Abstract
Agency attribution is a hallmark of mind perception; thus, diminished attributions of agency may disrupt social-cognition processes typically elicited by human targets. The current studies examine the effect of perceivers’ sexist attitudes on associations of agency with, and neural responses to, images of sexualized and clothed men and women. In study 1, male (but not female) participants with higher hostile sexism scores more quickly associated sexualized women with first-person action verbs (“handle”) and clothed women with third-person action verbs (“handles”) than the inverse, as compared to their less sexist peers. In study 2, hostile sexism correlated negatively with activation of regions associated with mental state attribution—mPFC, posterior cingulate, temporal poles—but only when viewing sexualized women. Heterosexual men best recognized images of sexualized female bodies (but not faces), as compared with other targets’ bodies; however, neither face nor body recognition were related to hostile sexism, suggesting the fMRI findings are not explained by more or less attention to sexualized female targets. Diminished mental-state attribution is not unique to targets that people prefer to avoid, as in dehumanization of stigmatized people. The current studies demonstrate that appetitive social targets may elicit a similar response depending on perceivers’ attitudes toward them.
Keywords: social cognition, theory of mind, sexism, fMRI
The use of sexual imagery in advertising is as old as the practice of advertising itself; alcohol, tobacco, fashion, music, and many other industries have long employed images of sexualized women to promote their products. Recent inquiries have called into question the efficacy of this strategy and, perhaps more importantly, the costs associated with it. Sexualized imagery of young women in the media has deleterious consequences not only for women’s mental and physical well-being, but also for men’s well-being and satisfaction (Report of the APA Task Force on the Sexualization of Girls Executive Summary, 2008; Schooler & Ward, 2006; Zillmann & Bryant, 1988).
A philosophical perspective suggests that sexualized targets are considered in terms of their usefulness and thus denied autonomy and agency typically afforded other people—a possible mechanism by which sexualization leads to a variety of negative consequences for individuals and society more broadly (Nussbaum, 1999). Psychologists have long understood that agency makes humans “unique in their power to shape their life circumstances” (Bandura, 2006). People are not simply acted upon; instead, they interact with, influence, and cognitively transcend their immediate environments (e.g., Buckner & Carroll, 2006). Humans are able to interact with one another most effectively when they attempt to understand each others’ minds (e.g., Adolphs, 2006; Frith & Frith, 2003). We suggest that sexualization and sexist attitudes together disrupt these spontaneous social cognition processes—cognitive processes that normally allow people to make inferences about the internal states of others.
Perceivers’ implicit associations and explicit attitudes about different social targets also influence behavioral, cognitive, affective, and neural responses to those targets. Several fMRI investigations have examined how individual differences in bias modulate neural responses to outgroup members (e.g., Phelps et al., 2000; Richeson et al., 2003), yet nearly all of these studies have focused on race as the category boundary of interest. The current investigation is the first of which we are aware to examine whether target sexualization and perceivers’ sexist attitudes influence associations of agency with, and neural responses to images of sexualized and clothed men and women.
Social Cognition and the Flexibility of Mental State Attribution
The ability to attribute mental states to others is referred to as “theory of mind” (Premack & Woodruff, 1978) or “mentalizing” (Frith & Frith, 2003). Mentalizing is a complex computation that comprises several cognitive processes; it is critical for understanding others and for effective communication. Because people do not have direct access to other people’s minds, they have to use cues (e.g., external cues, personal simulation) to infer the existence and contents of other agents’ mental states (e.g., intentions, beliefs, desires; Ames, 2004; Carruthers & Smith, 1996). In recent years, scores of cognitive neuroscience investigations have examined social cognition as it unfolds in the human brain. Some studies have used versions of the false belief paradigm or stories that require mentalizing to explain a target’s behavior (e.g., Gobbini, Koralek, Bryan, Montgomery, & Haxby, 2007; Grèzes, Bethoz, & Passingham, 2006; Grèzes, Frith, & Passingham, 2004; Mitchell, 2008; Saxe & Kanwisher, 2003, Saxe, Moran, Scholz, & Gabrieli, 2006, Saxe, Schuls, & Jiang, 2006); other studies use animations of geometric shapes inspired by Heider and Simmel (1944) (Castelli, Frith, Happé, & Frith, 2002; Gobbini et al., 2007; Schultz, Imamizu, Kawato, & Frith, 2004); yet another set of studies examines the neural basis of impression formation (Harris, Todorov, & Fiske, 2005; Heberlein & Saxe, 2005; Mitchell, Banaji, & Macrae, 2005a; Mitchell, Banaji, & Macrae, 2005b; Mitchell, Cloutier, Banaji, & Macrae, 2006), and a fourth examines inferences about intentions (Blakemore, den Ouden, Chowdgury, & Frith, 2007; Ciaramidaro et al., 2007; German, Niehaus, Roarty, Giesbrecht, & Miller, 2004; Kampe, Frith, & Frith, 2003; Walter et al., 2004). Across tasks and labs, social cognition engages medial prefrontal cortex (mPFC), right temporo-parietal junction (rTPJ), precuneus/posterior cingulate (PCC), superior temporal sulcus (STS), and anterior temporal poles with remarkable reliability.
In addition to its informative function for perceivers, mental state attribution is a hallmark of seeing another entity as human (Epley, Waytz, & Cacioppo, 2007; Epley & Waytz, 2009; Waytz, Epley, & Caccioppo, 2010; Waytz, Gray, Epley, & Wegner, 2010); however, not all humans are perceived as having mental states. Attribution of mind is a highly flexible process (Kwan & Fiske, 2008): sometimes people attribute less complex mental states to others than to themselves or their ingroup (Haslam, Bain, Douge, Lee, & Bastian, 2005; Haslam, Loughnan, Kashima, & Bain, 2008; Kozak, Marsh, & Wegner, 2006: Leyens et al., 2003) or altogether fail to attribute mental states to other people (Harris & Fiske, 2009; Haslam, 2006); other times people attribute mental states to inanimate objects (Heider & Simmel, 1944; Morewedge, Preston, & Wegner, 2007; Waytz et al., in press).
Because mind attribution is so flexible, recent research has started to ask which social targets fail to engage brain regions associated with social cognition and why? In one series of studies, participants “dementalized” social groups who elicit disgust (e.g., drug addicts, homeless). For example, participants used significantly fewer mental state verbs to describe a day in the life of “disgusting” targets, as compared with other social targets (Harris & Fiske, 2010), suggesting people spontaneously infer the contents of “disgusting” targets’ minds less than other people’s minds. Participants also viewed these “disgusting” outgroup members as less competent/autonomous, less warm/familiar, and less likely for interaction than other outgroup members. Moreover, passively viewing images of these targets fails to activate mPFC significantly above baseline (Harris & Fiske, 2006; 2007; 2009).1
Sexualization and Agency
Sexualization also demonstrably disrupts the typical course of social cognition.2 For example, viewing commercials with sexualized women facilitates men’s responses to sexist words (e.g., babe, bimbo) and slows responses to nonsexist words pertaining to women (e.g., mother, sister), increases stereotyped information acquisition about a female interviewee (i.e., participants remember more about physical behavior and appearance, less about personality, biographical information, and performance evaluation), and increases sexualized behavior in subsequent interaction with her (e.g., sitting closer than control subjects sit; Rudman & Borgida, 1995). More to the point, sexual objectification decreases attribution of complex mental states to targets (Loughnan et al., in press).
Because sexualization and sexual objectification refer to perceiving people in light of their usefulness for sex (Bartky, 1990; Fredrickson & Roberts, 1997), attributions of agency, in particular, to sexualized female targets may diminish (Nussbaum, 1999). In other words, considering one’s own intentions toward a target may interfere with recognition of the target’s status as an agent with intentions, beliefs, and desires of her own. This is important, because agency is a fundamental predictor of mind perception (Gray, Gray, & Wegner, 2007). No study of which we are aware has directly tested the hypothesis that sexualized female targets as compared to other social targets would be less closely associated with agency and less likely to engage brain regions associated with mental state attribution.
