Abstract
Important recent advances implicate a role of the carotid body (CB) chemoreflex in sympathetic and breathing dysregulation in several cardio-respiratory diseases, drawing renewed interest in its potential implications for clinical treatment. Evidence from both chronic heart failure (CHF) patients and animal models indicates that the CB chemoreflex is enhanced in CHF, and contributes to the tonic elevation in sympathetic nerve activity (SNA) and periodic breathing associated with the disease. Although this maladaptive change likely derives from altered function at all levels of the reflex arc, a change in afferent function of the CB is likely to be a main driving force. This review will focus on recent advances in our understanding of the pathophysiological mechanisms that alter CB function in CHF and their potential translational impact on treatment of chronic heart failure (CHF).
Keywords: Hypertension, Carotid body chemoreflex, CB, Heart failure, Animal models, Gasotransmitters, Blood flow, CB ablation, Angiotensin
Introduction
The carotid body (CB) chemoreflex plays an important role in oxygen homeostasis for the body. Specialized (glomus, type I) cells in the CB are stimulated by a reduction in arterial PO2 (PaO2) below 60 mm Hg, corresponding with the rapid declining phase in hemoglobin saturation and O2 content in blood. The increased neural input from the CB to the brainstem increases alveolar ventilation to avert significant hypoxemia from developing. This homeostatic reflex acts to ensure that gas exchange rate in the lungs continually matches metabolic demand for O2 uptake. Another important component of CB chemoreflex activation is an increase in sympathetic outflow to the vascular beds. This response aids in the maintenance of arterial pressure in the face of the direct vasodilatory effects of hypoxemia, and thus assists in the maintenance of arterial pressure for adequate blood flow and gas exchange in essential tissues.
Although the CB chemoreflex is generally regarded as a protective mechanism to avert significant hypoxemia, recent studies have underscored its important regulatory role in the eupneic drive to breathing [1] and control of blood flow during exercise [2, 3]. However, there is substantial evidence to indicate that the CB can be maladaptive. The CB chemoreflex is known to be exaggerated in sleep apnea [4], hypertension [5•], and chronic heart failure (CHF) [6], as well as other conditions [7] such as metabolic syndrome [8•] and renal failure [9•], and has been implicated as a contributing factor to the autonomic and respiratory dysregulation associated with these disorders.
In particular, CHF is characterized by tonic over-activation of sympathetic neural outflow that exacerbates its progression [10]. CHF is also characterized by the development of breathing instability with Cheyne-Stokes breathing and central apneas that further negatively impact autonomic and metabolic homeostasis. The CB clearly plays a potential role in these pathophysiological changes. Animal models [11] and patients [12] with CHF exhibit increased CB chemoreflex drive to increase sympathetic outflow and ventilation under both normoxic and hypoxic conditions. Moreover, the exaggerated CB chemoreflex is correlated with both the pathophysiology and prognosis of CHF in patients [13, 7].
Mechanisms Responsible for Enhanced CB Function in CHF
There is an enhanced tonic afferent input from CB chemoreceptors in CHF rabbits [14] and rats ([15•]), which provides a primary contribution to the augmentation of reflex function. The baseline discharge in the normoxic state and the magnitude of the afferent response to increasing levels of isocapnic hypoxia are enhanced well above normal in CHF animals. This enhancement occurs in both rapid pacing-induced [14] and myocardial infarct-induced [15•] models of CHF, and thus develops independently of the etiology of systolic failure. Furthermore, an intrinsic alteration within the CB, rather than a circulating factor, drives the increased chemoreceptor activity in the CHF state. These alterations are observed in isolated CB preparations as well as the intact (blood perfused) CB [14].
