Abstract
Background
Characterisation of the severity profile of human infections with influenza viruses of animal origin is a part of pandemic risk assessment, and an important part of the assessment of disease epidemiology. Our objective was to assess the clinical severity of human infections with avian influenza A H7N9 virus, which emerged in China in early 2013.
Methods
We obtained information about laboratory-confirmed cases of avian influenza A H7N9 virus infection reported as of May 28, 2013, from an integrated database built by the Chinese Center for Disease Control and Prevention. We estimated the risk of fatality, mechanical ventilation, and admission to the intensive care unit for patients who required hospital admission for medical reasons. We also used information about laboratory-confirmed cases detected through sentinel influenza-like illness surveillance to estimate the symptomatic case fatality risk.
Findings
Of 123 patients with laboratory-confirmed avian influenza A H7N9 virus infection who were admitted to hospital, 37 (30%) had died and 69 (56%) had recovered by May 28, 2013. After we accounted for incomplete data for 17 patients who were still in hospital, we estimated the fatality risk for all ages to be 36% (95% CI 26–45) on admission to hospital. Risks of mechanical ventilation or fatality (69%, 95% CI 60–77) and of admission to an intensive care unit, mechanical ventilation, or fatality (83%, 76–90) were high. With assumptions about coverage of the sentinel surveillance network and health-care-seeking behaviour for patients with influenza-like illness associated with influenza A H7N9 virus infection, and pro-rata extrapolation, we estimated that the symptomatic case fatality risk could be between 160 (63–460) and 2800 (1000–9400) per 100 000 symptomatic cases.
Interpretation
Human infections with avian influenza A H7N9 virus seem to be less serious than has been previously reported. Many mild cases might already have occurred. Continued vigilance and sustained intensive control efforts are needed to minimise the risk of human infection.
Funding
Chinese Ministry of Science and Technology; Research Fund for the Control of Infectious Disease; Hong Kong University Grants Committee; China–US Collaborative Program on Emerging and Re-emerging Infectious Diseases; Harvard Center for Communicable Disease Dynamics; US National Institute of Allergy and Infectious Disease; and the US National Institutes of Health.
Introduction
When a new influenza virus that can infect and cause disease in people emerges, such as the avian influenza A H7N9 virus, risk assessment is an urgent public health priority.1, 2, 3 In guidelines from the US Centers for Disease Control and Prevention,4 risk assessment metrics were broken down into ten criteria in three categories: properties of the virus, including transmission potential; properties of the population, including pre-existing immunity and the severity of human infections; and ecology and epidemiology in animals and human beings. The second category includes one of the most difficult criteria to assess, but the most important in terms of public health effects—namely, the clinical severity of human infections, which is estimated by measures such as the case fatality risk.5 The seriousness of infections has major implications for the potential overall severity of an influenza pandemic.3 However, assessment of severity is challenging because typically the most serious illnesses associated with infection have a much higher probability of being detected and laboratory confirmed than do mild illnesses.6
Human infections with the novel influenza A H7N9 virus were first identified in China in March, 2013, and the initial laboratory-confirmed cases were all patients with serious illness.7, 8, 9 The earliest laboratory-confirmed cases were clustered around the Yangtze River delta, while subsequent laboratory-confirmed cases occurred in neighbouring provinces to the south and north.10, 11 Most laboratory-confirmed cases occurred in urban areas in people who reported exposure to live poultry in the 7 days before illness onset.11 Investigation of live poultry markets in Huzhou, Zhejiang Province, identified a high prevalence of infection in poultry,12 and closure of live poultry markets seems to have been effective in the control of outbreaks.12, 13, 14 Intensive follow-up of more than 2500 close contacts of laboratory-confirmed cases identified just five potential secondary A H7N9 virus infections,11 which is consistent with low person-to-person transmissibility. Pre-existing immunity to influenza A H7N9 virus will probably be low in all age groups of the general population.15
Although initial estimates of clinical severity focused on laboratory-confirmed cases,8, 9, 10 they could give an incomplete picture.16 As the outbreak has continued, it has become clear that undetected cases of milder symptomatic infections have occurred. First, with the assumption that no immunity is present in any age group, the pattern in age-specific incidence of serious cases is not consistent with presumed patterns in exposure, implying that severity increases with age and that many undetected mild infections have occurred in adults.17 Second, six of the 131 laboratory-confirmed cases as of May 28, 2013, were identified via national sentinel influenza-like illness (ILI) surveillance (including the most recent case, reported on May 28 in Beijing), which is a population-wide system for measurement of patterns in consultation of patients with ILI and virological testing for influenza virus infections in a subset of patients.18, 19 Of the six cases (aged 2–26 years), only one developed pneumonia, and the other five cases had uncomplicated illness. Because this surveillance system is based on a sampling approach, laboratory confirmation of the six cases implies a substantial number of unconfirmed symptomatic infections with mild to moderate illness.
