly, more recently, Ben Ahmed et al. (2012) reported the involvement of a functional defect of peripheral CD4+CD25+ Treg cells in NSV patients, thus the precise role of CD4+CD25+FoxP3+ Treg cells in the pathogenesis of NSV warrants clarification.
Natural killer T (NKT) cells are another type of regulatory T cells that bridge the innate and adaptive immune systems. NKT cells are a heterogeneous subpopulation of T lymphocytes that co-express T-cell receptor (TCR) and NK lineage markers such as CD16, CD56, CD57, CD94, and CD161. Unlike conventional T cells, NKT cells recognize glycolipid antigens in the presence of the MHC class I-like antigen-presenting molecule CD1d (Linsen et al., 2005a). Type I classical or invariant NKT (iNKT) cells have a highly restricted TCR repertoire as they express an invariant Vα24-Jα18 rearranged TCRβ chain in humans, typically co-expressed with Vβ11-containing β chain; they can be identified by CD1d tetramers loaded with α-galactosylceramide (α-GalCer). The most remarkable property of iNKT cells is their ability to rapidly produced large amount of cytokines, such as IFN-γ and IL-4 (Linsen et al., 2005a; Matsuda et al., 2008). Consequently, iNKT cells have been shown to play crucial roles in a broad range of diseases, including infectious diseases, autoimmunity, and cancer (Bendelac et al., 2007; Wu and Van Kaer, 2009). A deficiency or dysfunction in iNKT cells has been found in different autoimmune diseases in human and mice, including rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes (Bendelac et al., 2007; Cho et al., 2011; Laloux et al., 2001; Mars et al., 2004). It should be noted that many autoimmune diseases are epidemiologically associated with NSV, which imply the potential involvement of iNKT cells in the pathogenesis of NSV. However, there has been no iNKT cell studies reported in NSV so far.
In this study, we evaluated the general immune phenotypes, including the percentages of naïve and memory CD4 and CD8 T cells and their cytokine production, and the percentages of circulating CD4+CD25+FoxP3+ Treg cells and iNKT cells as well as iNKT cytokine secretion functions, in a cohort of 43 NSV patients with active disease, and the results were compared to those obtained from race-, gender-, and age-matched healthy individual controls.
Results
Normal distribution of conventional T cells in the PBMCs of NSV patients
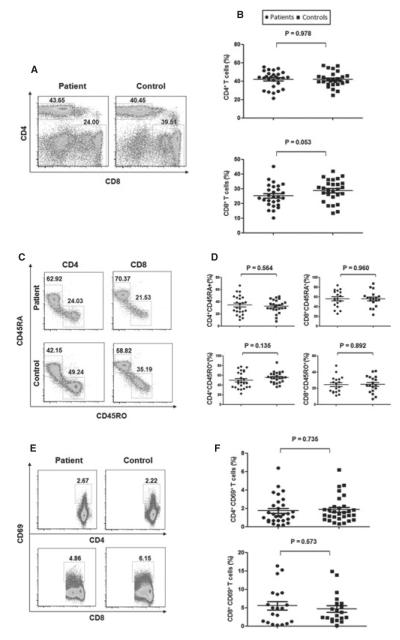
The immunophenotypes of circulating conventional T cells in our NSV patients were first characterized by flow cytometry. As shown in Figure 1A, B, the percentage of CD4+ T cells (41.94 ± 9.14%) and CD8+ T cells (25.26 ± 7.67%) in peripheral blood mononuclear cells (PBMCs) of NSV patients were comparable to healthy controls (41.90 ± 8.04% for CD4+ and 28.70 ± 7.53% for CD8+ cells, respectively), and the ratios of CD4/CD8 were also not significantly different (data not shown). The percentage of memory (CD45RO+) and naïve (CD45RA+) CD4+ and CD8+ cells were then evaluated (Figure 1C, D), and no significant difference was identified comparing patients with healthy controls (CD4+CD45RO+, 50.15 ± 16.82% versus 55.56 ± 11.32%; CD4+CD45RA+, 34.74 ± 15.35% versus 32.70 ± 10.45%; CD8+CD45RO+, 24.57 ± 10.10% versus 25.00 ± 10.24%; CD8+CD45RA+, 56.33 ± 17.10 versus 56.08 ± 16.20%, respectively, P > 0.05). T-cell activation based on CD69 expression was further analyzed. As shown in Figure 1E, F, no significant differences (P > 0.05) were observed in the percentages of CD4+ CD69+ (1.74 ± 1.46%) and CD8+CD69+ (5.32 ± 5.12%) T cells from NSV, compared to the percentages of CD4+CD69+ (1.85 ± 1.4%) and CD8+CD69+ (4.68 ± 4.04%) T cells from healthy controls. Thus, NSV patients from our large cohort had normal distribution and activation of conventional T cells in PBMCs.