The factors in the CB that enhance its excitability in CHF are complex. These include an enhanced excitatory effect of angiotensin II (Ang II) activation of NADPH oxidase (NOX) and superoxide (O2•−) production due to upregulation of angiotensin converting enzyme (ACE) and downregulation of CuZn- and Mn-SOD [16]; reduced inhibitory effects of Ang 1-7 [16], nitric oxide (NO) [17] and carbon monoxide (CO) [18] due to downregulation of ACE2, nNOS/eNOS and HO-2; and a supportive excitatory influence from H2S [15•].
Angiotensin in the CB in CHF
A locally generated Ang II system exists in the CB, and systemic and tissue Ang II levels are increased in CHF patients and animal models. Afferent recordings from the isolated CB confirm that a functional AT1R in the CB contributes to the elevated chemoreceptor afferent activity in CHF rabbits [19]. Pharmacological blockade of AT1R decreases CB chemoreceptor responses to hypoxia in the isolated CB in CHF rabbits, but not in sham rabbits. In addition, Ang II concentration and mRNA expression and protein levels of AT1R in the CB are increased in CHF animals.
As discussed previously [16], Ang II enhances the hypoxic sensitivity of the CB chemoreceptors, at least in part, by suppressing voltage-gated currents (IKv) in CB glomus cells [20]. Hypoxia inhibits IKv and this effect is enhanced in isolated CB glomus cells from CHF rabbits [20]. Blockade of AT1R alone is capable of reversing this enhanced hypoxic sensitivity of glomus cell IKv. In addition, Ang II mimics this effect of CHF on IKv when exposed to normal rabbit CB glomus cells. In the rabbit, the suppression of IKv observed in CHF appears to involve the Ca++-sensitive K+ channel (BK) [21], Kv3.4 and Kv4.3 [20, 11]. However, the specific types of K+ channels involved in glomus cell function vary by species, and other, not yet examined types of channels also may play a role.
Studies also confirm that the NOX-O2•− pathway mediates the effects of Ang II in the CB to enhance chemoreceptor sensitivity in the CHF state [16]. In the CB, expression of NOX2 subunits and O2•− production are enhanced in CHF rabbits [22]. AT1R antagonists, NOX inhibitors, and the O2•− scavenger tempol normalize the elevated O2•− [22]. In addition, NOX inhibitors and tempol normalize the enhanced sensitivity of IKv and CB chemoreceptor discharge to hypoxia in CB glomus cells in CHF [22].
Other angiotensin metabolites also modulate CB function in CHF [16]. The rate-limiting angiotensin converting enzyme (ACE) regulates Ang II production. Alternative pathways regulate Ang-(1-7) production. Ang II is converted to Ang-(1-7) by angiotensin converting enzyme 2 (ACE2) or from Ang I by either neutral endopeptidases or propylendopeptidases. Functionally, Ang-(1-7) activation of MasR increases nitric oxide (NO) via neural NOS (nNOS) in cultured catecholaminergic neuronal cells (CATH.a) [23] and within autonomic regions of the brain stem [24]. Ang-(1-7) activation of NO leads to increased IKv in CATH.a neurons [23]. This action may explain its sympatho-inhibitory effect. Ang-(1-7) similarly enhances IKv in CB glomus cells via MasR activation of nNOS and NO production: an effect that opposes the inhibitory effect of Ang II- O2•− on these channels [20].
Altered Redox Balance in the CB in CHF
CB function can be finely tuned by the balance of factors influencing Ang production and metabolism in the CB via the opposing effects of Ang II and Ang-(1-7) on CB chemo-sensitivity. Under normal conditions [16], Ang-(1-7) may act to maintain NOS expression and NO restraint of CB chemoreceptor activity. In CHF, elevated CB Ang II exerts an excitatory influence on CB chemoreceptor activity, which may override Ang-(1-7) and may even participate in the downregulation of MasR-NO effects seen in the CHF state. This balance is likely to be controlled by the relative expression of ACE and ACE2 activities in the CB [16]. Consistent with this notion, ACE2 and MasR are suppressed, whereas ACE and AT1R are upregulated in the CB in CHF rabbits [16]. These mechanisms may control redox balance in the CB such that oxidative stress enhances CB sensitivity in CHF, whereas inhibitory NO influences on CB sensitivity are downregulated.