Our objective was to assess the clinical severity profile of human infections with avian influenza A H7N9 virus on the basis of available information to inform assessment of potential pandemic risk. We estimated the clinical severity profile in terms of the risk of fatality, mechanical ventilation and admission to intensive care units (ICUs) for patients who had been admitted to hospital, and the risk of fatality in symptomatic cases.
Methods
Data sources
In China, all laboratory-confirmed cases of avian influenza A H7N9 virus infection are reported to the Chinese Center for Disease Control and Prevention (China CDC) through a national surveillance system. Case definitions, surveillance for identification of cases, and laboratory assays have been previously described.10 A joint team comprising staff from local or provincial CDC, or the China CDC, or both, did field investigations of the laboratory-confirmed cases of avian influenza A H7N9 virus infection. Demographic, epidemiological, and basic clinical data were obtained with standardised forms.
An integrated database was built by the China CDC, with detailed epidemiological information about each laboratory-confirmed case of avian influenza A H7N9 infection reported by May 28, 2013. We used information about age, sex, place of residence, dates of illness onset, hospital admission, ICU admission, mechanical ventilation, death, and recovery or discharge.
The National Health and Family Planning Commission ruled that the collection of data for laboratory-confirmed cases of avian influenza A H7N9 infection was part of a continuing public health investigation of an emerging outbreak and was therefore exempt from institutional review board assessment.
Statistical analysis
We used two approaches to characterise the severity of infection. First, for patients with laboratory-confirmed infection who required hospital admission for medical reasons, we examined the risk of death, admission to ICU, and mechanical ventilation. Second, we sought to estimate the number of symptomatic cases to form the denominator for the symptomatic case fatality risk. Together, these measures characterise the clinical severity profile of avian influenza A H7N9 and allow comparison with other influenza virus infections.
Garske and colleagues6 discussed two complications with characterisation of severity: the potential for underestimation of cases, and incomplete information about outcomes during a continuing outbreak. We attempted to circumvent the issue of underascertainment of cases and particularly shifts over time in case ascertainment by focusing on cases that required hospital admission. To allow for incomplete information about outcomes, we used survival analyses to allow the inclusion of all cases admitted to hospital in our analysis, incorporating data for patients who were still in hospital at the time of analysis that would be typical of any evolving, incomplete outbreak.
We estimated the fatality risk for patients admitted to hospital within a competing risks framework.20 Specifically, every patient admitted to hospital is assumed to either die of the disease or recover. We estimated the admission to death distribution (F1) and the admission to recovery distribution (F2) with a non-parametric approach, accounting for the competing nature of the outcomes and censoring. We calculated the hospital admission fatality risk with the fraction F1/(F1+F2) at 6 weeks after admission, and constructed 95% CIs with a bootstrap approach with 1000 resamples.20 We used the same non-parametric approach to estimate two other serious outcomes of hospital admission: ICU admission and mechanical ventilation. Because some patients died without requiring mechanical ventilation, we grouped two outcomes together to estimate the risk of mechanical ventilation or death, for which the alternative outcome is recovery without ventilation. For the same reason, we estimated risk of ICU admission, mechanical ventilation, or death versus recovery without ICU admission or mechanical ventilation, or both.
