Abstract
The relationship between the central nervous system (CNS) and the endocrine system have been known for many years. Indeed some of the hormone secreting glands are actually located in the brain. The notion that the CNS and hormones are also involved in the bi-directional cross-talk with the Immune System has been the target of intense research in the recent decades. In this manner, for example, psychological states can be closely related to changes in immune mediators, and not only they may influence the evolution of human diseases, but may in the future lead to novel therapeutic interventions. This is the subject of this review, with particular emphasis on the role of psychoneuroimmunology (PNI) in psoriasis.
Keywords: Central nervous system, Endocrine system, Hormones, Psychoneuroimmunology, Psoriasis
Decades of research into mind-body connections has firmly established that there is an orchestrated bi-directional communication that occurs between the central nervous system (CNS) and the immune system which has a significant impact on both physical and psychological health and well-being. The CNS, along with the hard-wired sympathetic nervous system (SNS), provide signals to the immune system that are important aspects of the surveillance network that maintains an organism’s health following infectious or antigenic challenge. Much of our understanding of this communication, the subject of the field of psychoneuroimmunology (PNI), comes from both experimental animal manipulations and clinical observations that demonstrate that a wide variety of psychological, physical or physiological stressors can affect innate and adaptive immune function as examined either in vivo or in vitro.
The CNS response to stressors occurs chiefly via two pathways, the hypothalamo-pituitary adrenal (HPA) axis and the SNS. In response to stressors, the hypothalamus secretes corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) into the hypophyseal portal system. The increase in CRH results in downstream release of peptides from the pituitary that are produced by the differential cleavage of pro-opiomelanocortin (POMC), with the most relevant peptide for the stress response being adrenocorticotropic hormone (ACTH). ACTH is secreted into the circulating blood supply, and induces release of glucocorticoids (GC), which regulates glucose metabolism, from the adrenal cortex. CRH also stimulates noradrenergic neurons in the CNS. Activation of the noradrenergic pathways by CRH results in secretion of norepinephrine (NE) by the peripheral sympathetic nervous system and induces release of NE and epinephrine (EPI) from the adrenal medulla. When an animal experiences stress, then, the body prepares to respond quickly, via catecholamine-driven increases in heart rate and blood pressure, and glucocorticoid-mediated changes in glucose metabolism — as in the classic “fight-flight” response first proposed by Walter Cannon.1
As daily stress waxes and wanes throughout the day, there are fluctuating peripheral levels of CRH, ACTH, GC, NE and Epi, as well as other neurohormones and transmitters that occur as a function of both natural rhythms and stimuli that are registering at the level of the CNS. Not only are these hormones important for regulating the metabolic response to stress, but they also act as messengers to cells of the immune system. As well, there is hard-wiring of immune organs, including the skin, by autonomic nervous system fibers. That is, in both primary and secondary lymphoid organs, there is an abundance of NE-containing sympathetic nerve fibers in tissue parenchyma.
In addition to availability of these neurohormones to cells of the immune system, virtually every hormone traditionally thought of as neuroendocrine in nature has been shown to affect both T and/or B lymphocytes and monocytes, regulating proliferation, cytokine production, and/or a host of effector functions.2 The cells of the immune system express receptors for a wide array of hormones, including growth hormone, catecholamines, glucocorticoids, prolactin, opioids, and thyroid hormones, to name just a few.3, 4 As well, hormones once thought of as neurally-derived, such as CRH, can themselves be produced by cells of the immune system.5 Further, T cells and the brain have long-been known to share other surface molecules. Indeed, when the first mouse and rat pan T lymphocyte reagent was made (anti-Thy 1), which was quite an advancement for the field of immunology, it was derived from a rabbit immunized with rat brain, not mouse or rat T cells.6 Thus, given the similarities between the CNS and the immune system, it is not surprising that the same signals which grab the attention of the brain — stressors, for example — would also cause changes in immunity. Such similarities between systems have often prompted researchers to think of the immune system as the “roving brain.”