Sexist Attitudes
Responses to sexualized female targets should also vary depending on the perceiver; that is, not all people may diminish attributions of agency to sexualized female targets. For example, power is automatically associated with sex, but only for those men who admit a tendency to sexually harass (Bargh, Raymond, Pryor, & Strack, 1995). Thus, some people more than others may be especially prone to seeing sexualized women as possessing less agency than other social targets and may demonstrate stronger modulation of neural activity in regions associated with social cognition.
Ambivalent Sexism Theory (Glick & Fiske, 1996) contends that sexism combines complementary gender ideologies, held by both men and women worldwide. Benevolent sexism (BS) is a subjectively positive, paternalistic ideology that views women as subordinate; they need to be protected, cherished, and revered for their virtue. In contrast, hostile sexism is a combative ideology maintaining that women seek to control men and use sexuality or feminist ideology as a means to achieving status. Individuals who score high on Hostile Sexism (HS) are more likely to deny that women possess positive, uniquely human, secondary emotions (e.g., compassion, hopefulness, and nostalgia; Viki & Abrams, 2003). Therefore, we expect high HS individuals to demonstrate pronounced modulation of neural responses, specifically in networks associated with mental state attribution, in response to viewing sexualized female targets.
Overview and Hypotheses
To examine the proposition that hostile sexists would more easily regard sexualized women as the objects of action as opposed to agents enacting actions, a first study assessed associations between images of sexualized women and first-person action verbs, in comparison with associations between images of clothed women and third-person action verbs, as a function of hostile sexism. Our hypothesis was that participants higher on hostile sexism would be faster than less sexist participants at associating first-person verbs with the sexualized women—because instruments are the objects of one’s own actions—and the control women with third-person verbs—because only agents, not objects, can be the authors of their actions—as compared with the inverse pairing.
In the second study, twenty-one heterosexual men viewed 200 ms exposures of sexualized and fully-clothed men and women during an fMRI scan. In contrast to previous neuroimaging investigations using sexually arousing stimuli (e.g., explicit erotic scenes, pictures of genitals; Ferretti et al., 2005; Ponseti et al., 2006; Stoleru et al., 1999; Walter et al., 2008), we employ images representative of the sort that are frequently observed in public spaces (e.g., advertisements, billboards). After the scan, separate surprise recognition tasks assessed participants’ memory for targets’ faces and bodies, respectively, and questionnaires assessed participants’ hostile and benevolent attitudes toward women. Our hypothesis was that for participants with high HS scores, passively viewing images of sexualized women should elicit relatively less activity in brain regions associated with social cognition and mentalizing (i.e., medial prefrontal cortex (MPFC), temporo-parietal junction, precuneus/posterior cingulate, superior temporal sulcus, and temporal poles; e.g., Frith & Frith, 2003,; Mitchell, 2008) as compared to looking at sexualized men or clothed women, for example.
Study 1: Implicit Association Test of Agency
Method
The Implicit Association Test (IAT; Greenwald, McGhee, & Schwartz, 1998) indexes the strength of one pair of associations (i.e., sexualized female targets/first-person verbs, clothed female targets/third-person verbs) relative to the strength of the reverse pair of associations (sexualized female targets/third-person verbs, clothed female targets/first-person verbs) by recording the length of time participants need to sort categories and features to different sides of the computer screen (labels: bikini/clothed; “I” verbs/”She” verbs).
Participants
Twenty-four female and 31 male participants (Mage = 25.9) completed the IAT online. Electronic informed consent and experimental procedures complied with the guidelines of Princeton University’s Institutional Review Board. Participants were recruited online via Amazon.com’s Mechanical Turk web service, and were paid for their participation. Six participants reported sexual orientations other than heterosexual, and two participants reported technical troubles with the program so they were excluded from the sample, leaving 20 women and 27 men.
Stimuli
We collected 20 images per stimulus class (sexualized female, clothed female). All of the images were uniform such that targets were smiling, and gazing directly at the camera. We cropped the images (from mid-thigh to top of head), standardized their sizes (380 × 450 pixels), and eliminated all background information. We also altered images to minimize detail in whatever clothing targets were wearing.
The following action verbs constituted our associated verbs, in first- and third-person: use(s), push(es), pull(s), squeeze(s), turn(s), press(es), fold(s), flip(s), grasp(s), handle(s), control(s), treat(s), hold(s), manage(s), operate(s), drive(s), lead(s), steer(s), grab(s), roll(s).
We used Princeton’s “Create Your Own IAT” program to run the study (http://iat.princeton.edu/iat/). The first two blocks were practice trials in which participants either sorted only images of sexualized and fully clothed women and then only first- and third-person verbs, or vice versa. During blocks 3 and 4, participants sorted first-person verbs and sexualized women on one side of the computer screen and third-person verbs and clothed women on the other side. During block 5 participants sorted only pictures of sexualized and clothed women again, this time on different sides of the screen. We doubled the number of practice trials in block 5 to eliminate practice effects (Nosek, Greenwald, & Banaji, 2006). Finally, in blocks 6 and 7, participants sorted first-person verbs and clothed women on one side of the screen and third-person verbs and sexualized women on the other side. Order of image and verb presentation within blocks was randomized across subjects. Blocks 3 and 4 were switched with blocks 6 and 7 for half of the subjects, to control further for order effects. At no point in the instructions or task were the words “sex” or “sexism” included.
Measures
Ambivalent sexism
We assessed participants’ ambivalent attitudes toward women using the Ambivalent Sexism Inventory (ASI; Glick & Fiske, 1996). The ASI consists of 22 statements that respondents rate on a scale ranging from 1 (strongly disagree) to 6 (strongly agree). Eleven items assess hostile sexism (e.g., “Once a woman gets a man to commit to her, she usually tries to put him on a tight leash”), and the other eleven items measure benevolent sexism (e.g., “A good woman should be set on a pedestal by her man”). Participants were not told that the items were designed to measure sexist attitudes.
Experimental Design
Participants were recruited online and redirected to the IAT website. Participants first completed the IAT, then the ASI, followed by a brief demographics questionnaire assessing gender, age, and sexual orientation.