Figure 1.
Distribution of conventional T cells in the peripheral blood. (A) Flow cytometry analysis of CD4+ and CD8+ T cells in peripheral blood mononuclear cells (PBMC) from non-segmental vitiligo (NSV) patients and healthy controls. (B) Summary plots showing individual results of the frequency of CD4+ and CD8+ T cells described. (C) Flow cytometry analysis of memory (CD45RO+) and naïve (CD45RA+) CD4+ and CD8+ T cells in PBMCs. (D) Summary plots showing individual results of the frequency of CD45RA+ and CD45RO+ CD4+ and CD8+ T cells in NSV patients versus healthy controls. (E) Representative FACS dot plots for CD69 expression in CD4+ and CD8+ T cells. (F) Summary plots showing individual results of the frequency of CD69+ CD4+ T cells and CD69+ CD8+ T cells in NSV patients versus healthy controls.
No alteration in the frequencies of CD4+Foxp3+ Treg cells in the PBMCs of NSV patients
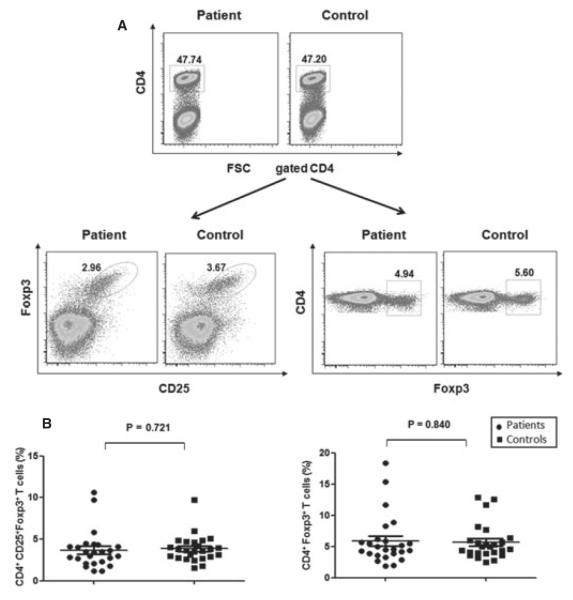
We next addressed whether the percentage of Treg cells in PBMCs was altered in NSV patients compared to healthy controls. PBMCs were stained with antihuman CD4, CD25, and Foxp3 antibodies. Lymphocytes gated on CD4+ T cells were analyzed for the expression of combined CD25 and FoxP3 or FoxP3 expression alone (Figure 2A). Non-segmental vitiligo patients had no significant difference in the percentage of CD4+CD25+Foxp3+ Treg cells (3.69 ± 2.26%) compared with healthy controls (3.85 ± 1.62; P > 0.05). Furthermore, no difference was found in the percentage of CD4+Foxp3+ Treg cells between NSV patients and controls (5.68 ± 2.87% versus 5.87 ± 3.97%; P > 0.05) (Figure 2B). Additionally, when the ratio of memory/activated T cells (CD4+CD45RO+ cells) to CD4+Foxp3+CD25+ Treg cells was analyzed in NSV patients and controls, no significant difference was observed (15.87 ± 5.69% in controls and 15.50 ± 7.66% in NSV patients, P > 0.05). Thus, the percentage of Treg cells in PBMCs was not altered in NSV patients.
Figure 2.
Flow cytometry analysis of peripheral blood Foxp3+CD4+ Treg cells. (A) Peripheral blood mononuclear cells (PBMCs) were stained with anti-CD4, CD25, and Foxp3. Gated CD4+ lymphocytes were selected for CD25+Foxp3+ or Foxp3+ cell analyses, respectively. (B) Summary plots showing individual results of the frequency of CD25+Foxp3+CD4+ and Foxp3+CD4+ in non-segmental vitiligo (NSV) patients versus healthy controls.
Cytokine-producing T cells in the peripheral blood are not altered in NSV patients
Previous work has suggested a possible role for Th1 cells and inflammatory cytokine IFN-γ in the development of NSV in humans as well as in mice (Gregg et al., 2010; Van Den Wijngaard et al., 2000). We then analyzed the percentages of IL4- and IFN-γ-producing CD4+ and CD8+ T cells in PBMCs. As shown in Figure 3A, B, there were no significant differences in the percentages of IFN-γ+CD4+ (8.02 ± 4.65% in NSV and 9.35 ± 6.71% in control; P > 0.05) and IFN-γ+CD8+ T cells (28.88 ± 12.77% in NSV and 35.72 ± 15.44% in controls; P > 0.05), or IL4+CD4+ (2.23 ± 2.17% in NSV and 2.44 ± 1.93% in controls; P > 0.05) and IL4+CD8+ T cells (1.74 ± 1.26% in NSV and 1.62 ± 1.46% in controls; P > 0.05) between NSV and controls. Thus, NSV patients had comparable IL4- and IFN-γ-producing T cells in PBMCs compared to that from healthy controls.