Stress and immunity
In considering the effects of stress on immune function, a number of other factors must be considered, such as individual differences (genetic and environmental), the type and intensity of the stressor, and the chronicity of the stressful experience. In general, it is well accepted that chronic stress dampens adaptive immune function, as well as innate natural killer (NK) cell activity. In contrast, chronic stress induces a persistent upregulation of the innate, proinflammatory cytokine cascade, via activation of the hypothalamo-pituitary-adrenal axis and the autonomic nervous system.7–9 With repeated chronic stressor administration, the burden of accumulated life stress, termed allostatic load by McEwen et al.,10 can lead to dysregulation of stress-responsive systems, and chronically elevated levels of these proinflammatory proteins. This profile of chronic inflammation is also associated with increasing indices of metabolic syndrome, which leads to high rates of diabetes and cardiovascular disease.11 At the extreme, elevated IL-6 is associated with mortality over a 7-year period in older adults.12
It is important to note that these chronic elevations in proinflammatory cytokines, acting as messengers from the periphery back to the brain, may also have significant consequences for the CNS and psychological well-being.13 It is now thought that depression, or at least certain subtypes of depression, may be causally mediated by chronic increases in proinflammatory cytokines,14–16 which instruct the hypothalamus to initiate a robust constellation of depressive-like symptoms, including fever, fatigue, anhedonia, and decreased activity, originally known as “sickness behavior.” Elevations in these proinflammatory cytokines, particularly IL-6 and tumor necrosis factor (TNF)-α, are now implicated in major depressive disorder in humans, as well as in animal models.16–19
Relevant to this paper are studies of chronic stress, proinflammatory cytokines and wound healing in humans. In a study of marital relationships in couples married for over a decade, Kiecolt-Glaser et al. observed experimental blister wounds were slower to heal and local levels of inflammatory cytokines (which are important for successful skin repair) production was lower following a laboratory-induced marital conflict.20, 21 Further, for those couples who exhibited a stable relationship pattern defined as high hostile, larger increases in circulating proinflammatory cytokines were observed the morning after the marital conflict task compared to low hostile couples. Thus, chronic relationship stress can result in dysregulated proinflammatory cytokines, which over time can become a factor in a number of diseases, including metabolic syndrome and cardiovascular disease.
In contrast to the pernicious effects of chronic stress on immune function, acute stress is often considered to induce increased adaptive immunity, as an organism prepares to deal with, for example, infection caused by a wound inflicted by an oncoming predator.22 Using a mouse model of delayed type hypersensitivity (DTH), Dhabhar proposed that acute stress induces migration of lymphocytes to skin, resulting in an enhanced DTH response (as measured by ear swelling following an antigenic challenge).23 It is hypothesized that this response is evolutionarily adaptive during a classic stress response.
Behavioral conditioning of immune responses
Even before the work of the last several decades demonstrating that psychological stressors can modify both adaptive and innate immune responses, another part of the foundation of PNI was being solidified. In the mid-1970s, Robert Ader and his colleague Nicholas Cohen published a seminal paper that proved that it was possible to behaviorally condition immunosuppression in the rat by repeatedly pairing a novel taste with injection of an immunosuppressive drug (cyclophosphamide). Exposure to the novel taste (saccharin) and injection of saline in place of cyclophosphamide resulted in significant decreases in both lymphocyte numbers, as well as function.24 In this maimer, the “placebo” was capable of mimicking the physiological response produced by the action of an immunosuppressant as long as the rats i) had been previously received cyclophosphamide together with the saccharin (conditioned stimulus) and ii) were intermittently re-exposed to the immunosuppressant (unconditioned stimulus)
Since that time, Ader has turned his attention to harnessing the principles of conditioning to effectively reduce the amount of noxious drugs that patients take for a variety of diseases, including psoriasis. Recently, Ader and colleagues 25 used a partial reinforcement (PR) schedule for treatment of psoriasis patients who had been being treated with 0.1% acetonide triamcinolone. The PR patients were treated with a full dose of drug only 25–50% of the time, compared to dose control (DC) patients who were treated continuously with 25% to 50% of the initial dose. Importantly, the frequency of psoriasis relapse under PR (26.7%) did not differ from the full-dose drag treatment, and was significantly lower was lower than in the DC patients (61.5%) and did not differ from patients who were maintained on continuous full-dose treatment (22.2%). In this study, all patients were treated with the application of triamcinolone to target plaques; once they cleared, they were randomized to 3 groups:
continued with the initial regimen of steroid;
received steroid one day and placebo the subsequent three days;
received daily triamcinolone but only 25% of the initial dose, to match the total amount used by subjects in group 2).