Hospital admission dates were unavailable for a few patients. Additionally, the exact date of ICU admission or ventilation was not available in our dataset for a few patients. Therefore, we used multiple imputation with 20 replications to allow for unknown dates of hospital admission, ICU admission, or ventilation.21 We first estimated the distributions of time from illness onset to hospital admission, and from hospital admission to ICU admission or ventilation by complete case analysis. Then, we used the derived distributions, truncated by the duration of follow-up for a specific patient, to impute event dates. We estimated risks of fatality, ICU admission, and mechanical ventilation with 95% CIs from the 20 imputed datasets, and estimated the pooled means across the imputed datasets with Rubin's formula.22
Additionally, we investigated the risk of fatality, ICU admission, and ventilation for patients admitted to hospital aged younger than 60 years and those aged 60 years or older. To assess the information about severity that was available early in the outbreak for patients who had been admitted to hospital, we did retrospective analyses of the overall fatality risk on the basis of data available on different cutoff dates in April and May, 2013.
To put the clinical severity profile into proper context—ie, by providing a denominator consisting of all infected and symptomatic cases—we estimated the number of symptomatic influenza A H7N9 virus infections in Shanghai and Nanjing (Jiangsu Province) by May 28, on the basis of the numbers of cases detected by routine virological surveillance at ILI sentinel sites. We combined these data with the daily number of all ILI cases reported and specimens tested by ILI surveillance in the two cities to infer the number of infected individuals who would have sought medical care at ILI sentinels (NILI-S; appendix). We then used two alternative methods to estimate the number of symptomatic infections in Shanghai and Nanjing. With method 1, we assumed that health-care-seeking behaviour of individuals with ILI associated with infection with the influenza A H7N9 virus was the same as health-care-seeking behaviour for those with ILI associated with the 2009 influenza A H1N1 pandemic virus infection. We used data from a nationwide serosurvey and ILI surveillance of the 2009 influenza A H1N1 pandemic virus in China from June, 2009, to January, 2010,23 to estimate the proportion of individuals with symptomatic infections who sought medical care at ILI sentinels. We divided NILI-S by this proportion (appendix). With method 2, we assumed that all cases of ILI associated with infection with influenza A H7N9 virus sought medical care, and we estimated the number of symptomatic cases on the basis of the proportions of outpatient consultations at the sentinel locations compared with the total number of consultations in Shanghai and Nanjing (appendix). After estimating the number of mild cases in Shanghai and Nanjing, we extrapolated our estimate to the rest of mainland China on the basis of the proportion of patients with H7N9 admitted to hospital in those two cities (ie, pro rata). Statistical analyses were done in R (version 3.0.1).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The joint first authors had full access to all the data in the study, and the corresponding authors had final responsibility for the decision to submit for publication.
Results
As of May 28, 2013, 131 laboratory-confirmed human cases of avian influenza A H7N9 infection had been officially recorded in mainland China. Of those, 123 patients had to be admitted to hospital for medical reasons and were included in our analyses. Four of the other eight individuals were diagnosed after they had already recovered from mild illness, and four who had mild illness were admitted to hospital for observation (one had been identified through ILI surveillance).
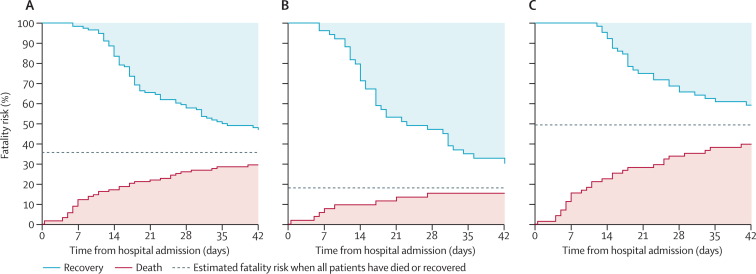
71 (58%) of the 123 individuals who had to be admitted to hospital were aged at least 60 years, and 87 (71%) were male (table 1 ). Table 2 shows estimated overall fatality risk and risk of other adverse outcomes. Fatality risk was higher for individuals aged 60 years or older than for younger individuals (p=0·0019; figure 1 , table 2). For the 37 individuals who died, median time to death was 11 days (IQR 6–23). For the 65 individuals who recovered, median time to recovery was 18 days (14–29).