Figure 3.
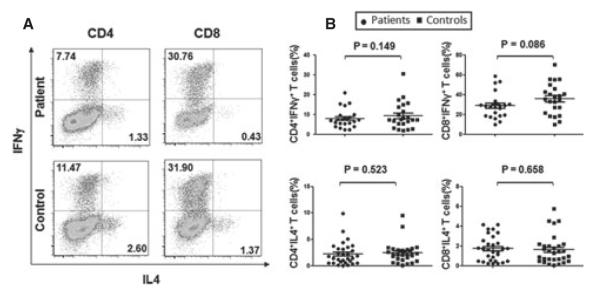
Cytokine-producing T cells from peripheral blood. Peripheral blood mononuclear cells (PBMCs) isolated from non-segmental vitiligo (NSV) patients and healthy controls were stimulated with phorbol myristate acetate (PMA) and ionomycin for 5 h in the presence of Golgi stop. Then, the IFNγ- and IL-4-producing T cells were determined by intracellular staining and flow cytometry analysis. (A) Representative FACS dot plots for IL-4- and IFN-γ-producing CD4+ and CD8+ T cells gated on CD4-positive and CD8-positive cells. (B) Summary plots showing individual results of the frequency of IL4- and IFN-γ-producing CD4+ and CD8+ T cells in NSV patients versus healthy controls.
Reduced frequency of circulating iNKT cells in NSV patients
Invariant natural killer T cells have been shown to play crucial roles in the development of human autoimmune diseases (Bendelac et al., 2007; Wu and Van Kaer, 2009). αGalCer-loaded CD1d tetramer is the best reagent currently available to accurately identify human iNKT cells in terms of specificity and sensitivity (Berzins et al., 2011; Lee et al., 2002b). The percentage of peripheral blood iNKT cells was determined for the 43 NSV patients and 43 healthy individuals using CD1d-loaded αGalCer tetramers. As shown in Figure 4, the percentage of iNKT cell in PBMCs from both NSV patient and healthy individual groups varied widely, ranging from 0.001 to 0.58%. However, the percentage of iNKT cells in NSV patients (0.065 ± 0.015%) was significantly reduced compared to that in healthy controls (0.108 ± 0.02%, P = 0.0359). This result suggests that defective iNKT cells may be involved in NSV development.
Figure 4.
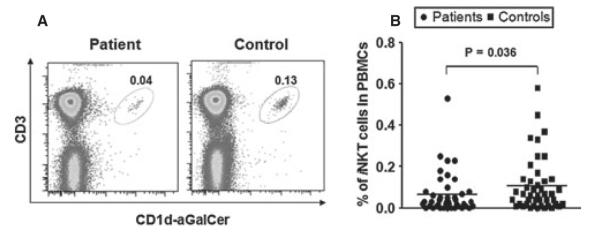
Invariant natural killer T (iNKT) cell frequency in peripheral blood mononuclear cells (PBMCs) of non-segmental vitiligo (NSV) patients. Peripheral blood mononuclear cells were separated by Ficoll gradient, and flow cytometry was performed using CD1d-aGalCer tetramers and anti-mouse CD3 antibody. (A) Representative FACS dot plots for iNKT cells from NSV patient sand healthy controls. (B) Summary plots showing individual results of iNKT cell frequency in NSV patients versus healthy controls.
Phenotypes of iNKT in NSV patients
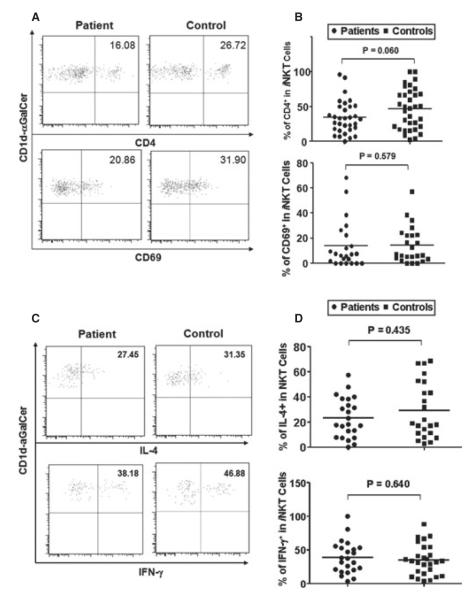
Mature human iNKT cells can be divided into functionally distinct CD4+ and CD4− two subsets and CD69 was used as a marker characterizing iNKT cell maturation and activation status. To investigate whether iNKT cells from NSV patients exhibited phenotypic abnormalities, we first analyzed the percentages of CD4+ iNKT subset and CD69+ iNKT cells in PBMCs. Figure 5A shows the representative dot plots from the analysis of CD4+ subset and CD69+ iNKT cells. As shown in Figure 5B, there were no significant differences detected between NSV patients and healthy controls. However, iNKT cells from NSV patients exhibit a trend toward decreased CD4+ subset (P = 0.06). Activated iNKT cells release large amounts of cytokines, including IL-4 and IFN-γ. As shown in Figure 5C, there was a strong induction of IFN-γ and IL-4 from activated iNKT cells. However, the percentages of IFN-γ- and IL-4- producing iNKT cells from both NSV patients and control groups varied widely, and no significant difference was identified in the capacity of peripheral iNKT cells cytokine secretion between NSV patients and healthy controls (Figure 5D).
