Abstract
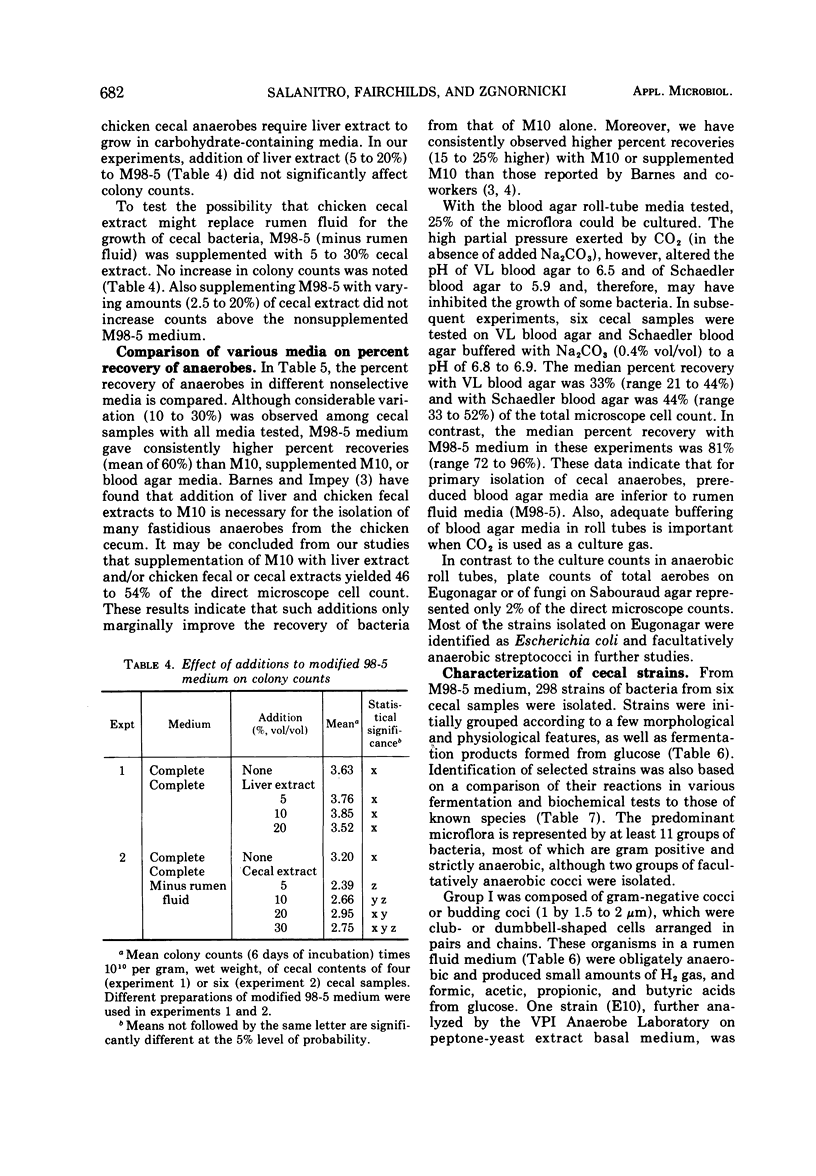
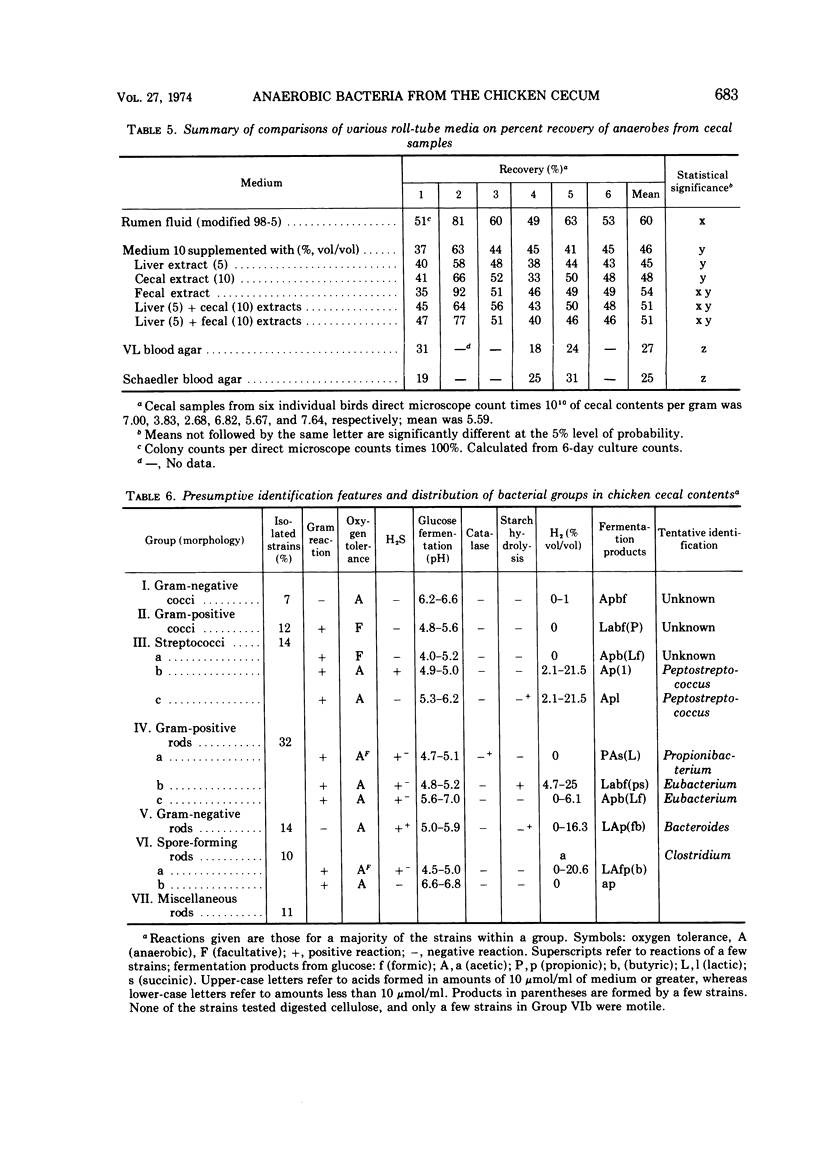
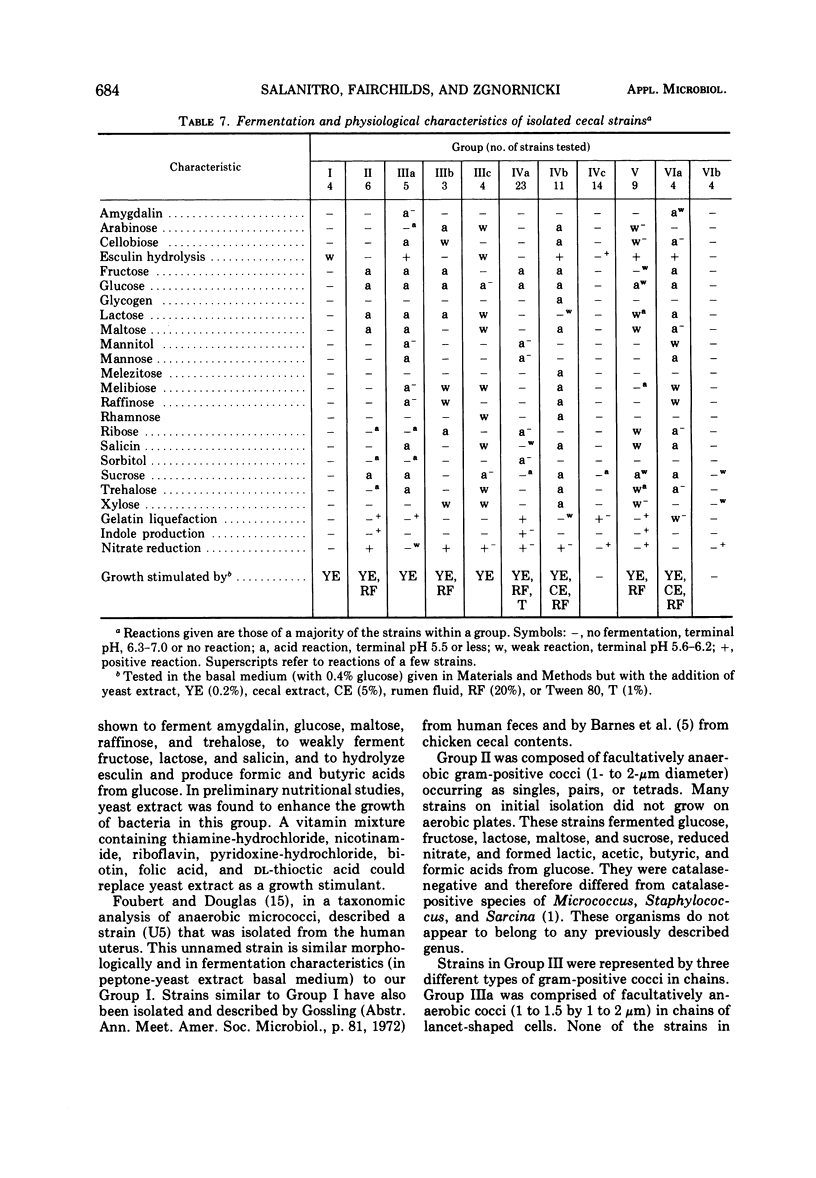
Studies on the anaerobic cecal microflora of the 5-week-old chicken were made to determine a suitable roll-tube medium for enumeration and isolation of the bacterial population, to determine effects of medium components on recovery of total anaerobes, and to identify the predominant bacterial groups. The total number of microorganisms in cecal contents determined by direct microscope cell counts varied (among six samples) from 3.83 × 1010 to 7.64 × 1010 per g. Comparison of different nonselective media indicated that 60% of the direct microscope count could be recovered with a rumen fluid medium (M98-5) and 45% with medium 10. Deletion of rumen fluid from M98-5 reduced the total anaerobic count by half. Colony counts were lower if chicken cecal extract was substituted for rumen fluid in M98-5. Supplementing medium 10 with liver, chicken fecal, or cecal extracts improved recovery of anaerobes slightly. Prereduced blood agar media were inferior to M98-5. At least 11 groups of bacteria were isolated from high dilutions (10-9) of cecal material. Data on morphology and physiological and fermentation characteristics of 90% of the 298 isolated strains indicated that these bacteria represented species of anaerobic gram-negative cocci, facultatively anaerobic cocci and streptococci, Peptostreptococcus, Propionibacterium, Eubacterium, Bacteroides, and Clostridium. The growth of many of these strains was enhanced by rumen fluid, yeast extract, and cecal extract additions to basal media. These studies indicate that some of the more numerous anaerobic bacteria present in chicken cecal digesta can be isolated and cultured when media and methods that have been developed for ruminal bacteria are employed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD-PARKER A. C. A classification of micrococci and staphylococci based on physiological and biochemical tests. J Gen Microbiol. 