Results
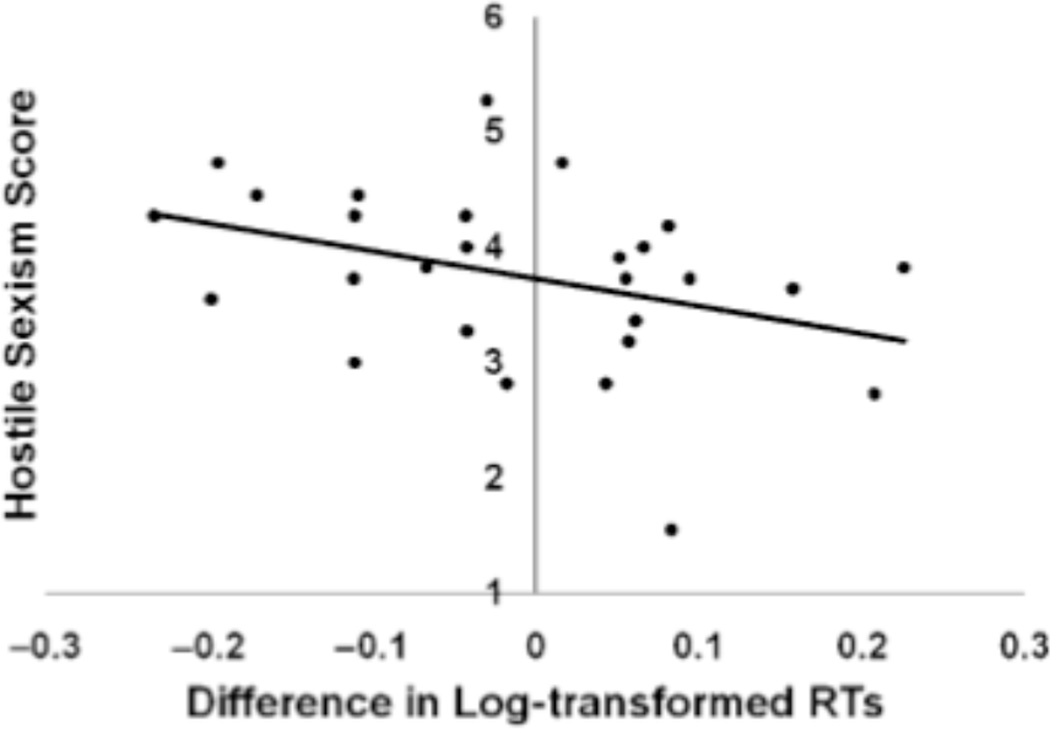
We log transformed the response times (see Bargh & Chartrand, 2000) and conducted a paired samples t-test between sexualized-first person verb/clothed-third person verb RTs versus sexualized-third person/clothed first-person RTs. Though men and women were in general slightly faster to pair images of sexualized female targets with first-person action verbs (e.g., push) and clothed female targets with third-person action verbs (e.g., pushes) than the inverse, the difference between the two pairings was not significant for either sample. We created a difference score of the two RT values (i.e., sexualized-first person/clothed-third person RTs minus sexualized-third person/clothed first-person RTs) and examined the relationship between response time differences and sexist attitudes, benevolent and hostile. Neither female nor male participants’ BS scores were correlated with RT differences, r(18) = −.04 and r(25) = .05, respectively. In contrast, male participants’ HS scores were negatively correlated with RT differences, r(25) = −.38, p = .05: male participants with higher HS scores were faster to pair images of sexualized female targets with first-person action verbs and clothed female targets with third-person action verbs than the inverse, whereas male participants with lower HS scores demonstrated no RT difference between the two conditions or faster RTs for the inverse pairing (Figure 1). Female participants’ HS scores were not related to RT differences, r(18) = −.05, ns.
Figure 1.
Correlation between hostile sexism scores and RT differences for male participants in Study 1, r(25) = −.38, p = .05. Negative values on the x-axis indicate participants who were faster to pair images of sexualized female targets with first-person action verbs and clothed female targets with third-person action verbs than the inverse.
Study 2: fMRI Investigation
Method
Participants
Participants were 22 healthy male undergraduates. This study used only heterosexual male participants because the Study 1 data suggested women, irrespective of their hostile sexism scores were not associating sexualized women with less agency compared to clothed women. Furthermore, previous research has shown substantial differences between heterosexual men and women in the desirability of sex as a goal (Clark & Hatfield, 1989) and in terms of neural activity associated with sexual arousal (Canli & Gabrieli, 2004). All participants were right-handed, native English speakers with no history of psychiatric or neurological problems, and had normal or corrected vision (Mage = 21.05, SD = 3.17). Written informed consent from each participant and experimental procedures complied with the guidelines of Princeton University’s Institutional Review Board. Participants were paid for their participation. All but one participant reported being heterosexual, so the final N = 21. We were not able to obtain ASI scores for two participants, so all analyses including HS and BS, N = 19.
Stimuli
We collected 20 images per stimulus class (sexualized female, clothed female, sexualized male, clothed male), which participants viewed in the scanner, as well as an additional 10 foil faces and 10 foil bodies per stimulus class for face and body recognition tasks. Stimuli were pre-tested to make sure all stimuli and foils were matched in terms of facial attractiveness. For the stimuli used in the scanner: there was no main effect of gender F(1, 119) = 1.67, ns, nor of clothing style F(1, 119) = 2.41, ns, and the interaction between gender and clothing style was not significant, F(1, 119) = 0.65, ns. Comparing stimuli used in the scanner to foil stimuli in the recognition task: Tukey’s post-hoc tests revealed that the clothed targets, sexualized targets, and foil targets had equivalent facial attractiveness ratings.
All of the images were uniform, such that targets were smiling, and gazing directly at the camera. We cropped the images (from mid-thigh to top of head), standardized their sizes (380 × 450 pixels), and eliminated all background information. We also altered images to minimize detail in whatever clothing targets were wearing. Participants saw each stimulus twice, yielding 160 trials over the course of 8 runs. The order in which the images appeared was randomized between participants using AFNI’s “rsfgen” program.
All 80 images viewed in the recognition tasks (10 original and 10 foils per stimulus class) were standardized. For the face recognition task: images were cropped identically, from chin to top of forehead (200 × 220 pixels). For the body recognition task: images were cropped from neck down to mid-thigh (275 × 320 pixels). We computed discriminability scores (d’) for each stimulus category, for each task, separately.
Measures
Ambivalent sexism
We assessed participants’ ambivalent attitudes toward women using the Ambivalent Sexism Inventory (ASI; Glick & Fiske, 1996).
Experimental Design
Participants came to the lab, were screened for participation, and gave their consent. Before the scan, participants completed a practice run; their task was to indicate when they had seen the picture by pressing a button. Once participants were in the scanner, stimuli were projected onto a screen at the rear of the bore of the magnet and viewed via an angled mirror placed above participants’ eyes. Again, they were asked to indicate when they had seen each stimulus by pressing a button. For each image presentation trial, participants saw a white fixation cross on black background for either 6000 or 10000 ms (jittered inter-stimulus intervals), saw the target for 200 ms, and then saw a green fixation cross for 1800 ms. We used 200 ms exposure in order to avoid confounding visual input with stimulus class; the brief presentation did not allow subjects enough time to saccade from the fixation cross. The fixation cross was always situated where targets’ necks would appear, to reduce bias for the face versus the body, depending on which stimulus appeared. Stimuli were presented using E-prime 1.2 (Psychology Software Tools, Inc.: http://www.pstnet.com), and participants responded in the scanner using a fiber-optic touchpad, which they held in their right hands.
After the scan, participants completed the face and body recognition tasks on a lab computer using E-prime 1.2 (order of tasks was counterbalanced across participants). For the recognition tasks: Participants were simply instructed to say whether they had just seen that face (or body with the head cropped off) in the scanner or not and to move as quickly through the images while making as few mistakes as possible. Image orders were randomized between all participants for both recognition tasks. Finally, all participants completed the ASI and a demographics questionnaire assessing age, ethnicity, and sexual orientation.
fMRI Acquisition
At the beginning of each scan session, a high-resolution T-1 weighted anatomical image (T1-MPRAGE, 0.5 × 0.5 × 1.0 mm) was acquired for use in registering activity to each participant’s anatomy and for spatially normalizing data across participants. Echo-planar images were acquired using a 3.0 T Siemens Allegra head-dedicated scanner (Siemens, Erlangen, Germany) with a standard ‘‘bird-cage’’ head coil (TR = 2000 msec, TE = 30 msec, 196mm FOV, matrix size = 64 × 64). Near whole-brain coverage was achieved with 32 interleaved 3-mm axial slices.
fMRI Preprocessing and Data Analysis
We used Analysis of Functional Neuro-Images (AFNI; Cox, 1996) to preprocess and analyze data. Participant motion was corrected using a six-parameter 3-D motion-correction algorithm following slice scan-time correction. We then subjected the data to spatial smoothing with an 8-mm FWHM Gaussian kernel. Finally, the signal was normalized to percent signal change from the mean.