The results showed that those in groups 1) and 2) remained free of disease whereas those in group 3) relapsed. This study suggests that in the future, placebos administered under principles of classical conditioning (Pavlovian) may be interspersed with active medication leading to similar therapeutic effects, utilizing significantly less active drag, and thus minimizing some of the deleterious side effects of such drags. Studies are underway to examine if the same principle can be applied to the systemic therapy of psoriasis as well as other diseases. It is obvious that in order for partial reinforcement schedules to be effective they must involve interactions between the CNS and the target of the active drag (in this case, between CNS and the immune system).
The immunological underpinnings of psoriasis
Immune regulation plays a central pathophysiological role in the development of psoriasis, which is considered to be an immunological condition with a complex genetic basis.26 Psoriasis is characterized histologically by increased proliferation of keratinocytes, and by inflammatory leukocyte cell infiltration into the epidermis and the underlying dermis. For many years, psoriasis has been understood as a T lymphocyte mediated autoimmune condition, largely driven by production of cytokines. T helper (Th)1 cells and the cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α were the focus of most early studies.
Accumulating evidence currently points to a central role for the Th17 cytokine network in the development of psoriatic lesions. Within this network, the cytokines interleukin (IL)-6 and transforming growth factor (TGF)-β (produced by a variety of cell types, including regulatory T cells and keratinocytes) 27 drive maturation of naïve T cells into Th17 cells. IL-23 produced by dendritic cells, macrophages, Langerhans cells and keratinocytes within the skin induces expansion of these Th17 cells, and induces their production of IL-22, which regulates proliferation and differentiation of keratinocytes.28–31 The activated Th17 cells also secrete increased levels of IL-17, which attracts neutrophils to the tissue site.29 Finally, IFN-γ and TNF-α derived either from Th128 or a subset of Th17 cells 32 are also elevated in psoriatic lesions and act to amplify inflammation.
Inflammatory cytokines and psychological distress
Not surprisingly, psoriasis patients commonly report a significant decrease in quality-of-life, and a range of negative psychosocial consequences, including depression, suicidal thoughts, increased perceived stress levels, social stigmatization, and employment problems.33, 34 Upwards of 40% of patients meet criteria for probable mood disorders,35 and prevalence of depression and/or anxiety disorder is reported in a range of 30% 36 to as many as 58% of subjects.37 Thus, psoriasis is not only an immune-driven disease, but is also often characterized by significant mental health issues, in particular, depression.
Over the past two decades, significant progress has been made in elucidating the relationship between the immune system and neuropsychiatric disease. These developments have emphasized the clinical significance of psychoneuroimmunology and opened doors for novel translational work. Largely due to the work of Andrew H. Miller and colleagues, there is now substantial evidence to demonstrate a link between inflammation and the development of major depressive disorder. While there is significant overlap between the symptoms of idiopathic depression in medically-healthy individuals and inflammatory, or cytokine-induced, depression, subtle differences are emerging, especially with respect to the effects of inflammation on psychomotor activity and cognitive performance.38 On a molecular basis, patients with depression have shown evidence of peripheral markers of inflammation, including cytokines, which have access to and can act in the CNS,39 although much of the mechanistic details remain out of the scope of this article.
In brief, both medically-ill and medically-healthy patients with major depressive disorder have been shown to exhibit several features of inflammation, with literature supporting elevated markers of the innate immune response, e.g. cytokines including interleukin (IL)-1, IL-6, and TNF-α, in peripheral blood and cerebrospinal fluid, and acute phase reactants, chemokines, and inflammatory mediators in peripheral blood.39, 40 There is evidence that administration of cytokines or cytokine inducers can elicit symptoms of depression and anxiety, including depressed mood, fatigue, poor sleep quality, cognitive dysfunction, and psychomotor slowing, and that symptom severity is correlated with increases in peripheral cytokine concentration.39, 41–43 Of note, depressed patients with increased levels of inflammatory markers have been found to be more likely to demonstrate treatment resistance, and antidepressant therapy has been shown to be associated with a decreased inflammatory response.39 There is also evidence for increased markers of T cell activation in depressed patients, which suggests a possible additional role for an acquired immune response.39 In addition, associations between individual depressive symptoms, such as poor sleep quality and cognitive dysfunction, and increased markers of inflammation have been described.44 Of particular interest in dermatology is the role of immune-targeted therapies; a study of the anti-TNF-α biologic, etanercept, demonstrated efficacy in treating both psoriasis and depression in a double-blind, placebo-controlled trial,45 and the effect on depression was independent of improvement in cutaneous disease activity.