1963 Mar;30:409–427. doi: 10.1099/00221287-30-3-409. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. Anaerobic gram negative nonsporing bacteria from the caeca of poultry. J Appl Bacteriol. 1968 Dec;31(4):530–541. doi: 10.1111/j.1365-2672.1968.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. Some properties of the nonsporing anaerobes from poultry caeca. J Appl Bacteriol. 1972 Jun;35(2):241–251. doi: 10.1111/j.1365-2672.1972.tb03696.x. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. The isolation and properties of the predominant anaerobic bacteria in the caeca of chickens and turkeys. Br Poult Sci. 1970 Oct;11(4):467–481. doi: 10.1080/00071667008415842. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Mead G. C., Barnum D. A., Harry E. G. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972 May;13(3):311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert E. L., Douglas H. C. Studies on the Anaerobic Micrococci: I. Taxonomic Considerations. J Bacteriol. 1948 Jul;56(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON A. P., Jr, HANSEN P. A. The bacterial flora of the cecal feces of healthy turkeys. J Bacteriol. 1950 Feb;59(2):197–210. doi: 10.1128/jb.59.2.197-210.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala E., Weaver M. L. Separation and quantitative determination of lactic, pyruvic, fumaric, succinic, malic, and citric acids by gas chromatography. Anal Biochem. 1969 Jul;30(1):32–39. doi: 10.1016/0003-2697(69)90370-4. [DOI] [PubMed] [Google Scholar]
- LEV M., BRIGGS C. A., COATES M. E. The gut flora of the chick. 3. Differences in caecal flora between infected, uninfected and penicillin-fed chicks. Br J Nutr. 1957;11(4):364–372. doi: 10.1079/bjn19570057. [DOI] [PubMed] [Google Scholar]