Other changes also contribute to altered redox balance in the CB in CHF. Both copper/zinc superoxide dismutase (CuZnSOD) and manganese SOD (MnSOD), endogenous scavengers of O2•−, are suppressed in glomus cells of the CB in CHF [25, 26]. A dual role of both cytosolic and mitochondrial O2•− in the Ang II-NOX signaling cascade has been demonstrated within CB glomus cells. Gene transfer to restore expression of either of the SOD isoforms in the CB reverses the suppression of glomus cell IKv and subsequent enhancement of CB chemoreceptor and chemoreflex activation observed in CHF [25, 26].
Gasotransmitters in the CB in CHF
NO is thought to play an important role in the tonic control of CB chemosensitivity [27]. Both nNOS and endothelial NOS (eNOS) are present in the CB. NO enhances IKv and inhibits CB activity [14, 21]. NO suppresses CB chemoreceptor discharge in normoxic conditions because O2 is essential for biosynthesis of NO [14]. Basal NO production and nNOS/eNOS expression within the CB are depressed in CHF [18, 28, 11]. Thus, the tonic inhibitory effect of NO on the activity of CB chemoreceptors and its enhancement of IKv in glomus cells under normoxic conditions are virtually absent in CHF rabbits [21] Adenoviral gene transfer of nNOS to the CB in CHF rabbits reverses the enhanced resting CB chemoreceptor activity seen in the CHF state and reduces resting renal sympathetic nerve activity [17].
The gasotransmitters CO and H2S also influence CB chemoreceptor sensitivity. CO is known to be an important signaling molecule in the CB [29, 30]. The constitutive heme-oxygenase enzyme (HO-2) is expressed in neuronal and chemosensing tissues, including CB chemoreceptor (glomus) cells [30]. Inducible HO-1 is not activated in the CB in CHF animals, whereas HO-2 protein expression is markedly decreased in the CB [18]. CO, similar to NO, plays a functional role in restraining hypoxic sensitivity of the CB in the normal condition and CO deficiency in the CB contributes to enhanced peripheral chemoreflex function and sympathetic activation in CHF animals [18]. In addition, there is a functional interaction between CO and NO pathways in the CB. The exaggerated hypoxia-induced chemoreflex response in CHF rabbits is blunted by co-administration of CO + NO donors, and this effect is additive to the effect of administering either donor alone [18]. Conversely, in normal rabbits, HO + NOS inhibition induces greater enhancement of sympathetic activation to hypoxia than either inhibitor alone. Thus the CO and NO pathways in the CB have cumulative effects to enhance peripheral chemoreflex function in pacing-induced CHF rabbits.
H2S derived from cystathionine γ-lyase (CSE) also contributes to the CB chemosensory response to hypoxia [31]. CSE expression is maintained in the CB during CHF, and CSE inhibition reduces CB afferent responsiveness, suggesting that H2S contributes to the maintenance of exaggerated CB afferent responsiveness in CHF [15•]. H2S causes a rapid reversible increase in intracellular Ca2+ in CB type 1 cells [32, 33]. It has been shown that H2S excites type 1 cells through the inhibition of background K+ (TASK) channels in rats (5) and BKCa currents in mice (19). Recent studies [31] suggest that H2S function in the CB may be linked to HO-2 due to an inhibitory influence of CO on CSE enzyme activity [31]. This concept is supported by our prior studies [18, 34]. We have shown previously that HO-2 is downregulated in the CB in CHF [18], which would promote CSE activity and H2S-mediated modulation of CB chemoreceptor afferent activity as observed in the CHF rats under both normoxic and hypoxic conditions [15•]. Nevertheless, the observation that CSE expression is not altered in the CB in CHF [15•] suggests that H2S probably plays a supportive rather than causative role in sensitization of the CB in CHF.