Task-related activity was measured using a window of 2 s, beginning with the onset of target image. For each participant, we computed the BOLD signal for each stimulus occurrence in its respective 2 s TR. For statistical analysis, each stimulus time series was convolved with a hemodynamic response function to create a unique regressor for each stimulus class. Regressors of noninterest were included in the multiple regression model to factor out variance associated with mean, linear, quadratic, and cubic trends in each run as well as participant head motion. We used the nine-parameter landmark method of Talairach and Tournoux (1988) to spatially normalize the activation maps across participants.
Whole brain exploratory analyses were performed with a voxel-wise significance threshold of p < .001. We used the AlphaSim program in AFNI in order to correct for multiple comparisons. A Monte Carlo simulation determined a minimum cluster size of 918 mm3 to achieve corrected significance of p < .05 for whole-brain contrasts, with a voxel-wise threshold of p < .001.
Exploratory whole-brain contrasts
We used AFNI’s 3dANOVA2 program to determine which voxels were more responsive to passively viewing sexualized female targets as compared to the other 3 types of targets. We conducted a 4 (sexualized female, clothed female, sexualized male, clothed male) × 21 (participants) mixed-effects ANOVA, which allowed us to specify a (+3 −1 −1 −1) contrast (for precedent, see Harris & Fiske, 2006).
Correlations with HS
We defined regions that correlated with HS using the Williams’ t-test for the difference between two dependent correlations, which tests the null hypothesis of equality between two dependent product-moment correlations. We tested whether the correlation between HS and activity in response to sexualized female targets differed from the correlation between HS and activity in response to sexualized male targets, and selected only voxels that demonstrated significantly different correlations (Williams’ t(17) = 3.2, p < .005). We chose sexualized men as the comparison target because pilot testing indicated that after sexualized women, sexualized men were perceived as having the least control over their own lives. Because they were most similar to sexualized women on the agency dimension, sexualized men constituted the most conservative comparison group.
Though the Williams’ t-test is informative with regard to selecting voxels that respond differently to sexualized female as compared with sexualized male targets as a function of HS, the precise nature of the relationship between HS and activity in the two conditions is not described. We computed the product-moment correlation between participants’ HS scores and activity averaged across all voxels (not peak values) in the mPFC, PCC, left and right temporal poles, separately, when viewing sexualized female targets and when viewing sexualized male targets, respectively. However, selecting voxels with a correlation threshold and then reporting the strength of the correlation in those voxels with the same data introduces the problem of non-independence. In order to avoid artificial inflation of the correlations, we conducted the Williams’ t-test again, this time on one subset of the data. Specifically, we identified regions using one subset of the data (runs 1, 3, and 5 for subjects 1–10; runs 2, 4, and 6 for subjects 11–21) and computed the correlations using the other half of the data, following Vul and colleagues’ (2009) recommendation.
Results
Behavioral Data
A pilot study conducted with a separate group of male participants confirmed the appetitive and relatively less agentic nature of the sexualized female stimuli as compared to clothed women and sexualized and clothed men (Table 1). Images of sexualized women, as compared to the other three targets, made men feel most pleasant and most emotionally aroused. Men were also most sexually attracted to and least likely to avoid sexualized women. Most germane to the current investigation, sexualized women were perceived as having the least control over their own lives.
Table 1.
Ratings of Stimuli
| Attribute | Control Male | Sexualized Male |
Control Female |
Sexualized Female |
|---|---|---|---|---|
| Pleasant | 3.98 (.44) | 3.04 (.48) | 4.61 (.32) | 4.71 (.50) |
| Emotionally Arousing | 2.75 (.39) | 3.38 (.57) | 3.59 (.54) | 4.58 (.39) |
| Sexually Attractive | 1.29 (.46) | 1.37 (.48) | 3.91 (.80) | 4.67 (.76) |
| Likely to Avoid | 3.64 (.70) | 4.79 (.78) | 2.46 (.47) | 2.71 (.82) |
| In Control of Own Life | 4.73 (.41) | 3.78 (.37) | 4.78 (.33) | 3.62 (.31) |
Note. N = 79. Scale range is 1–7 for all items. Standard deviations appear in parentheses.
A one-way, within-subjects ANOVA examined participants’ discriminability scores (d’) for targets’ faces (all analyses treat the data as a single-factor design with 4 levels so that we could specify +3 −1 −1 −1—deviant cell—contrasts for both the behavioral and exploratory imaging data). The deviant-cell contrast demonstrated that sexualized female targets’ faces were not better remembered than the other 3 targets’ faces, F(1,20) < .1, ns, ηp2 = .007. We followed up with paired samples t-tests, which revealed that sexualized female targets’ faces were not better recognized than clothed female, sexualized male, or clothed male targets’ faces, respectively, all ts < 1, ns, all ηp2 < .03.
The same ANOVA examined participants’ d’ scores for targets’ bodies with the heads removed. The deviant-cell contrast confirmed that participants were significantly better at recognizing sexualized female targets’ bodies as compared with the other 3 types of bodies, F(1,20) = 9.42, p < .01, ηp2 = .32. We followed up with paired samples t-tests, which revealed that sexualized female targets’ bodies were better recognized than clothed female, t(20) = 2.43, p < .05, ηp2 = .23, sexualized male, t(20) = 2.10, p < .05, ηp2 = .18, and clothed male targets’ bodies, t(20) = 5.23, p < .05, ηp2 = .58.
Note that discriminability scores for sexualized female faces and bodies, respectively, were not significantly related to benevolent or hostile sexism scores: correlation between sexualized female face discriminability scores and hostile sexism, r(17) = .11; sexualized female face discriminability and benevolent sexism, r(17) = .13; sexualized female body discriminability and hostile sexism, r(17) = .09; and sexualized female body discriminability and benevolent sexism, r(17) = .08; all rs = ns. Discriminability scores for sexualized male targets’ faces and bodies demonstrated similar null relationships with HS and BS: r(17) = .17, −.15, −.07, −.21, respectively; all rs = ns. This divergence provides preliminary evidence that the imaging findings related to hostile sexism cannot be explained by more or less attention to the sexualized female and male stimuli.
fMRI Data
Exploratory analyses
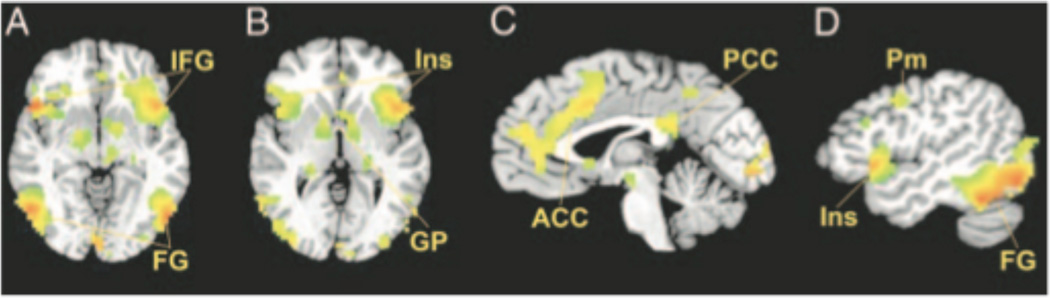
The whole-brain deviant-cell contrast demonstrated that passively viewing images of sexualized female targets activated bilateral fusiform gyrus, bilateral inferior frontal regions including insula, anterior cingulate, bilateral globus pallidus, left thalamus/pulvinar, posterior cingulate, and bilateral premotor cortex, when contrasted against the other 3 classes of stimuli (Table 2; Figure 2).
Table 2.