Cytokines, even when peripherally administered, may access the CNS, generate an inflammatory response, and have profound effects on the patho-physiology of depression (Figure 1). These molecules can alter the metabolism of monoamine neurotransmitters, (e.g. serotonin, dopamine, and norepinephrine), which can lead to reduced transmitter availability and synthesis and increased reuptake, with potential effects on mood and behavior.39, 46, 47 Cytokines also affect neuroendocrine function by stimulating the release of corticotrophin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and Cortisol, all of which are increased in patients suffering from depression 39,48. In times of prolonged activation, cytokines can disrupt neural plasticity through decreasing neurotrophic support, neurogenesis, and glutamate reuptake, while increasing oxidative stress and apoptosis. These effects are ultimately reflected in the loss of glial cells, including astrocytes and oligodendrocytes, that characterize the neuropathologic findings seen in patients with major depression.39
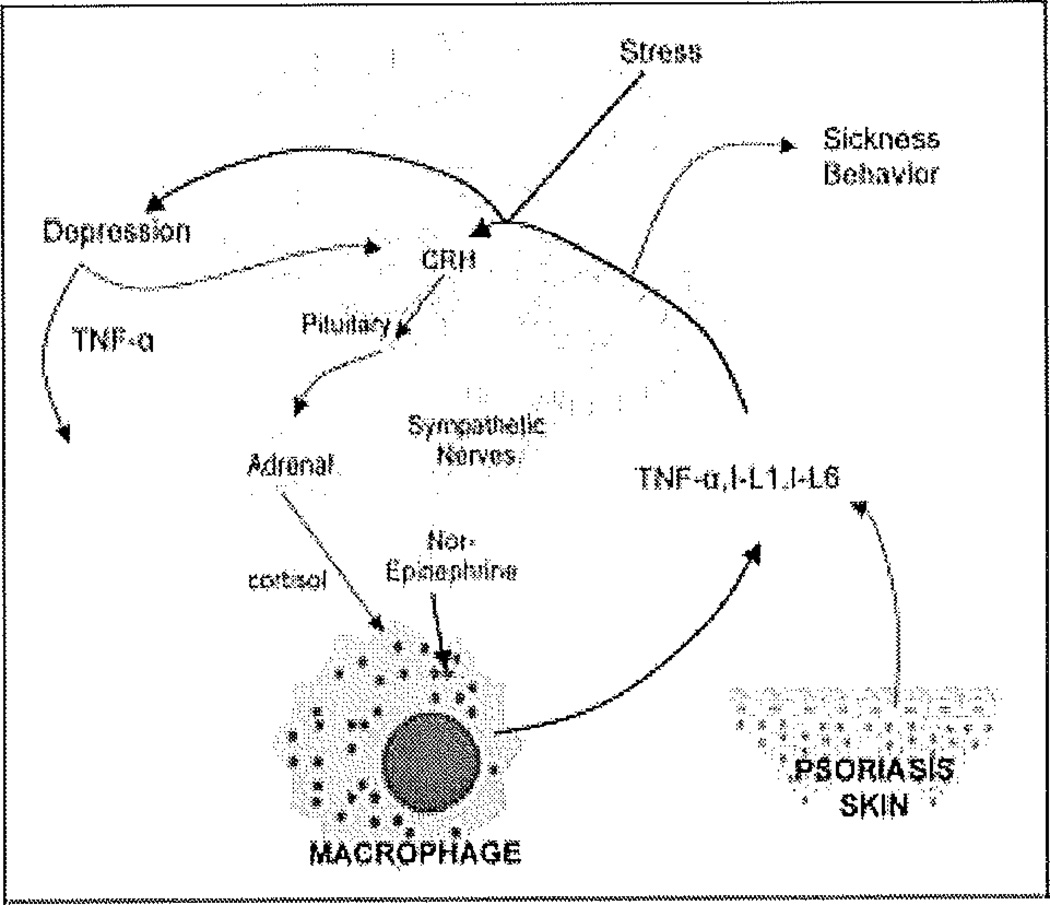
Figure 1.