Role of Blood Flow in CB Function in CHF
The factors responsible for upregulation of local ACE and Ang II-AT1R- O2•− signaling and downregulation of ACE2 and Ang-(1-7)-MasR-NO and HO-2/CO in CB glomus cells during CHF are not clear. Adaptations to hypoxia are not likely to play a major role. CB sensitivity increases progressively during the development of CHF while arterial PO2 remains stable. Intermittent hypoxia can result from Cheyne-Stokes breathing and central apneas induced by CHF, but breathing instability in animal models of CHF appears only after alterations in CB function are evident [35]. This raises the question whether impaired blood flow secondary to cardiac failure may play a role. Although acute changes in systemic hemodynamics are known to have little effect on chemoreceptor activity, chronic changes in blood flow could impact the CB via alterations in endothelial function and signaling pathways.
Chronic reduction in blood flow to the CB induces changes in CB afferent function similar to those seen in CHF [36]. A study in which a sustained reduction in CB blood flow was imposed over 3 weeks with adjustable cuff occlusion of the carotid arteries in rabbits revealed enhanced CB afferent chemoreceptor discharge and reflex hypoxic ventilatory and sympathetic responses [36]. The reduction in blood flow and functional CB effects are similar to changes observed in CHF rabbits over a similar time course. The chemoreflex and CB afferent activity were not affected by shorter duration (1 day) carotid occlusion, emphasizing the chronic nature of the effect. Immunohistochemical examination of the CB after chronic carotid occlusion revealed an increase in AT1R and decrease in nNOS expression.
These results suggest that a chronic reduction in blood flow to the carotid body may precipitate altered CB function in CHF [16]. However, the link between reduced CB flow and altered signaling pathways in the CB remains to be identified. The transcriptional regulation of ACE and eNOS expression (and possibly ACE2 expression) in vascular endothelial cells is influenced by changes in blood flow to alter shear stress [37, 38]. This association raises the question whether an endothelial response to changes in blood flow in the CB plays an important role in regulating Ang metabolism and NO/CO effects on CB chemoreceptor function in CHF. Studies are needed to better understand the relation of CB blood flow to endothelial function and CB sensory activity in CHF.
Clinical Impact of Enhanced CB Function in CHF
Can normalization of CB function have a beneficial effect in CHF, and how might this be achieved? These are important translational questions that have yet to be adequately addressed. High CB chemosensitivity is an independent predictor of early death from CHF [13, 39]. Enhanced CB chemosensitivity contributes to sympathetic activation and breathing instability, exercise intolerance, impaired baroreflex function, and reduced heart rate variability: conditions that are maladaptive in the CHF state. Thus, it is reasonable to suggest that normalizing CB function would be efficacious in CHF.
Transient hyperoxic inhibition of the CB reduces sympathetic activity and breathing instability and improves exercise tolerance in CHF patients, but has limited efficacy in an ambulatory setting. Pharmacological approaches such as opiates or dopamine can reduce CB chemoreflex drive in CHF patients, but have contraindications [40]. ACE inhibitors and AT1R blockers (ARBs) are routinely used to treat CHF with variable success [41], but there has been no effort to relate the clinical efficacy of these drugs to effects on chemoreflex function. Further, the potential adverse implications of CB inhibition in patients with marked hypoxemia or tenuous cardio-respiratory status must be realized.
Exercise conditioning has come to light as an effective therapeutic modality in CHF patients. Moderate regular exercise has significant beneficial effects in CHF [42] that include enhancement of endothelial function and normalization of sympatho-vagal balance. The effects of regular exercise on CB chemoreflex function in CHF; therefore, they are of obvious relevance. Regular exercise is effective in normalizing CB chemoreflex function in CHF rabbits via enhancing NO and reducing Ang II- O2•− redox influences on the CB [43•]. These changes may be due to the effects of exercise on endothelial function in the CB. These findings illustrate the beneficial effect of exercise on CB function in CHF, but further studies are needed to assess its long-term impact on cardiac function and mortality.