Brain Regions Exhibiting Differential Activity for Sexualized Female As Compared to 3 Other Targets
| Regions | Right/Left | Brodmann’s Area |
Max t Score (df = 20) |
Cluster Size (mm3) |
Talairach Coordinates (x, y, z) |
|---|---|---|---|---|---|
| Fusiform Gyrus | L | 37 | 8.60 | 26514 | −38, −56, −5 |
| Fusiform Gyrus | R | 37 | 7.57 | 24192 | 42, −55, −6 |
| Inferior Frontal Gyrusa | L | 47 | 8.20 | 17388 | −34, 18, 0 |
| Anterior Cingulate Gyrus | R/L | 24/32 | 7.94 | 13338 | −2, 22, 30 |
| Inferior Frontal Gyrus b | R | 45/47 | 6.05 | 9396 | 39, 21, 3 |
| Medial Globus Pallidus | L | 6.23 | 4077 | −9, −3, −1 | |
| Medial Globus Pallidus | R | 6.70 | 3996 | 11, −2, 0 | |
| Posterior Cingulate | R/L | 23/30 | 5.83 | 1755 | 0, −38, 24 |
| Thalamus (Pulvinar nucleus) | L | 4.85 | 1053 | −20, −25, 4 | |
| Premotor Cortex | R | 6/9 | 4.86 | 999 | 47, 3, 31 |
| Premotor Cortex | L | 6 | 5.27 | 810 | −46, 0, 38 |
Note. Voxelwise significance threshold, p < .001.
Cluster extends to include left anterior insula
Cluster extends to include right anterior insula
Figure 2.
Selected brain regions (see Table 2) exhibiting significantly greater activity in response to passively viewing sexualized female targets as compared to the other 3 stimulus classes: (A) Bilateral fusiform (FG) and bilateral inferior frontal gyrus (IFG); axial slice plane is z = −4. (B) Bilateral insula (Ins) and bilateral globus pallidus (GP); axial slice plane is z = 0. (C) Anterior cingulate (ACC) and posterior cingulate (PCC); sagittal slice plane is x = 0; (D) Left premotor (Pm), insula (Ins), and fusiform (FG); sagittal slice plane is x = −46. Statistical maps of voxelwise t scores were thresholded for significance (p < .001). Images A and B are reversed right to left according to radiologic convention.
Regions of activation correlating with HS
The Williams’ t-test of dependent correlations selected voxels that responded differently to sexualized female as compared to sexualized male targets as a function of HS. We created maps in which each voxel contained two values: the correlation between an individual’s HS score and own activity when viewing sexualized women, and the correlation between HS and activity when viewing sexualized men. Again, we chose sexualized men as the comparison target because pilot testing indicated that after sexualized women, sexualized men were perceived as having the least control over their own lives.3
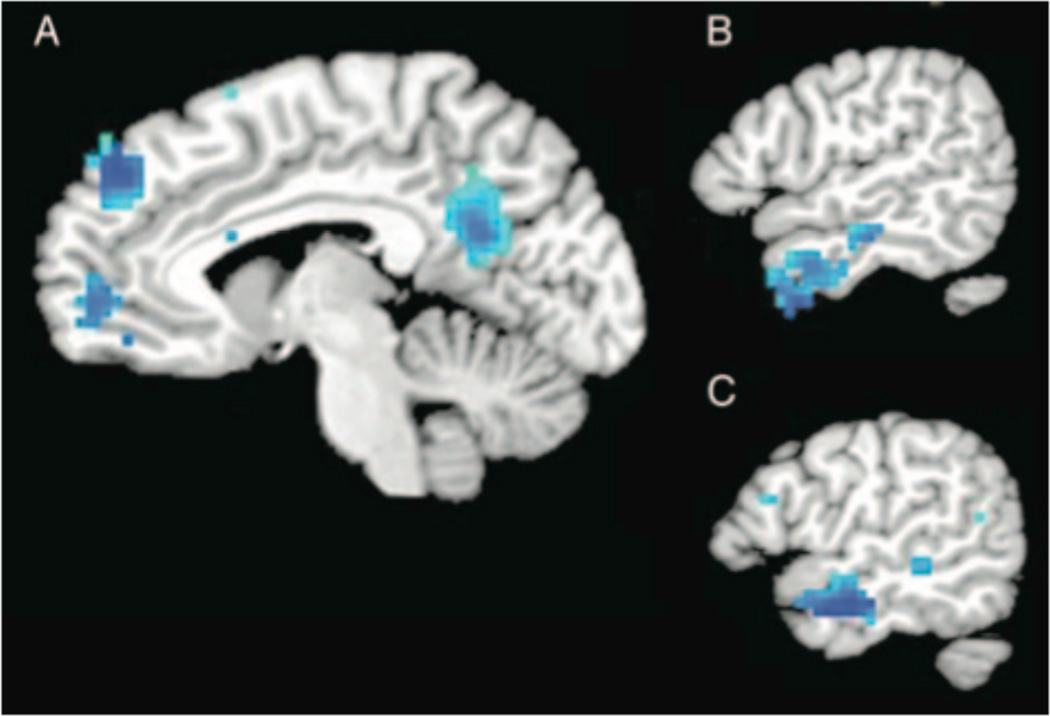
The correlation between HS and activity in response to looking at sexualized female targets was significantly stronger than the correlation between HS and activity in response to looking at sexualized male targets in the following predicted regions, t(17) = 3.2, p < .005: prefrontal cortex (1 cluster in left BA 8, 1 cluster in medial BA 10), posterior cingulate cortex (BA 23/31), and bilateral temporal poles (BA 38/21) (Table 3; Figure 3). These regions represent the five largest clusters identified by the Williams’ t-test; none of the other clusters identified by this analysis surpassed the threshold designated by AlphaSim. Consistent with the findings of Study 1, the Williams’ t-test did not identify any clusters in which the correlation between BS and sexualized female targets was significantly different from the correlation between BS and sexualized male targets.
Table 3.
Brain Regions Exhibiting Differential Activity for Williams’ t-test of Dependent Correlations: (Correlation between HS and Activity in Response to Sexualized Female Target) – (Correlation between HS and Activity in Response Sexualized Male Target)
| Regions | Right/Left | Brodmann’s Area |
Max t Score (df = 17) |
Cluster Size (mm3) |
Talairach Coordinates (x, y, z) |
|---|---|---|---|---|---|
| Posterior Cingulate | R/L | 23/31 | −4.61 | 8316 | 4, −48, 24 |
| Middle Temporal Gyrus | R | 38/21 | −5.42 | 6372 | 50, 1, −24 |
| Middle Temporal Gyrus | L | 38/21 | −5.44 | 5157 | −52, −1, −17 |
| Medial Frontal Gyrus | L | 8 | −5.53 | 1917 | −11, 45, 37 |
| Medial Frontal Gyrus | R/L | 10 | −6.06 | 1458 | 1, 52, 3 |
Minimum t(17)= 3.2, p <.005.
Figure 3.
Selected brain regions (see Table 3) exhibiting significantly greater negative correlation between hostile sexism and activation while viewing sexualized female as compared to sexualized male targets: (A) Medial frontal gyrus (BA 8, BA 10) and posterior cingulate (BA 23/31); sagittal slice plane is x = −2. (B) Right temporal pole (BA 38/21); sagittal slice plane is x = 50. (C) Left temporal pole (BA 38/21); sagittal slice plane is x = −52. Maps are thresholded at t(17)= 3.2, p <.005.