Table 1.
Characteristics of 123 patients with laboratory-confirmed infection with avian influenza A H7N9 virus who were admitted to hospital
| Died (n=37) | Recovered (n=69) | Unresolved (n=17)* | ||
|---|---|---|---|---|
| Age (years) | ||||
| 0–15 | 0 | 2 (3%) | 0 | |
| 16–59 | 8 (22%) | 35 (51%) | 7 (41%) | |
| 60–74 | 16 (43%) | 21 (30%) | 7 (41%) | |
| ≥75 | 13 (35%) | 11 (16%) | 3 (18%) | |
| Men | 28 (76%) | 47 (68%) | 12 (71%) | |
| Delay from illness onset to hospital admission (days)† | ||||
| 0–2 | 9 (24%) | 11 (17%) | 3 (18%) | |
| 3–6 | 21 (57%) | 49 (74%) | 8 (47%) | |
| ≥7 | 7 (19%) | 6 (9%) | 6 (35%) | |
| Illness onset date (%) | ||||
| Feb 19–March 31, 2013 | 18 (49%) | 11 (16%) | 5 (29%) | |
| April 1–14, 2013 | 15 (41%) | 44 (64%) | 8 (47%) | |
| April 15–May 3, 2013 | 4 (11%) | 14 (20%) | 4 (24%) | |
| Residence | ||||
| Urban | 32 (86%) | 42 (61%) | 14 (82%) | |
| Rural | 5 (14%) | 27 (39%) | 3 (18%) | |
Data are n (%).
As of May 28, 17 patients had not died but their disease had not resolved either.
Admission date not known for three patients who recovered.
Table 2.
Risks of adverse outcomes for patients with laboratory-confirmed infection with avian influenza A H7N9 virus who were admitted to hospital
| All ages | Aged <60 years | Aged ≥60 years | |
|---|---|---|---|
| Fatality risk | 36% (26–45) | 18% (6–29) | 49% (36–63) |
| Risk of mechanical ventilation* or fatality | 69% (60–77) | 53% (39–68) | 80% (71–90) |
| Risk of admission to intensive care unit†, mechanical ventilation*, or fatality | 83% (76–90) | 75% (63–87) | 89% (81–97) |
Data in parentheses are 95% CIs. 95% CIs were estimated with bootstrapping with 1000 resamples. Accounting for incomplete data for 17 patients who were still in hospital as of May 28, 2013.
Data not available for 15 patients (five aged <60 years; ten aged ≥60 years).
Data not available for 13 patients (four aged <60 years; nine aged ≥60 years).
Figure 1.
Fatality risk for patients with laboratory-confirmed infection with avian influenza A H7N9 virus who were admitted to hospital
(A) 123 patients of all ages, (B) 52 patients younger than 60 years, and (C) 71 patients aged at least 60 years. As of May 28, 2013, 17 patients were still in hospital; the solid lines will converge when these cases resolve.
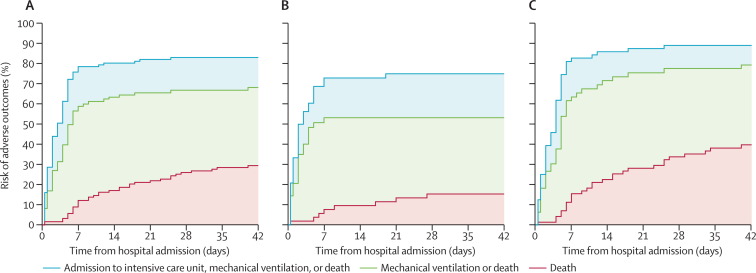
71 (66%) of 108 patients for whom detailed clinical information was available required mechanical ventilation, and 83 (75%) of 110 were admitted to ICU. We used multiple imputation to account for missing data for dates of ICU admission for one patient and for mechanical ventilation for 23 patients, and censored in 17 patients who were still in hospital as of May 28. We estimated that risks of ICU admission (p=0·08) and mechanical ventilation (p=0·0067) were higher for patients aged 60 years or older than for younger patients (figure 2 , table 2). We recorded some evidence that disease progressed or resolved faster in patients younger than 60 years than in older individuals (Figure 1, Figure 2), but the small sample size meant that we did not have sufficient statistical power to warrant further investigation.