The interconnection between psoriasis, stress, depression and inflammation. The presence of a stressor is relayed to the para ventricular nucleus of hypothalamus, inducing the release of corticotropin releasing hormone (CRH), which is transported to the anterior pituitary resulting in the secretion of corticotropin (ACTH). The latter is released to the circulation, stimulation the release of Cortisol from the adrenal glands. Simultaneously, the locus ceruleus mediates the activation of the sympathetic system resulting in the release of nor-epinephrine. The opposing effects of Cortisol and catecholamines on macrophages is mostly tilted in favor of the latter, since glucocorticosteroid receptors may eventually become insensitive to the continued effects of Cortisol. Proinflammatory cytokines such as TNF-α, IL-1 and IL-6 secreted predominantly from macrophages and additionally from psoriatic skin reach the brain through leaky blood brain barrier areas, or through signaling the vagus nerve. These cytokines induce sickness behavior (fatigue, fever and somnolence), activation of the hypothalamic stress response through CRH and the sympathetic system, and mediate depression, which is also found to result in elevated cytokines.
Psychosocial stress is a known precipitant and exacerbant of mood disorders, but can also stimulate a peripheral and CNS inflammatory response through a complex loop. Stress-induced inflammation involves activation of the hypothalamic pituitary adrenal (HPA) axis via CRH and the sympathetic nervous system, which leads to increases in levels of nuclear factor kappa B (NF-κB) and the release of inflammatory mediators and cytokines that can access the CNS and affect monoamine metabolism.39 Stress-related causes of inflammation are particularly useful when considering medically-healthy depressed patients, in which there is no clear medical source of an inflammatory process (Figure 1).
Current data suggests that the inhibition of proinflammatory cytokines may improve depressed mood and increase treatment response to the currently available antidepressant medications.45, 49 Given that patients with idiopathic major depression and medically ill patients with cytokine-induced depression present with similar symptoms, inflammatory biomarkers may help stratify patient populations and identify those who may best respond to such immunologically-targeted therapy.38 In addition, cytokines can be used to trend and monitor patient response to treatment.
These same cytokines are also elevated in response to both physiological and psychological stressors via activation of the hypothalamo-pituitary-adrenal axis and the autonomic nervous system.7–9, 50 Indeed, recent studies indicate that psoriasis patients are at increased risk for these inflammation-associated diseases or syndromes, and the peripheral systemic inflammatory response may well be exacerbated by high levels of psychological distress.51, 52 Further, inflammatory processes occurring in the periphery may set up a “vicious cycle” that actually serves to exacerbate psychological distress, depression, and anxiety in psoriasis patients.18, 19
The role of the central and peripheral nervous systems and hormones in psoriasis
Psychological or life stressors have been reported to precede the onset of psoriasis,53 as well as to precipitate flares.37, 54 Richards et al.(2005) showed that psoriasis patients who reported stress-related exacerbations of the disease were observed to have lower baseline (9:00 AM) and experimental stressor-induced Cortisol levels representing an altered hypothalamic deficiency similar to previously reported in atopic patients.55 Studies involving patients with moderate psoriasis who were not differentiated by reports of stress-related exacerbations have not found evidence of altered Cortisol levels.56 Thus, the role of the classic stress hormone Cortisol in psoriasis remains unclear. Buske-Kirschbaum et al.56 did, however, observe that circulating epinephrine and norepinephrine were significantly increased by an experimental stressor in psoriasis patients, suggesting altered sympathetic nervous system activation in this patient group. Further, Buske-Kirschbaum and colleagues found that stressor-induced increases in circulating monocytes and CD4+ cells were greater in psoriasis patients compared to controls, and a significant decrease in the percentage of activated T cells (CD3+/CD25+) was observed in the patient population.57
Further evidence that psoriasis may be associated with dysregulation of the peripheral nervous system comes from the observation by Farber and colleagues that in patients who suffered traumatic severance of sensory innervation, the plaques of psoriasis present in the areas innervated by the sectioned nerves resolved, and only reappeared when nerve fibers regenerated and the sensitivity returned.58 This observation highlights the role played by sensory cutaneous nerves, leading to the hypothesis that locally secreted neuropeptides contribute to the maintenance of psoriatic disease.59–62 Subsequently, it was found that psoriatic plaques display increased nerve fiber density and altered content of neuropeptides, including calcitonin gene-related peptide (CGRP), substance P (SP), vasoactive intestinal peptide (VIP), and nerve growth factor (NGF).63 High expression of NGF mediates T cell and keratinocyte proliferation, mast cell migration, degranulation, and memory T cell chemotaxis, which are all hallmarks of psoriasis.62
Psychosocial intervention for psoriasis
Numerous psychosocial interventions aimed at the reduction of stress have proved to be successful for the treatment of psoriasis (as well as psychological symptoms). Hypnosis is one alternative therapy with evidence of utility for these patients.64, 65 Psoriasis patients improved significantly during hypnosis sessions, where they received suggestions that they were being exposed to “whatever they believed …would ameliorate their condition”.64
More traditional therapies have also been applied with some success to patients with psoriasis. Fortune et al., for example, showed that a short program of cognitive behavioral therapy (CBT) was associated with a decrease in the number and frequency of psoriasis symptoms reported even six months following the program’s end. It should be noted that this was not a randomized control trial (RCT); patients were allowed to choose CBT. Psychotherapy — including stress reduction and imagery—was also shown to have a positive effect on disease activity.66 Finally, in a very small group of randomized subjects, meditation resulted in improvement of psoriatic lesions, and the addition of imagery to meditation had no added effect.67 Kabat-Zinn et al. reported that the addition of Mindfulness Based Stress Reduction tapes during the time that the psoriasis patients were inside phototherapy booths markedly accelerated the time to clearance of the psoriasis plaques when compared to patients that received phototherapy without the tapes 68, 69. This study highlights the fact that brief psychosocial interventions may not only result in an enhanced overall wellbeing of the patients, but can specifically improve the psoriatic disease itself.