Figure 5.
Invariant natural killer T (iNKT) cell subsets, maturation, activation status, and cytokine production in non-segmental vitiligo (NSV) patients. Circulating iNKT cells were identified as CD1d-aGalCer tetramer+CD3+ cells, and then their subsets, maturation, and activation were further determined by the expression of CD4 and CD69, respectively. Intracellular IFN-γ and IL-4 production of circulating iNKT cells was analyzed after stimulation with phorbol myristate acetate (PMA) plus ionomycin in the presence of GolgiStop for 5 h. (A) Representative FACS dot plots for iNKT cell CD4 and CD69 expression in NSV patients and healthy controls. (B) Summary plots showing individual results of the frequency of CD4+ iNKT cell and CD69+ iNKT cells in NSV patients versus healthy controls. (C) Representative FACS dot plots for IL-4 and IFN-γ expression in gated iNKT cells. (D) Summary plots showing individual results of the frequency of IL4- and IFN-γ-producing iNKT cells in NSV patients versus healthy controls.
Discussion
Autoimmune diseases are characterized by failure in the mechanisms of tolerance to self-antigens. Immunoregulation by Treg cells, including CD4+FoxP3+CD25+Tregs and iNKT cells, has emerged as an important part of peripheral tolerance. Studies from our laboratory and others suggest that defects in the number and function of Treg cells contribute to autoimmune disease development (Ly et al., 2006; Zhou et al., 2011). Although the pathogenesis of NSV is still not yet fully understood, accumulated studies suggest that NSV is an autoimmune disease (Le Poole and Luiten, 2008; Spritz, 2011). The present study represents, to our knowledge, the first large-scale systemic analysis of basic immunophenotypes, from conventional T cells to Treg cells, in NSV patients with strictly matched healthy individual controls. We found that there were no differences in the percentages of conventional CD4/CD8, naïve or memory/active CD4/CD8 T cells, or their Th1/Th2 cytokine production, and that the percentages of CD4+CD25+ FoxP3+Treg cells were comparable between NSV patients and healthy controls. However, the percentages of peripheral iNKT cells were significantly decreased in NSV patients, suggesting the possible involvement of iNKT cells in the autoimmunity of NSV.
Previous studies indicated the potential abnormal subsets of circulating T cells in NSV (Basak et al., 2008; Mahmoud et al., 2002; Pichler et al., 2009). Mahmoud et al. (1998, 2002) reported that NSV patients from Japanese population had elevated percentages of memory (CD4+CD45RO+) T cells and decreased percentages of naive T cells (CD4+CD45RA+) compared to healthy controls, supporting the hypothesis of T-cell activation as a major feature of the disorder. Interestingly, we did not find the significant differences in our NSV patients compared to healthy controls. This may be related to different genetic background of patient and control subjects. The percentages of CD4+CD25+Treg cells in peripheral blood in most autoimmune diseases investigated to date have either been normal or decreased. On analyzing peripheral CD4+CD25+FoxP3+Treg cells in 13 NSV patients, Klarquist found no differences in the percentage and immune suppression function between NSV patients and controls, but CD4+CD25+FoxP3+Treg cells from NSV patients had defects on skin homing (Klarquist et al., 2010). More recently, Ben Ahmed et al. also reported the comparable frequency of Tregs (CD4+CD25high) between NSV (10 patients) and controls, but they stated that the suppressive function of CD4+CD25high Treg cells was decreased in four progressive NSV patients (Ben Ahmed et al., 2012). Nonetheless, it is now recognized that CD25+ alone is not the best marker for detecting CD4+Tregs, especially for individuals with progressive inflammatory diseases, in which CD4+CD25highTregs could potentially be contaminated with activated CD4+ T cells. In our study, we analyzed CD4+CD25+FoxP3+Treg cells in 43 progressive NSV. Our results further support previous findings (Ben Ahmed et al., 2012; Klarquist et al., 2010) that the percentages of peripheral CD4+CD25+FoxP3+Treg cells were not altered in NSV patients. However, the suppressive function of CD4+CD25+FoxP3+Tregs remains to be validated in future studies.