Carotid Body Ablation As An Effective Therapeutic Approach in CHF
What is apparent from our present knowledge is that the factors involved in sensitization of CB chemoreflex in CHF are multiplicative, redundant, and not yet fully realized. This complexity begs the question: to what extent is it possible to normalize CB function to assess its potential beneficial effect in CHF? Pharmacological and exercise approaches targeting the unique contribution of the CB in CHF are limited due to widespread (and possibly counter indicative) effects of these interventions outside of the CB. In recent studies, we addressed this issue by selective surgical ablation of the CB, leaving carotid baroreceptors and other neural reflexes intact in two models of systolic heart failure: the myocardial infarct [44••] and rapid-pacing [45••, 46••] models of CHF. CB ablation markedly reduced the elevated resting ventilation and the enhanced ventilatory responses to hypoxia in both animal models of CHF, while leaving baroreflex responsiveness intact [44••, 45••]. Carotid body ablation also normalized breath-to-breath breathing rate variability and apnea incidence in CHF animals. These results clearly indicate that input from the CB in CHF contributes to breathing instability and the development of periodic breathing associated with CHF.
Cardiac autonomic control also improved in CHF animals after CB ablation [44, 46]. Total spectral power of heart rate variability and the high frequency component of HRV, which are suppressed in CHF animals, increased toward normal levels after CB denervation. The incidence of arrhythmias in CHF was also markedly reduced following CB ablation. These results implicate the CB in the reduction of tonic vagal and increased sympathetic influences on the heart known to occur in CHF. Importantly, other indices of sympathetic hyperactivity in CHF, including increased renal sympathetic nerve activity [45••], activation of pre-sympathetic neurons in the RVLM [44••] and elevated sympatho-respiratory coupling, [45••] were also markedly reduced by CB ablation. These changes were accompanied by an improvement in baroreflex sensitivity and reduced low frequency oscillations in systolic blood pressure [44••, 46••]. These results strongly support a role for the CB in the tonic activation of sympathetic outflow and deactivation of vagal outflow and impairment in baroreflex function known to occur in CHF, and appear to be related to destabilization of control of breathing.
Of major clinical relevance, CB ablation was shown to improve cardiac function, reduce cardiac remodeling, and increase survival rate in the CHF animals [44••, 46••]. The mechanisms by which removal of the CB improves cardiac performance and survival remain to be elucidated, but are likely to involve improved autonomic control of hemodynamics and cardiac function and improved control of breathing (Fig. 1). Breathing disorders and heightened sympathetic outflow are two major hallmarks of CHF, and both are closely related to increased morbidity and mortality [47, 48]. Furthermore, it has been shown that higher chemoreflex drive is associated with higher mortality in CHF patients [13]. Cardiac remodeling and increases in cardiac arrhythmogenesis are both recognized to contribute to the progression of CHF. Our current findings indicate that the CB plays a seminal role in the pathological progression of CHF. Targeted ablation of the CB is a potentially valuable therapeutic strategy that can effectively reverse autonomic and respiratory dysfunction and improve survival of CHF patients.
Fig. 1.