- LEV M. The growth-promoting activity of compounds of the vitamin K group and analogues for a rumen strain of Fusiformis nigrescens. J Gen Microbiol. 1959 Jun;20(3):697–703. doi: 10.1099/00221287-20-3-697. [DOI] [PubMed] [Google Scholar]
- LOESCHE W. J., SOCRANSKY S. S., GIBBONS R. J. BACTEROIDES ORALIS, PROPOSED NEW SPECIES ISOLATED FROM THE ORAL CAVITY OF MAN. J Bacteriol. 1964 Nov;88:1329–1337. doi: 10.1128/jb.88.5.1329-1337.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi Y., Mitsuoka T., Sega T. Untersuchungen über die Darmflora des Huhnes. III. Die Entwicklung der Darmflora von Küken bis zum Huhn. Zentralbl Bakteriol Orig. 1964 Jun;193(1):80–95. [PubMed] [Google Scholar]
- Pulverer G., Ko H. L. Fermentative and serological studies on Propionibacterium acnes. Appl Microbiol. 1973 Feb;25(2):222–229. doi: 10.1128/am.25.2.222-229.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. W. OBSERVATIONS ON THE FLORA OF THE ALIMENTARY TRACT OF ANIMALS AND FACTORS AFFECTING ITS COMPOSITION. J Pathol Bacteriol. 1965 Jan;89:95–122. [PubMed] [Google Scholar]
- Starr S. E., Killgore G. E., Dowell V. R., Jr Comparison of Schaedler agar and trypticase soy-yeast extract agar for the cultivation of anaerobic bacteria. Appl Microbiol. 1971 Oct;22(4):655–658. doi: 10.1128/am.22.4.655-658.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS C. G., HARE R. The classification of anaerobic cocci and their isolation in normal human beings and pathological processes. J Clin Pathol. 1954 Nov;7(4):300–304. doi: 10.1136/jcp.7.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms L. Observations on the bacterial flora of the alimentary tract in three age groups of normal chickens. Br Vet J. 1968 Oct;124(10):470–477. doi: 10.1016/s0007-1935(17)39155-8. [DOI] [PubMed] [Google Scholar]