Another key immunoregulatory T cell is the iNKT cell. Understanding of the role of iNKT cells in the development of NSV is lacking. We used CD1d-tetramer staining, a specific method for iNKT identification, to accurately determine the percentage and function of iNKT cells. A significant decrease in peripheral blood iNKT cells was observed in NSV patients. Mahmoud previously reported that peripheral NKT cells (CD3+ CD16+CD56+) were significantly reduced in NSV patients compared to healthy controls (Mahmoud et al., 2002). Even though the NKT cells in their analysis contained both iNKT cells and other non-iNKT cells, our results further support their findings. Although iNKT cells constitute only a small fraction of lymphocytes, their ability to rapidly secrete large amounts of cytokines, including IFN-γ, IL-4, IL-10, and IL-13, makes them an important regulator of the Th1/Th2 cytokine balance in immune responses. Human iNKT cells segregate into CD4+ and CD4− subsets with distinct phenotypic and functional characteristics (Gumperz et al., 2002; Kim et al., 2002; Lee et al., 2002a). CD4+iNKT cells produce both Th1 and Th2 cytokines, whereas the CD4− subset exhibits a Th1 cytokine profile. We showed here that iNKT cells in NSV exhibited a trend of decreased CD4+ subset, even though the difference was not statistically significant. Significant progress has been made regarding the role of iNKT cells in the pathogenesis of human autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, type 1 diabetes (T1D), systemic lupus erythematosus, and adrenoleukodystrophy (Gautron et al., 2010; Illes et al., 2000; Kojo et al., 2001; Linsen et al., 2005b; Mars et al., 2004; Sumida et al., 1995; Van Der Vliet et al., 2001), suggesting a protective role of iNKT cells. We and others have previously shown that deficiencies in iNKT cell number and function mediate the development of T1D in nonobese diabetic (NOD) mice, and that αGalCer-induced NKT cell activation corrected these deficiencies and reduced the incidence of spontaneous and recurrent diabetes in NOD mice (Mi et al., 2003, 2004; Sharif et al., 2001). In studies using T1D mouse model, it has been demonstrated that Th1 cell-mediated tissue damage was initially regulated by iNKT cells and the protection conferred by iNKT cells was associated with a Th2 shift within the pancreatic islets, and IL-4 has also been implicated as a key mediator of immunoregulation (Hammond et al., 1998; Laloux et al., 2001). It has been shown that psoriasis is associated with decreased numbers of circulating iNKT cells (Koreck et al., 2002; Van Der Vliet et al., 2001), and a decreased percentage of CD3+CD56+ population of iNKT cells in patients with psoriasis tended to be even lower in those patients with frequently relapsing and treatment-resistant disease (Koreck et al., 2002). In multiple sclerosis, the critical role of iNKT phenotype in disease pathogenesis was demonstrated by the strong Th2 bias of the CD4+ iNKT cell lines from patients in remission compared with that from patients in relapse (Araki et al., 2003). Thus, taken together, the decreased peripheral blood iNKT cell percentage and the trend toward changed iNKT cell phenotype suggest a protective and immune regulatory role of iNKT cells in the pathogenesis of NSV.
As is the case for the association of iNKT cells with multiple other autoimmune diseases, key unanswered questions regarding the role of iNKT cells in NSV include whether iNKT cell deficiencies predispose individuals to disease and what the cause(s) of iNKT cell deficiencies is (are). Our current analysis has certain limitations; for example, in iNKT cell function analyses, phorbol myristate acetate (PMA) and ionomycin activated iNKT cells through bypass of the TCR upstream signal, which cannot elucidate whether iNKT cells responding to their TCR through a-GalCer are defective, including iNKT cell proliferation and cytokine production. To gain a better understanding of the significance of iNKT cell defects in the pathogenesis of NSV, a more comprehensive characterization of iNKT cells from blood, especially during both the relapse and remission phases of disease, will be needed. Studying the role of iNKT cells in skin lesions may further uncover how iNKT cells contribute to disease pathogenesis locally. However, only very few infiltrating cells could be isolated from vitiligo skin lesions; with the known low frequency of iNKT cells, it was not possible to accurately perform FACS analyses for iNKT cell number and subset, and to assess their cytokine production.