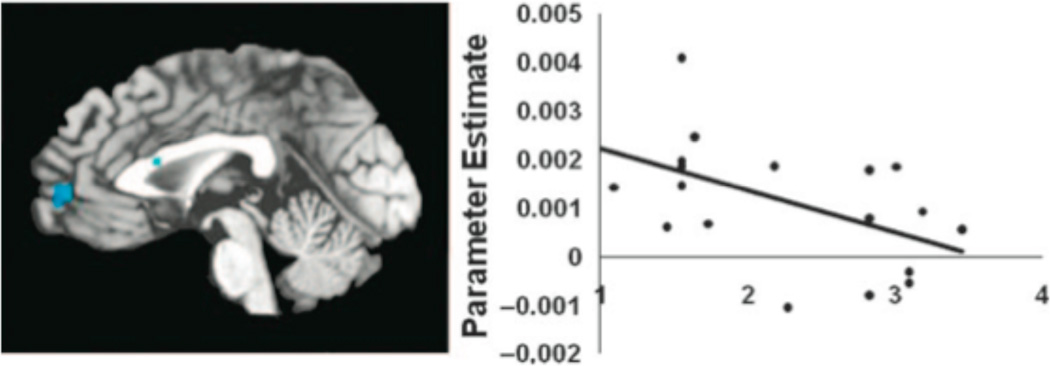
We computed the product-moment correlation between participants’ HS scores and average (not peak) values in mPFC (BA 10 and BA 8), PCC, and left and right temporal poles, separately. All of the regions listed in Table 2 demonstrated the same pattern: significant negative correlations between HS and neural activity when participants viewed sexualized women, but positive correlations between HS and activity in those voxels when participants viewed sexualized men. Specifically, HS was negatively correlated with activity in mPFC (BA 10), r(17) = −.52, p < .05; dorsal mPFC (BA8) r(17) = −.46, p < .05; and PCC, r(17) = −.50, p < .05. HS was also negatively correlated with right and left temporal poles, though the relationships were marginal, r(17) = −.35 and r(17) = −.38, p < .15, respectively. We plotted the correlation between HS and activity in mPFC when participants viewed sexualized female targets (Figure 4); participants who had low HS scores (Mscore ~1; scale’s range 1–6) demonstrated activation in mPFC while looking at sexualized female targets, whereas participants who had higher HS scores (Mscore ~ 4) failed to demonstrate activation above 0 in the same region. Dorsal mPFC, PCC, and temporal poles exhibited the same pattern. In contrast, the correlation between HS and average activity in mPFC (BA 8 and BA 10), PCC, and temporal poles when viewing sexualized male targets was positive, r(17) = .43, .42, .20, .51, and .32, respectively.
Figure 4.
Correlation between activity in mPFC (BA 10) when viewing sexualized female targets and participants’ hostile sexism (HS) scores, r(17) = −.52, p < .05; sagittal slice plane is x = 1.
Discussion
Two studies examined whether target sexualization and perceivers’ sexist attitudes influenced associations of agency with, and neural responses to, images of sexualized and clothed men and women. In line with our first hypothesis, male participants with high hostile sexism scores were faster to associate sexualized female targets with first-person action verbs and clothed female targets with third-person action verbs than the inverse. This suggests that sexualized women are more closely associated with being the objects, not the agents, of action as compared to clothed women, but only for men who possess hostile sexist attitudes. Female participants, irrespective of hostile sexism scores, did not demonstrate this pattern of associations. Neither men’s nor women’s benevolent sexism scores were related to response times. Study 1 provides preliminary evidence that for male perceivers with hostile sexist attitudes, sexualization decreases association of agency with female targets.
This paper is also the first to examine how sexist attitudes modulate neural responses to sexualized and clothed, male and female targets. Higher hostile sexism scores for men predicted decreased activation of mPFC (BA 8 and BA 10), posterior cingulate, and bilateral temporal poles in response to looking at sexualized women, indicating that more hostile attitudes predict less spontaneous activation of the network reliably associated with mentalizing. No such correlation emerged with benevolent sexism.
These mentalizing regions have also been defined as part of the default network, meaning these areas are more active when our minds are engaged in external-stimulus independent thought (Buckner & Carroll, 2007; Gusnard & Raichle, 2001; Mason et al., 2007). Thus an alternative explanation of our findings might be that these areas are less active for men with higher HS scores because high HS participants are relatively more vigilant to images of sexualized women compared to other participants. If indeed these participants were more vigilant to sexualized female targets, we would predict a positive correlation between HS and discriminability scores for sexualized female bodies (or faces), as increased attention at encoding is related to better subsequent memory. There is, however, almost no correlation between participants’ HS scores and their ability to later recognize sexualized women’s bodies or faces, suggesting that the HS-mentalizing network findings cannot be accounted for by increased attention to sexualized female targets. Though we attempted to control variation in saccades across conditions by minimizing stimulus exposure time, one limitation of the current study is that we did not directly measure attention to stimuli in the scanner. Future studies should include eye-tracking and skin conductance to control further for any attention/arousal differences between conditions.
We did not observe decreased activation of rTPJ or pSTS in response to sexualized female targets in participants with relatively higher hostile sexism scores. We believe higher HS scores were not related to decreased activation of rTPJ because participants did not have enough time, nor were they instructed to consider the beliefs of the different targets (Saxe & Kanwisher, 2003; Saxe, Moran, Scholz, & Gabrieli, 2006, Saxe, Schuls, & Jiang, 2006). Similarly, we did not observe this pattern in the pSTS presumably because the images of the targets were static and all targets’ gazes were directed at the camera; pSTS is particularly sensitive to gaze direction (Campbell et al., 2001; Cloutier et al., 2007; Puce, Allison, Bentin, Gore, & McCarthy, 1998; Schuermann et al., 2005) and biological motion (Beauchamp, Lee, Haxby & Martin, 2002, 2003; Blake & Shiffar, 2007; Giese & Poggio, 2003; Mar, Kelley, Heatherton, & Macrae, 2007; Michels, Lappe, & Vaina, 2005; Puce & Perrett, 2003), both of which were held constant across conditions.
In addition to the hypothesized findings, these data also replicate several other imaging studies that find increased neural activity in right inferior frontal cortex, inferior temporal cortex, left anterior cingulate, and right insula, in response to sexually arousing stimuli, irrespective of sexist attitudes (Arnow et al., 2002; Bocher et al., 2001; Ferretti et al., 2005; Hamann et al., 2004; Karama et al., 2002; Mouras et al., 2003; Park et al., 2001; Ponseti et al., 2006; Redouté et al., 2000; Stoleru et al., 1999; Walter et al., 2008).
General Discussion
Several factors predict diminished attribution of mind across a variety of social targets: race/ethnicity of the target, disability, likability, even patient-status in the medical domain (Kozak et al., 2006; Haslam, 2006). Diminished mental state attribution is important because it has implications for how targets are (mis)treated (e.g., Gray & Wegner, 2009; Gruenfeld et al., 2008; Kelman, 1976). The current investigation represents a first step in examining whether sexualized female targets fail to elicit spontaneous mental state attribution to the same extent as other social targets, specifically in perceivers who harbor hostile sexist attitudes.
We employed a variety of methods to assess the effects of hostile sexism on associations of agency with and neural responses to sexualized female targets. Because overtly objectifying others is socially undesirable, people may feel uncomfortable or unable to respond naturally in experimental settings. These dynamics have made studying objectification (and related phenomena such as dehumanization) a methodological challenge. Using indirect measures such as fMRI to complement implicit and explicit self-report helps to circumvent some of the hurdles associated with measuring socially undesirable emotions and behaviors.
In addition to providing converging evidence for the effects of sexist attitudes and sexualization on social cognition, the current fMRI findings add to a growing literature examining disruptions in social cognition as it unfolds in the brain. Previous work has shown decreased activation of mPFC to the most negatively regarded, typically avoided outgroups (e.g., homeless people, drug addicts; Harris & Fiske, 2006). Here we demonstrate decreased mPFC activation to a group to which men feel especially attracted and are least likely to avoid. Though approach-driven objectification is conceptually distinct from avoidance-driven dehumanization, the current findings suggest a common neural response related to diminished attributions of mental states. Note that prior theory distinguishes disgusted dehumanization from objectification (Fiske, Cuddy, & Glick, 2007; Gruenfeld et al., 2008; Haslam, 2006). Rather than demonstrating that objectification and dehumanization are the same process, these data suggest that different social cognitive processes may be associated with similar neural responses (i.e., reduced activation of networks associated with mentalizing).