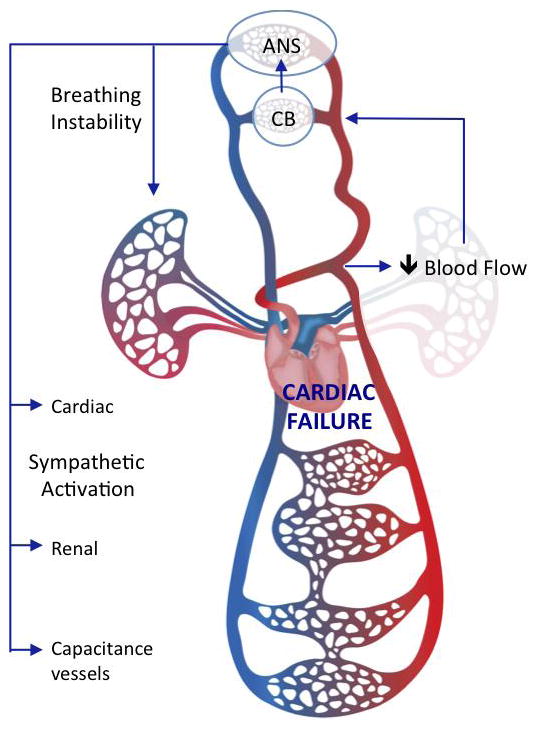
Illustrative model of the impact of carotid body (CB) chemoreceptor afferents on cardio-respiratory function in CHF. Elevated neural input from the CB to the autonomic nervous system (ANS) contributes to breathing instability and sympathetic activation in CHF. CB ablation reduces the sympatho-respiratory dysfunction to improve cardiac performance. Background image from Dreamstime.com
Conclusion
The causes of enhanced CB function in CHF are multifactorial. CB afferent activity is tonically elevated as a result of a shift in redox balance in the CB from inhibitory NO/CO pathways to excitatory oxidative pathways. Altered angiotensin metabolism and signaling in the CB plays an important role in this shift. These alterations are mediated by upregulation of ACE, Ang II, AT1R and NOX2 and downregulation of ACE2, Ang 1-7, NOS and NO in the CB. Gasotransmitters CO and H2S also play a role. A precipitating factor involved in altering CB function in CHF may be related to reduced blood flow and its hindrance of normal endothelial function and signaling in the CB. The beneficial effect of exercise to normalize CB function in CHF may be related to improving endothelial function and counteracting these phenomena in the CB. The translational impact of these effects has been recently clarified. These studies confirm that the elevated CB activity in CHF contributes to sympathetic activation, breathing instability, impaired baroreflex function, impaired cardiac sympatho-vagal balance, and deteriorating cardiac function. Surgical ablation of the CB normalizes autonomic and respiratory balance in CHF animals to improve cardiac function and increase survival. Selectively targeting of the CB in addition to other current treatment modalities may prove to be an effective therapeutic approach in the management of CHF.
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed.
Compliance with Ethics Guidelines
Conflict of Interest
Harold D. Schultz, Noah J. Marcus, and Rodrigo Del Rio declare that they have no conflict of interest to disclose regarding funding or compensation from industry for this review or for the authors’ research cited in this paper. All authors collectively provided consultancy to Coridea NCI (now Cibiem) regarding their interests in the efficacy of carotid body denervation in a rabbit model of CHF.
References
Recently published papers of particular importance have been highlighted as:
*Of importance
**Of major importance
- 1.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106(5):1564–73. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol. 2012;590(Pt 17):4129–44. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586(6):1743–54. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kara T, Narkiewicz K, Somers VK. Chemoreflexes--physiology and clinical implications. Acta Physiol Scand. 2003;177(3):377–84. doi: 10.1046/j.1365-201X.2003.01083.x. 1083 [pii] [DOI] [PubMed] [Google Scholar]
- *5.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, et al. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590(Pt 17):4269–77. doi: 10.1113/jphysiol.2012.237800. The study demonstrates the efficacy of CB ablation to reduce blood pressure in spontaneously hypertensive rat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50(1):6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. HYPERTENSIONAHA.106.076083 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Schmidt H, Francis DP, Rauchhaus M, Werdan K, Piepoli MF. Chemo- and ergoreflexes in health, disease and ageing. Int J Cardiol. 2005;98(3):369–78. doi: 10.1016/j.ijcard.2004.01.002. S0167527304000932 [pii] [DOI] [PubMed] [Google Scholar]
- *8.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013 doi: 10.2337/db12-1463. The study demonstrates the efficacy of CB ablation to prevent the development of insulin resistance and hypertension induced by hypercaloric diet in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Hering D, Zdrojewski Z, Krol E, Kara T, Kucharska W, Somers VK, et al. Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens. 2007;25(1):157–61. doi: 10.1097/HJH.0b013e3280102d92. doi:10.1097/HJH.0b013e3280102d92 00004872-200701000-00023 [pii] The study demonstrates the efficacy of acute CB inhibition (breathing 100 % O2) to reduce the elevated muscle sympathetic nerve activity in patients with chronic renal failure. [DOI] [PubMed] [Google Scholar]