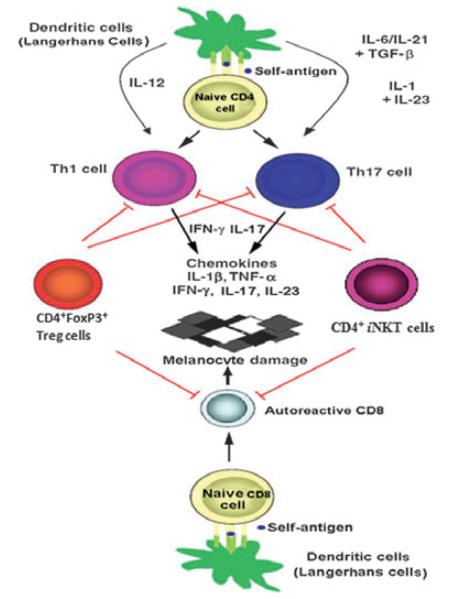
Using an iNKT cell-deficient mouse model, a recent study demonstrated that iNKT cells play an important role in limiting the development of the Th17 lineage, suggesting that iNKT cells provide a natural barrier against Th17 responses (Mars et al., 2009). Interestingly, additional studies indicate that in patients with NSV, serum IL-17 levels were positively correlated with the extent of body area involvement (Basak et al., 2009) and that Th17 cells were increased in NSV lesions (Bassiouny and Shaker, 2011; Kotobuki et al., 2012; Wang et al., 2011). Thus, iNKT cell deficiency in NSV may not only contribute to Th1 and autoreactive CD8 T cells, but also to dysregulated Th17 cells. Taken together, our data suggest that iNKT cells may play a role in the pathogenesis of NSV. As shown in Figure 6, both iNKT cells and CD4+Foxp3+ Treg cells may work together to regulate autoreactive Th1, Th17, and CD8+ T cells during NSV development. Additional studies are clearly needed to further test this hypothesis.
Figure 6.
A model for immunopathogenesis of autoimmune nonsegmental vitiligo (NSV). Dendritic cells (Langerhans cells in epidermis) present self-antigen to activate naïve CD4+ and CD8+ T cells and induce naïve CD4+ T cells to differentiate into Th1 and Th17, which mediate the destruction of melanocytes. CD4+CD25+Foxp3+ Tregs and invariant natural killer T (iNKT) cells can block these processes through the secretion of protective cytokine (IL-4 and IL10) or cell–cell contact to suppress Th1, Th17, and autoreactive CD8+ T-cell function. Defective frequencies and functions of CD4+CD25+Foxp3+ Tregs and iNKT cells, with up-regulated autoreactive Th1, Th17, and CD8+ T cells, contribute to immune destruction of melanocytes in autoimmune NSV.
Methods
Patients
The study cohort included 43 progressive NSV patients and 43 age-, gender-, and race-matched healthy controls. Patients with NSV were recruited with the following criteria: (i) a clinical diagnosis of generalized NSV with related criteria (Taieb and Picardo, 2007), (ii) discontinuation of any systemic therapy for at least 4 weeks, and (iii) development of new lesions within the past 3 months. Clinical characteristics of the patients are summarized in Table 1. The study was approved by the Institutional Review Board of Henry Ford Health System, and written informed consent was obtained from all participants.
Table 1.
Clinical characteristics of NSV patients and healthy controls
| Characteristics | Healthy controls (n = 43) |
NSV patients (n = 43) |
|---|---|---|
| Sex (male/female) | 15/28 | 15/28 |
| Age, median (range) | 40 (18–71) | 45 (18–72) |
| Autoimmune diseases (positive/negative) |
ND | 13/30 |
| Family history of NSV (positive/negative) |
ND | 16/27 |
| Percent of body surface area involved, Median (range) |
ND | 15 (5–100) |
NSV, non-segmental vitiligo.
T-cell stimulation
Peripheral blood mononuclear cells (2 × 106/ml) were cultured in RPMI 1640 medium in 24-well plate and stimulated for 5 h at 37°C with 50 ng/ml of PMA and 1 μmol/ml of Ionomycin in the presence of 0.67 μl/ml Golgi stop (BD Bioscience, San Jose, CA, USA). After incubation, cells were collected for flow cytometry analysis.
Flow cytometry analyses
To analyze CD4, CD8, Tregs, and iNKT cell frequencies and phenotypes, PBMCs were stained with the following antihuman antibodies: anti-CD3 (clone SK7), anti-CD4 (RPA-T4), anti-CD8 (RPA-T8), anit-CD25 (M-A251), anti-CD69 (FN50), CD45RA (HI100), CD45RO (UCHL1), and CD1d-αGalCer tetramers (NIH Tetramer Facility). For intracellular Foxp3 and cytokine staining, PBMCs were fixed and permeabilized and with fixation/permeabilization solution, and then stained with antihuman IFN-γ (4S.B3), IL-4 (MP4-25D2), and Foxp3 (M-A251). All antibodies were purchased from BD Biosciences or eBioscience. Stained cells were analyzed either on a FACSCalibur or FACSAriaII (BD Bioscience) using FlowJo software (Tree Star Inc, Ashland, OR, USA). To make the data from patients and healthy controls maximally comparable, patient and matched control PBMCs were activated and then stained with antibodies and analyzed side by side.