Finally, we would like to note that our findings applied only to the sexualized female targets; the HS-mentalizing network relationship did not emerge for clothed female targets, highlighting that these responses are unique to the combination of sexist male perceivers viewing sexualized targets. Furthermore, we do not believe these findings are unique to men; women may just as easily perceive men as useful (e.g., as function of a man’s status; Kenrick, Sadalla, Groth, & Trost, 1990), a hypothesis we intend to test in future experiments. In accord with prior theory distinguishing distinct kinds of dehumanization (Fiske, Cuddy, & Glick, 2007; Haslam, 2006), the current findings suggest that sexualization, along with sexist attitudes, fundamentally alters the course of social cognition.
Acknowledgments
The authors thank the Russell Sage Foundation and Princeton Neuroscience Institute, for their generous support of this research project. We also gratefully acknowledge the financial support of the University Center for Human Values and a Charlotte Elizabeth Procter Fellowship at Princeton University awarded to Mina Cikara. Finally, we thank Chris Said for statistical advice and assistance. Portions of this paper were presented at the 2009 meeting of the Society for Personality and Social Psychology and at the 2009 meeting of the American Association for the Advancement of Science.
Footnotes
Note however, that when participants are explicitly asked to infer these same targets’ preferences, mPFC is engaged to an extent similar to that in response to other social targets (Harris & Fiske, 2007), highlighting again the flexibility of these responses.
Dehumanization and sexualization are not synonymous: the former is reserved for targets who elicit an avoidance response, whereas the latter is reserved for sexually instrumental, or appetitive targets (Gruenfed, Inesi, Magee, & Galinsky, 2008).
We also conducted the Williams’ t-test comparing correlations between HS and activity in response to sexualized female targets as compared to clothed female targets. Results looked similar to the contrast against sexualized male targets, though the clusters were smaller in volume and did not exceed the minimum threshold for statistical significance for whole brain comparisons: posterior cingulate (486 mm3), left middle temporal gyrus (621 mm3), right middle temporal gyrus (918 mm3), mPFC (BA 10; 540 mm3).
References
- Adolphs R. How do we know the minds of others? Domain-specificity, simulation, and inactive social cognition. Brain Research. 2006;1079:25–35. doi: 10.1016/j.brainres.2005.12.127. [DOI] [PubMed] [Google Scholar]
- American Psychological Association, Task Force on the Sexualization of Girls. Report of the APA Task Force on the Sexualization of Girls. Washington, DC: American Psychological Association; 2007. Retrieved from: www.apa.org/pi/wpo/sexualization.html. [Google Scholar]
- Ames DR. Inside the mind reader’s tool kit: Projection and stereotyping in mental state inference. Journal of Personality and Social Psychology. 2004;87:340–353. doi: 10.1037/0022-3514.87.3.340. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, Lue TF, Atlas SW. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bandura A. Toward a psychology of human agency. Perspectives on Psychological Science. 2006;1:164–180. doi: 10.1111/j.1745-6916.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chartrand TL. The mind in the middle: A practical guide to priming and automaticity research. In: Reis HT, Judd CM, editors. Handbook of research methods in social and personality psychology. New York: Cambridge University Press; 2000. pp. 253–285. [Google Scholar]
- Bargh JA, Raymond P, Pryor JB, Strack F. Attractiveness of the underling: An automatic power-sex association and its consequences for sexual harassment and aggression. Journal of Personality and Social Psychology. 1995;68:768–781. doi: 10.1037//0022-3514.68.5.768. [DOI] [PubMed] [Google Scholar]
- Bartky SL. Femininity and domination: Studies in the phenomenology of oppression. New York: Routledge; 1990. [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and pointlight displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, Mishani E, Bonne O. Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O-H2O PET study in heterosexual men. Neuroimage. 2001;14:105–117. doi: 10.1006/nimg.2001.0794. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Science. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Campbell R, MacSweeney M, Surguladze S, Calvert G, McGuire P, Suckling J, Brammer MJ, David AS. Cortical substrates for the perception of face actions: an fMRI study of the specificity of activation for seen speech and for meaningless lower-face acts. Cognitive Brain Research. 2001;12:233–243. doi: 10.1016/s0926-6410(01)00054-4. [DOI] [PubMed] [Google Scholar]
- Canli T, Gabrieli JD. Imaging gender differences in sexual arousal. Nature Neuroscience. 2004;7:325–326. doi: 10.1038/nn0404-325. [DOI] [PubMed] [Google Scholar]
- Carruthers P, Smith PK, editors. Theories of theories of mind. Cambridge, England: Cambridge University Press; 1996. [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, Erk S, Pia L, Bara BG, Walter H. The intentional network: How the brain reads varieties of intentions. Neuropsychologia. 2007;45:3105–3113. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Clark RD, Hatfield E. Gender differences in receptivity to sexual offers. Journal of Personality and Human Sexuality. 1989;2:39–55. [Google Scholar]
- Cloutier J, Turk DJ, Macrae CN. Extracting variant and invariant information from faces: The neural substrates of gaze detection and sex categorization. Social Neuroscience. 2007;3:69–78. doi: 10.1080/17470910701563483. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Epley N, Waytz A, Cacioppo JT. On Seeing Human: A three-factor theory of anthropomorphism. Psychological Review. 2007;114:864–886. doi: 10.1037/0033-295X.114.4.864. [DOI] [PubMed] [Google Scholar]
- Epley N, Waytz A. Mind Perception. In: Fiske ST, Gilbert DT, Lindsay G, editors. The Handbook of Social Psychology. 5th ed. New York: Wiley; 2009. pp. 498–541. [Google Scholar]
- Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, Pizzella V, Pompa P, Rigatti P, Rossini PM, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086–1096. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P. Universal dimensions of social cognition: Warmth, then competence. Trends in Cognitive Science. 2007;11:77–83. doi: 10.1016/j.tics.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Frederickson BL, Roberts T-A. Objectification theory. Psychology of Women Quarterly. 1997;21:173–206. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TP, Niehaus JL, Roarty MP, Giesbrecht B, Miller MB. Neural correlates of detecting pretense: Automatic engagement of the intentional stance under covert conditions. Journal of Cognitive Neuroscience. 2004;16:1805–1817. doi: 10.1162/0898929042947892. [DOI] [PubMed] [Google Scholar]
- Giese MA, Poggio T. Neural mechanisms for the recognition of biological movements. Nature Reviews Neuroscience. 2003;4:179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- Glick P, Fiske ST. The Ambivalent Sexism Inventory: Differentiating hostile and benevolent sexism. Journal of Personality and Social Psychology. 1996;70:491–512. [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: A comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Gray K, Wegner DM. Moral typecasting: Divergent perceptions of moral agents and moral patients. Journal of Personality and Social Psychology. 2009;96:505–520. doi: 10.1037/a0013748. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurons in the human brain: an fMRI study. NeuroImage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Berthoz S, Passingham RE. Amygdala activation when one is the target of deceit: Did he lie to you or to someone else? NeuroImage. 2006;30:601–608. doi: 10.1016/j.neuroimage.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: An fMRI study. NeuroImage. 2004;21:744–750. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- Gruenfeld DH, Inesi ME, Magee JC, Galinsky AD. Power and the objectification of social targets. Journal of Personality and Social Psychology. 2008;95:111–127. doi: 10.1037/0022-3514.95.1.111. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle MA. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Dehumanizing the lowest of the low: neuro-imaging responses to extreme out-groups. Psychological Science. 2006;17:847–853. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social groups that elicit disgust are differentially processed in the mPFC. Social Cognitive Affective Neuroscience. 2007;2:45–51. doi: 10.1093/scan/nsl037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social neuroscience evidence for dehumanised perception. European Review of Social Psychology. 2009;20:192–231. [Google Scholar]