Figure 2.
Risks of adverse outcomes for patients with laboratory-confirmed infection with avian influenza A H7N9 virus who were admitted to hospital
(A) 123 patients of all ages, (B) 52 patients younger than 60 years, and (C) 71 patients aged at least 60 years.
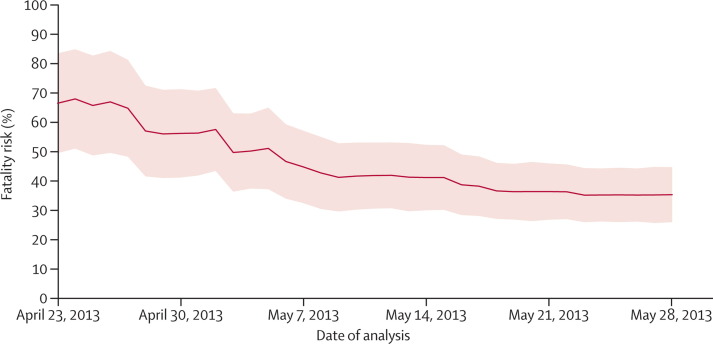
When we estimated the fatality risk of patients admitted to hospital on the basis of information available on different dates, we noted that the estimated risk gradually decreased (figure 3 ). Uncertainty was initially substantial, but decreased with time (figure 3), because of the increasing number of cases and follow-up of individuals admitted to hospital.
Figure 3.
Real-time estimates of the fatality risk for patients with laboratory-confirmed infection with avian influenza A H7N9 virus who were admitted to hospital
Shading shows 95% CIs. On the basis of available information about officially announced cases to each date from April 23, to May 28, 2013.
We estimated that 23 (95% credible interval [CrI] seven to 58) symptomatic individuals infected with avian influenza A H7N9 sought medical care at ILI sentinels in Shanghai up to May 28, on the basis that two cases were identified there by ILI surveillance. Additionally, we estimated that 40 (seven to 129) sought medical care at ILI sentinels in Nanjing on the basis that one case was identified by ILI surveillance. With method 1, we estimated that about 0·75% of individuals with symptomatic 2009 influenza A H1N1 pandemic virus infection sought medical care. With the assumption that a similar proportion of symptomatic individuals infected with avian influenza A H7N9 would have attended ILI sentinels, we estimated that about 3020 (95% CrI 900–7800) symptomatic infections had occurred in Shanghai and 5310 (880–17 300) in Nanjing (appendix). Pro-rata extrapolation on the basis of 40 total hospital admissions in Shanghai and Nanjing, as of May 28, suggested that 27 000 (95% CrI 9530–65 000) symptomatic infections might have occurred throughout the country as of May 28. This number corresponds to a symptomatic case fatality risk of 160 (63–460) per 100 000 symptomatic cases.
About 21% of outpatient visits in the internal medicine, paediatrics, and emergency departments in Shanghai, and 11% in Nanjing, occurred in sentinel ILI sites. With method 2 and allowing for this coverage, we estimated that about 107 (95% CrI 33–273) symptomatic infections had occurred in Shanghai and 367 (61–1200) in Nanjing (appendix). Pro-rata extrapolation on the basis of number of hospital admissions in these cities (as in method 1) suggested that 1500 (95% CrI 470–4050) symptomatic infections might have occurred throughout the country as of May 28. This number corresponds to a symptomatic case fatality risk of 2800 (1000–9400) per 100 000 symptomatic cases.