Statistical analysis
Data are presented as mean ± SD, frequencies, and percentages as appropriate. For data that were not normally distributed, comparison of quantitative variables between the study groups was performed using the Mann–Whitney U test. For all cases, P < 0.05 was considered significant. All statistical calculations were performed using the Prism program (GraphPad, La Jolla, CA, USA).
Significance.
The present study represents, to our knowledge, the first large-scale systemic analyses of peripheral T-cell immunophenotypes, from conventional T cells to regulatory T cells (Tregs), in a large cohort of progressive non-segmental vitiligo (NSV) patients. Our results show that there are no significant differences in the basic immunophenotypes of conventional CD4/CD8 T cells and the percentages of CD4+CD25+FoxP3+ Tregs between NSV patients and healthy controls. However, the percentages of peripheral invariant natural killer T (iNKT) cells, which have been thought to play a major role in autoimmune diseases, were significantly decreased in NSV patients. Thus, our results highly suggest that iNKT cells may be involved in the immnuopathogeneis of NSV.
Acknowledgements
The authors thank all patients who participated in this study, and Matthew Weiland and Le Qu for their help in processing the PBMCs. This work was supported in part by the grants from National Institutes of Health (AR059976), Vitiligo Research Foundation, the Shahani Foundation, and Henry Ford Health System Research Grant for the Immunology Program (T71016 and T71017).
Footnotes
Conflict of interest No conflict of interest disclosures.
References
- Araki M, Kondo T, Gumperz JE, Brenner MB, Miyake S, Yamamura T. Th2 bias of CD+ NKT cells derived from multiple sclerosis in remission. Int. Immunol. 2003;15:279–288. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB. Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J. Eur. Acad. Dermatol. Venereol. 2008;22:970–976. doi: 10.1111/j.1468-3083.2008.02681.x. [DOI] [PubMed] [Google Scholar]
- Basak PY, Adiloglu AK, Ceyhan AM, Tas T, Akkaya VB. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J. Am. Acad. Dermatol. 2009;60:256–260. doi: 10.1016/j.jaad.2008.09.048. [DOI] [PubMed] [Google Scholar]
- Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin. Exp. Dermatol. 2011;36:292–297. doi: 10.1111/j.1365-2230.2010.03972.x. [DOI] [PubMed] [Google Scholar]
- Ben Ahmed M, Zaraa I, Rekik R, et al. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res. 2012;25:99–109. doi: 10.1111/j.1755-148X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- Birlea SA, Jin Y, Bennett DC, et al. Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J. Invest. Dermatol. 2011;131:371–381. doi: 10.1038/jid.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YN, Kee SJ, Lee SJ, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology (Oxford) 2011;50:1054–1063. doi: 10.1093/rheumatology/keq457. [DOI] [PubMed] [Google Scholar]
- Ezzedine K, Lim H, Suzuki T, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–E13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron AS, Giquel B, Beaudoin L, Autrusseau E, Speak A, Platt F, Kemp S, Pujol A, Aubourg P, Lehuen A. Invariant NKT cells in adrenoleukodystrophy patients and mice. J. Neuroimmunol. 2010;229:204–211. doi: 10.1016/j.jneuroim.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J. Immunol. 2010;184:1909–1917. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. Alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NK T cell V alpha 24J alpha Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 2000;164:4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- Jin Y, Mailloux CM, Gowan K, Riccardi SL, Laberge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- Jin Y, Birlea SA, Fain PR, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N. Engl. J. Med. 2010;362:1686–1697. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516–519. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–286. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ1+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–1138. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Koreck A, Suranyi A, Szony BJ, Farkas A, Bata-Csorgo Z, Kemeny L, Dobozy A. CD3+CD56+ NK T cells are significantly decreased in the peripheral blood of patients with psoriasis. Clin. Exp. Immunol. 2002;127:176–182. doi: 10.1046/j.1365-2249.2002.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, Fujimoto M, Serada S, Naka T, Katayama I. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25:219–230. doi: 10.1111/j.1755-148X.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- Laloux V, Beaudoin L, Jeske D, Carnaud C, Lehuen A. NK T cell-induced protection against diabetes in V alpha 14-J alpha 281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J. Immunol. 2001;166:3749–3756. doi: 10.4049/jimmunol.166.6.3749. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr. Dir. Autoimmun. 2008;10:227–243. doi: 10.1159/000131485. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Van Den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am. J. Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 2002a;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J. Clin. Invest. 2002b;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T cells. Hum. Immunol. 