In conclusion, the past decades have seen an explosion of scientific advancements showing the close interconnection between the central and the peripheral nervous, the immune, and the endocrine systems. More recent and ongoing translational studies have highlighted that the discipline of psychoneuroimmunology may be of significant relevance to the generation and evolution of human diseases, including cutaneous ones such as psoriasis.
References
- 1.Cannon W. The wisdom of the body. New York: Norton Pubs; 1932. [Google Scholar]
- 2.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 3.Khansari DN, Murgo AJ, Faith RE. Effects of stress on the immune system. Immunol Today. 1990;11:170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 5.Stephanou A, Jessop DS, Knight RA, Lightman SL. Corticotrophin-releasing factor-like immunoreactivity and mRNA in human leukocytes. Brain Behav Immun. 1990;4:67–73. doi: 10.1016/0889-1591(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 6.Morris RJ, Williams AF. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975;5:274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- 7.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 8.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 9.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 10.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.10.002. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickx H, McEwen BS, Ouderaa F. Metabolism, mood and cognition in aging: the importance of lifestyle and dietary intervention. Neurobiol Aging. 2005;26(Suppl 1):1–5. doi: 10.1016/j.neurobiolaging.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Walston J, Arking DE, Fallin D, Li T, Beamer B, Xue Q, et al. IL-6 gene variation is not associated with increased serum levels of IL-6, muscle, weakness, or frailty in older women. Exp Gerontol. 2005;40:344–352. doi: 10.1016/j.exger.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glassman AH, Miller GE. Where there is depression, there is inflammation… sometimes! Biol Psychiatry. 2007;15:280–281. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 17.Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 19.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 21.Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann NY Acad Sci. 2003;992:205–217. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72:192–197. doi: 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdimarsson H. The genetic basis of psoriasis. Clin Dermatol. 2007;25:563–567. doi: 10.1016/j.clindermatol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H) 17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 29.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 30.Ma HL, Liang S, Li J, Brown T, Benoit S, Senices M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–244. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 32.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 33.Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383–392. doi: 10.2165/00128071-200506060-00005. [DOI] [PubMed] [Google Scholar]
- 34.Schmid-Ott G, Malewski P, Kreiselmaier I, Mrowietz U. [Psychosocial consequences of psoriasis—an empirical study of disease burden in 3753 affected people] Hautarzt. 2005;56:466–472. doi: 10.1007/s00105-005-0906-9. [DOI] [PubMed] [Google Scholar]
- 35.Richards HL, Fortune DG, Griffiths CE, Main CJ. The contribution of perceptions of stigmatisation to disability in patients with psoriasis. J Psychosom Res. 2001;50:11–15. doi: 10.1016/s0022-3999(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 36.Hughes JE, Barraclough BM, Hamblin LG, White JE. Psychiatric symptoms in dermatology patients. Br J Psychiatry. 1983;143:51–54. doi: 10.1192/bjp.143.1.51. [DOI] [PubMed] [Google Scholar]