- 10.Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010;108(2):227–37. doi: 10.1152/japplphysiol.00832.2009. 00832.2009 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Schultz HD, Li YL. Carotid body function in heart failure. Respir Physiol Neurobiol. 2007;157(1):171–85. doi: 10.1016/j.resp.2007.02.011. S1569-9048(07)00055-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponikowski P, Banasiak W. Chemosensitivity in chronic heart failure. Heart Fail Monit. 2001;1(4):126–31. [PubMed] [Google Scholar]
- 13.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104(5):544–9. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 14.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol. 1999;86(4):1273–82. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- *15.Del Rio R, Marcus NJ, Schultz HD. Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol. 2013;114(9):1141–1150. doi: 10.1152/japplphysiol.01503.2012. The study demonstrates that systemicinhibition of H2S production improves control of breathing and autonomic function in heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz HD. Angiotensin and carotid body chemoreception in heart failure. Current opinion in pharmacology. 2011;11(2):144–9. doi: 10.1016/j.coph.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, et al. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res. 2005;97(3):260–7. doi: 10.1161/01.RES.0000175722.21555.55. 01.RES.0000175722.21555.55 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, Li YL, Schultz HD. Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits. J Appl Physiol. 2008;105(1):14–23. doi: 10.1152/japplphysiol.01345.2007. 01345.2007 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, et al. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71(1):129–38. doi: 10.1016/j.cardiores.2006.03.017. S0008-6363(06)00144-1 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol. 2006;575(Pt 1):215–27. doi: 10.1113/jphysiol.2006.110700. jphysiol.2006.110700 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YL, Sun SY, Overholt JL, Prabhakar NR, Rozanski GJ, Zucker IH, et al. Attenuated outward potassium currents in carotid body glomus cells of heart failure rabbit: involvement of nitric oxide. J Physiol. 2004;555(Pt 1):219–29. doi: 10.1113/jphysiol.2003.057422. doi:10.1113/jphysiol.2003.057422 jphysiol.2003.057422 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res. 2007;75(3):546–54. doi: 10.1016/j.cardiores.2007.04.006. S0008-6363(07)00161-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang RF, Yin JX, Li YL, Zimmerman MC, Schultz HD. Angiotensin-(1-7) Increases Neuronal Potassium Current via a Nitric Oxide-Dependent Mechanism. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00369.2010. ajpcell.00369.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106(2):373–82. doi: 10.1161/CIRCRESAHA.109.208645. CIRCRESAHA.109.208645 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Li YL, Zimmerman MC, Davisson RL, Schultz HD. Role of CuZn superoxide dismutase on carotid body function in heart failure rabbits. Cardiovasc Res. 2009;81(4):678–85. doi: 10.1093/cvr/cvn350. cvn350 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y, Li YL, Zimmerman MC, Schultz HD. Elevated mitochondrial superoxide contributes to enhanced chemoreflex in heart failure rabbits. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R303–11. doi: 10.1152/ajpregu.00629.2009. 00629.2009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. 2010;95(6):657–67. doi: 10.1113/expphysiol.2009.049312. expphysiol.2009.049312 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Li YL, Zheng H, Ding Y, Schultz HD. Expression of neuronal nitric oxide synthase in rabbit carotid body glomus cells regulates large-conductance Ca2+-activated potassium currents. J Neurophysiol. 2010;103(6):3027–33. doi: 10.1152/jn.01138.2009. jn.01138.2009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P. Sensing hypoxia in the carotid body: from stimulus to response. Essays in biochemistry. 2007;43:43–60. doi: 10.1042/BSE0430043. [DOI] [PubMed] [Google Scholar]