2005a;66:1193–1202. doi: 10.1016/j.humimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Linsen L, Thewissen M, Baeten K, Somers V, Geusens P, Raus J, Stinissen P. Peripheral blood but not synovial fluid natural killer T cells are biased towards a Th1-like phenotype in rheumatoid arthritis. Arthritis Res. Ther. 2005b;7:R493–R502. doi: 10.1186/ar1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly D, Mi QS, Hussain S, Delovitch TL. Protection from type 1 diabetes by invariant NK T cells requires the activity of CD4+CD25+ regulatory T cells. J. Immunol. 2006;177:3695–3704. doi: 10.4049/jimmunol.177.6.3695. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Abul H, Al-Saleh Q, Haines D, Burleson J, Morgan G. Peripheral T-cell activation in non-segmental vitiligo. J. Dermatol. 1998;25:637–640. doi: 10.1111/j.1346-8138.1998.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Abul H, Haines D, Al-Saleh C, Khajeji M, Whaley K. Decreased total numbers of peripheral blood lymphocytes with elevated percentages of CD4+CD45RO+ and CD4+CD25+ of T-helper cells in non-segmental vitiligo. J. Dermatol. 2002;29:68–73. doi: 10.1111/j.1346-8138.2002.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, Debenedette MA. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J. Invest. Dermatol. 2003;121:550–556. doi: 10.1046/j.1523-1747.2003.12413.x. [DOI] [PubMed] [Google Scholar]
- Mars LT, Novak J, Liblau RS, Lehuen A. Therapeutic manipulation of iNKT cells in autoimmunity: modes of action and potential risks. Trends Immunol. 2004;25:471–476. doi: 10.1016/j.it.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Mars LT, Araujo L, Kerschen P, Diem S, Bourgeois E, Van LP, Carrie N, Dy M, Liblau RS, Herbelin A. Invariant NKT cells inhibit development of the Th17 lineage. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6238–6243. doi: 10.1073/pnas.0809317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr. Opin. Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi QS, Meagher C, Delovitch TL. CD1d-restricted NKT regulatory cells: functional genomic analyses provide new insights into the mechanisms of protection against Type 1 diabetes. Novartis Found. Symp. 2003;252:146–160. discussion 160–4, 203–10. [PubMed] [Google Scholar]
- Mi QS, Ly D, Zucker P, Mcgarry M, Delovitch TL. Interleukin-4 but not interleukin-10 protects against spontaneous and recurrent type 1 diabetes by activated CD1d-restricted invariant natural killer T-cells. Diabetes. 2004;53:1303–1310. doi: 10.2337/diabetes.53.5.1303. [DOI] [PubMed] [Google Scholar]
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90–100. doi: 10.1034/j.1600-0749.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J. Invest. Dermatol. 2001;117:326–332. doi: 10.1046/j.1523-1747.2001.01408.x. [DOI] [PubMed] [Google Scholar]
- Pichler R, Sfetsos K, Badics B, Gutenbrunner S, Berg J, Aubock J. Lymphocyte imbalance in vitiligo patients indi cated by elevated CD4+/CD8+ T-cell ratio. Wien. Med. Wochenschr. 2009;159:337–341. doi: 10.1007/s10354-009-0699-z. [DOI] [PubMed] [Google Scholar]
- Quan C, Ren YQ, Xiang LH, et al. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat. Genet. 2010;42:614–618. doi: 10.1038/ng.603. [DOI] [PubMed] [Google Scholar]
- Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat. Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med. 2010;2:78. doi: 10.1186/gm199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA. Recent progress in the genetics of generalized vitiligo. J. Genet. Genomics. 2011;38:271–278. doi: 10.1016/j.jgg.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J. Invest. Dermatol. 2012;132:268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. Selective reduction of T cells bearing invariant V alpha 24J alpha Q antigen receptor in patients with systemic sclerosis. J. Exp. Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A, Picardo M. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- Taieb A, Picardo M. Clinical practice. Vitiligo. N. Engl. J. Med. 2009;360:160–169. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- Van Den Boorn JG, Konijnenberg D, Dellemijn TA, Van Der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Invest. Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- Van Den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab. Invest. 2000;80:1299–1309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet HJ, Von Blomberg BM, Nishi N, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- Visseren MJ, Van Elsas A, Van Der Voort EI, Ressing ME, Kast WM, Schrier PI, Melief CJ. CTL specific for the tyrosinase autoantigen can be induced from healthy donor blood to lyse melanoma cells. J. Immunol. 1995;154:3991–3998. [PubMed] [Google Scholar]
- Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, Gilleaudeau P, Cohen JA, Krueger JG. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS ONE. 2011;6:e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankowicz-Kalinska A, Van Den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab. Invest. 2003;83:683–695. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- Zhou L, Park JJ, Zheng Q, Dong Z, Mi Q. MicroRNAs are key regulators controlling iNKT and regulatory T-cell development and function. Cell. Mol. Immunol. 2011;8:380–387. doi: 10.1038/cmi.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]