- 37.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009 doi: 10.1016/j.jad.2009.02.017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of major depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brydon L, Harrison N, Walker C, Steptoe A, Critchley H. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-α treatment: The Role of IL-6 and sleep quality. Brain, Behavior, and Immunity. 2009 doi: 10.1016/j.bbi.2009.07.001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capuron L, Miller A. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 45.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomized phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 46.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain, Behavior, and Immunity. 2008;23:148–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pariante C, Miller A. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 49.Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo-controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 50.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb AB, Dann F. Comorbidities in patients with psoriasis. Am J Med. 2009;122:1150, el151–e1159. doi: 10.1016/j.amjmed.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatolog Treat. 2008;19:5–21. doi: 10.1080/09546630701364768. [DOI] [PubMed] [Google Scholar]
- 53.Naldi L, Peli L, Parazzini F, Carrel CF. Family history of psoriasis, stressful life events, and recent infectious disease are risk factors for a first episode of acute guttate psoriasis: results of a case-control study. J Am Acad Dermatol. 2001;44:433–438. doi: 10.1067/mjd.2001.110876. [DOI] [PubMed] [Google Scholar]
- 54.Fortune DG, Richards HL, Griffiths CE, Main CJ. Psychological stress, distress and disability in patients with psoriasis: consensus and variation in the contribution of illness perceptions, coping and alexithymia. Br J Clin Psychol. 2002;41:157–174. doi: 10.1348/014466502163949. [DOI] [PubMed] [Google Scholar]
- 55.Buske-Kirschbaum A, Jobst S, Hellhammer DH. Altered reactivity of the hypothalamus-pituitary-adrenal axis in patients with atopic dermatitis: pathologic factor or symptom? Ann N Y Acad Sci. 1998;840:747–754. doi: 10.1111/j.1749-6632.1998.tb09613.x. [DOI] [PubMed] [Google Scholar]
- 56.Buske-Kirschbaum AEM, Kern S, Hellhammer DH. Endocrine stress responses in TH1-mediated chronic inflammatory skin disease (psoriasis vulgaris)—do they parallel stress-induced endocrine changes in TH2-mediated inflammatory dermatoses (atopic dermatitis)? Psychoneuroendocrinology. 2006;31:439–446. doi: 10.1016/j.psyneuen.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Buske-Kirschbaum A, Kern S, Ebrecht M, Hellhammer DH. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain Behav Immun. 2007;21:92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418–420. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 59.Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2 Pt 1):305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- 60.Raychaudhuri SP, Farber EM. Neuroendocrine influences on the pathogenesis of psoriasis. new york: Academic Press; 2000. [Epub ahead of print]. [Google Scholar]
- 61.Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriatic lesions? J Am Acad Dermatol. 1993;28:488–489. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- 62.Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470–477. doi: 10.1111/j.1749-6632.2009.04652.x. [DOI] [PubMed] [Google Scholar]
- 63.Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22–31. doi: 10.1111/j.1529-8019.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 64.Tausk F, Whitmore SE. A pilot study of hypnosis in the treatment of patients with psoriasis. Psychother Psychosom. 1999;68:221–225. doi: 10.1159/000012336. [DOI] [PubMed] [Google Scholar]
- 65.Shenefelt PD. Hypnosis in dermatology. Arch Dermatol. 2000;136:393–399. doi: 10.1001/archderm.136.3.393. [DOI] [PubMed] [Google Scholar]
- 66.Zachariae R, Oster H, Bjerring P, Kragballe K. Effects of psychologic intervention on psoriasis: a preliminary report. J Am Acad Dermatol. 1996;34:1008–1015. doi: 10.1016/s0190-9622(96)90280-7. [DOI] [PubMed] [Google Scholar]
- 67.Gaston L, Crombez JC, Lassonde M, Bernier-Buzzanga J, Hodgins S. Psychological stress and psoriasis: experimental and prospective correlational studies. Acta Derm Venereol Suppl. 1991;156:37–43. [PubMed] [Google Scholar]
- 68.Kabat-Zinn J, Wheeler E, Light T, Skillings A, Scharf MJ, Cropley TG, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB), and photochemotherapy (PUVA) Psychosom Med. 1998 Sep-Oct;60:625–632. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 69.Benhard JD, Kristeller J, Kabat-Zinn J. Effectiveness of relaxation and visualization techniques as an adjunct to phototherapy and photochemotherapy of psoriasis. J Am Acad Dermatol. 1988;19:572–574. doi: 10.1016/s0190-9622(88)80329-3. [DOI] [PubMed] [Google Scholar]