- 30.Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respiration physiology. 1999;115(2):161–8. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakar NR. Carbon monoxide (CO) and hydrogen sulfide (H(2)S) in hypoxic sensing by the carotid body. Respir Physiol Neurobiol. 2012;184(2):165–9. doi: 10.1016/j.resp.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012;463(5):743–54. doi: 10.1007/s00424-012-1089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makarenko VV, Nanduri J, Raghuraman G, Fox AP, Gadalla MM, Kumar GK, et al. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol. 2012;303(9):C916–23. doi: 10.1152/ajpcell.00100.2012. doi:10.1152/ajpcell.00100.2012 ajpcell.00100.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz HD, Del Rio R, Ding Y, Marcus NJ. Role of neurotransmitter gases in the control of the carotid body in heart failure. Respir Physiol Neurobiol. 2012;184(2):197–203. doi: 10.1016/j.resp.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus NJ, Schultz HD. Role of Carotid Body Chemoreflex Function in the Development of Cheyne-Stokes Respiration During Progression of Congestive Heart Failure. FASEB J. 2011;25:841.7. [Google Scholar]
- 36.Ding Y, Li YL, Schultz HD. Role of Blood Flow in Carotid Body Chemoreflex Function in Heart Failure. J Physiol. 2010 doi: 10.1113/jphysiol.2010.200584. jphysiol.2010.200584 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167(2):609–18. doi: 10.1016/S0002-9440(10)63002-7. S0002-9440(10)63002-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyakawa AA, de Lourdes Junqueira M, Krieger JE. Identification of two novel shear stress responsive elements in rat angiotensin I converting enzyme promoter. Physiol Genomics. 2004;17(2):107–13. doi: 10.1152/physiolgenomics.00169.2003. doi:10.1152/physiolgenomics.00169.2003 00169.2003 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53(21):1975–80. doi: 10.1016/j.jacc.2009.02.030. S0735-1097(09)00745-1 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Van De Borne P, Somers VK. Dopamine and congestive heart failure: pharmacology, clinical use, and precautions. Congest Heart Fail. 1999;5(5):216–21. [PubMed] [Google Scholar]
- 41.Morrissey RP, Czer L, Shah PK. Chronic heart failure: current evidence, challenges to therapy, and future directions. Am J Cardiovasc Drugs. 2011;11(3):153–71. doi: 10.2165/11592090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Downing J, Balady GJ. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58(6):561–9. doi: 10.1016/j.jacc.2011.04.020. S0735-1097(11)01780-3 [pii] [DOI] [PubMed] [Google Scholar]
- *43.Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol. 2008;105(3):782–90. doi: 10.1152/japplphysiol.90533.2008.. 90533.2008 [pii]The study demonstrates the efficacy of exercsie conditioning to normalize CB chemoreflex function and reduce sympathetic activity in heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of autonomic function. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.079. in press. The study demonstrates the efficacy of CB ablation to improve cardio-respiratory function and survival in the myocardial infarct model of heart failure in rat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Marcus NJ, Del Rio R, Schultz HD. Carotid body denervation attenuates oscillations in respiratory rate and sympathetic nerve activity, and decreases apnea/hypopnea index in congestive heart failure. FASEB J. 2013;27:1137.7. The study demonstrates the efficacy of CB ablation to improve control of breathing and reduce respiratory-sympathetic coupling in heart failure. [Google Scholar]
- **46.Marcus NJ, Del Rio R, Schultz HD. Carotid Body Denervation attenuates increased sympathetic nerve activity in congestive heart failure. The Physiologist. 2012;55:C13. The study demonstrates the efficacy of CB ablation to reduce sympatheteic outflow and improve cardiac function in the pacing model of heart failure in rabbit. [Google Scholar]
- 47.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153(1):272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]