- Harris LT, Fiske ST. People use fewer mental-state verbs to describe extreme outgroup minds, which appear less competent-autonomous, less warm-familiar, and less likely for interaction. New York University; 2010. Unpublished manuscript. [Google Scholar]
- Harris LT, Todorov A, Fiske ST. Attributions on the brain: Neuro-imaging dispositional inferences, beyond theory of mind. NeuroImage. 2005;28:763–769. doi: 10.1016/j.neuroimage.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Haslam N. Dehumanization: An integrative review. Personality and Social Psychological Review. 2006;10:252–264. doi: 10.1207/s15327957pspr1003_4. [DOI] [PubMed] [Google Scholar]
- Haslam N, Bain P, Douge L, Lee M, Bastian B. More human than you: Attributing humanness to self and others. Journal of Personality and Social Psychology. 2005;89:937–950. doi: 10.1037/0022-3514.89.6.937. [DOI] [PubMed] [Google Scholar]
- Haslam N, Bain P, Loughnan S, Kashima Y. Attributing and denying humanness to others. European Review of Social Psychology. 2008;19:55–85. [Google Scholar]
- Heberlein AS, Saxe RR. Dissociation between emotion and personality judgments: Convergent evidence from functional neuroimaging. NeuroImage. 2005;28:770–777. doi: 10.1016/j.neuroimage.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psychology. 1944;57:243–259. [Google Scholar]
- Kampe KKW, Frith CD, Frith U. “Hey John:” Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. The Journal of Neuroscience. 2003;23:5258–5263. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman HC. Violence without restraint: Reflections on the dehumanization of victims and victimizers. In: Kren GM, Rappoport LH, editors. Varieties of psychohistory. New York: Springer; 1976. pp. 282–314. [Google Scholar]
- Kenrick DT, Sadalla EK, Groth G, Trost MR. Evolution, traits, and the stages of human courtship: Qualifying the parental investment model. Journal of Personality. 1990;58:97–116. doi: 10.1111/j.1467-6494.1990.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Kozak M, Marsh AA, Wegner DM. What do I think you're doing? Action identification and mind attribution. Journal of Personality and Social Psychology. 2006;90:543–555. doi: 10.1037/0022-3514.90.4.543. [DOI] [PubMed] [Google Scholar]
- Kwan VSY, Fiske ST. Missing links in social cognition: The continuum from nonhuman agents to dehumanized humans. Social Cognition. 2008;26:125–128. [Google Scholar]
- Leyens JP, Cortes BP, Demoulin S, Dovidio J, Fiske ST, Gaunt R, et al. Emotional prejudice, essentialism, and nationalism. European Journal of Social Psychology. 2003;33:703–718. [Google Scholar]
- Loughnan S, Haslam N, Murnane T, Vaes J, Reynolds C, Suitner C. Objectification leads to depersonalization: The denial of mind and moral concern to objectified others. European Journal of Social Psychology. (in press) [Google Scholar]
- Mar RA, Kelley WM, Heatherton TF, Macrae CN. Detecting agency from the biological motion of veridical versus animated agents. Social Cognitive and Affective Neuroscience. 2007;2:199–205. doi: 10.1093/scan/nsm011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Lappe M, Vaina LM. Visual areas involved in the perception of human movement from dynamic form analysis. NeuroReport. 2005;16:1037–1041. doi: 10.1097/00001756-200507130-00002. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Contributions of functional neuroimaging to the study of social cognition. Current Directions in Psychological Science. 2008;17:142–146. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005a;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005b;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Cloutier J, Banaji MR, Macrae CN. Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Social Cognitive and Affective Neuroscience. 2006;1:49–55. doi: 10.1093/scan/nsl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morewedge CK, Preston J, Wegner DM. Timescale bias in the attribution of mind. Journal of Personality and Social Psychology. 2007;93(1):1–11. doi: 10.1037/0022-3514.93.1.1. [DOI] [PubMed] [Google Scholar]
- Mouras H, Stoleru S, Bittoun J, Glutron D, Pelegrini-Issac M, Paradis AL, Burnod Y. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. NeuroImage. 2003;20:855–869. doi: 10.1016/S1053-8119(03)00408-7. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Greenwald AG, Banaji MR. The Implicit Association Test at age 7: A methodological and conceptual review. In: Bargh JA, editor. Social psychology and the unconscious: The automaticity of higher mental processes. Psychology Press; 2006. pp. 265–292. [Google Scholar]
- Nussbaum MC. Sex and social justice. New York: Oxford University Press; 1999. [Google Scholar]
- Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. International Journal of Impotence Research. 2001;13:73–81. doi: 10.1038/sj.ijir.3900649. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Mehdorn HM, Buchel C, Siebner HR. A functional endophenotype for sexual orientation in humans. NeuroImage. 2006;33:825–833. doi: 10.1016/j.neuroimage.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences. 1978;1:515–526. [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation of humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philosophical Transactions Royal Society London. Biological Sciences. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, Le Bars D, Forest MG, Pujol JF. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11:162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, et al. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;16:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Rudman LA, Borgida E. The afterglow of construct accessibility: The behavioral consequences of priming men to view women as sexual objects. Journal of Experimental Social Psychology. 1995;31:493–517. [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of temporoparietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: Dissociating theory of mind and executive control in the brain. Social Neuroscience. 2006;1:284–298. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- Schooler D, Ward LM. Average Joes: Men’s relationships with media, real bodies, and sexuality. Psychology of Men and Masculinity. 2006;7:27–41. [Google Scholar]
- Schuermann M, Hesse MD, Stephan KE, Saarela M, Zilles K, Hari R, Fink GR. Yearning to yawn: the neural basis of contagious yawning. NeuroImage. 2005;24:1260–1264. doi: 10.1016/j.neuroimage.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Schultz J, Imamizu H, Kawato M, Frith CD. Activation of the human superior temporal gyrus during observation of goal attribution by intention objects. Journal of Cognitive Neuroscience. 2004;16:1695–1705. doi: 10.1162/0898929042947874. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, Lavenne F, Le Bars D, Vernet-Maury E, Rada H, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of Sexual Behavior. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain (M. Rayport, Trans.) New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Viki GT, Abrams D. Infra-humanization: Ambivalent sexism and the attribution of primary and secondary emotions to women. Journal of Experimental Social Psychology. 2003;39:492–499. [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enici I, Pia L, Bara BG. Understanding intentions in social interactions: The role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schlitz K, Tempelman C, Rotte M, Heinze HJ, Bogerts B, Northoff G. Distinguishing specific sexual and general emotional effects in fMRI—Subcortical and cortical arousal during erotic picture viewing. NeuroImage. 2008;40:1482–1494. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Waytz A, Morewedge CK, Epley N, Monteleone G, Gao J-H, Cacioppo JT. Making sense by making sentient: Effectance motivation increases anthropomorphism. Journal of Personality and Social Psychology. doi: 10.1037/a0020240. (in press) [DOI] [PubMed] [Google Scholar]
- Waytz A, Epley N, Cacioppo JT. Social cognition unbound: Psychological insights into anthropomorphism and dehumanization. Current Directions in Psychological Science. 2010;19:58–62. doi: 10.1177/0963721409359302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A, Gray K, Epley N, Wegner DM. Causes and consequences of mind perception. Trends in Cognitive Science. 2010;14:383–388. doi: 10.1016/j.tics.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Zillmann D, Bryant J. Pornography’s impact on sexual satisfaction. Journal of Applied Social Psychology. 1988;18:438–453. [Google Scholar]