Discussion
Our findings represent the most complete picture of the clinical severity profile of avian influenza A H7N9 virus infections in China so far. We have shown that the fatality risk of infected patients admitted to hospital is roughly 36%, and that it increases with age. Our findings put the early reports of severe laboratory-confirmed cases of avian influenza A H7N9 virus infection7, 8, 9, 10 into perspective. Although previous clinical case series have focused on the potential for avian influenza A H7N9 virus infection to cause severe illness,7, 8, 24 we have estimated that many mild cases might have occurred. Our results thus support continued vigilance and sustained intensive control efforts against the virus to minimise risk of human infection, which is greater than previously recognised.
Notably, our analysis did not contain a metric of confirmed-case fatality risk. We have previously recommended that this term be avoided for epidemiological analysis of pandemic influenza viruses,25 and now argue that this term should also be avoided for avian influenza A H7N9 virus. Confirmed cases are apparently biased towards infections associated with serious illnesses,17 although a few confirmed cases of avian influenza A H7N9 virus infection were identified through sentinel ILI surveillance. Because of this underascertainment, the confirmed-case fatality risk would apply neither to patients admitted to hospital (for whom the fatality risk is increased), nor to those with mild illness. Shifts in ascertainment of cases with time—eg, by increasing the proportion of ILI specimens tested every week in affected regions—would change the confirmed-case fatality risk, perhaps substantially, without any actual change in the underlying clinical severity profile the infection.
Instead, we used a two-stage approach that was also recommended in the 2009–10 pandemic of influenza A H1N1 virus to provide a stable and robust assessment of severity: estimation of fatality risk and then estimation of number of symptomatic infections.26, 27 Our estimation of fatality risk for patients admitted to hospital (36%) is higher than the widely reported risk of less than 25%.9, 10, 28, 29, 30 Our estimate accounted for the unknown outcomes of patients who were still in hospital at time of data cutoff. Our estimate is lower than that for cases of influenza A H5N1 virus infection that has been reported in China (65%)31 and worldwide (60% for all laboratory-confirmed cases),32 but higher than that for 2009 influenza A H1N1 pandemic virus in China (21%).33 Our estimate that between 1500 and 27 000 symptomatic infections with avian influenza A H7N9 virus might have occurred as of May 28, 2013, is much larger than the number of laboratory-confirmed cases. The proportion of patients with symptomatic infections with the virus who sought medical care was probably higher than was assumed with method 1 (which corresponds to the upper limit of our estimates) because laboratory-confirmed cases seemed to have faster and more severe disease progression than did those of infection with the 2009 pandemic virus, and also because residents of the highly developed cities of Shanghai and Nanjing were more likely to seek medical care than the general population of China.
The fatality risk of patients admitted to hospital differed substantially by age. Increasing age is also associated with greater severity of patients infected with seasonal influenza and the 2009 pandemic virus.34, 35 However, age-specific ILI surveillance data were not available to allow assessment of age-specific risk of hospital admission for cases of avian influenza A H7N9 virus infection. When possible, an ILI surveillance system that is capitated—ie, based on known population denominators36 rather than on floating consultation denominators—would enable improved characterisation of rates of ILI in the population; laboratory data for a subset of patients could be used to extrapolate the proportion of illnesses associated with influenza.37 However, this system would be challenging in most settings without a defined population catchment.
Reasonable estimates of the fatality risk were available by mid-May. During the epidemic of severe acute respiratory syndrome, reports of the case fatality risk were low when based on number of deaths divided by number of cases cross-sectionally before complete resolution of all cases, leading to substantial discussion and methodological development during and after that epidemic.20, 38, 39 10 years later, during the present H7N9 outbreak, case fatality risks of about 20–25% have frequently been communicated,9, 10, 29, 30 which is an underestimate. Clearly, more work to disseminate scientific methodological findings and the wider application in public health practice is necessary. We recorded a decrease in the estimated fatality risk over time, because death generally occurred more quickly than recovery. This issue is a limitation of existing methods, and methodological developments would be welcome.
Our analyses have some limitations. First, our estimates are real time, calculated during the ongoing outbreak of H7N9 while some patients remained critically ill in hospital. Our estimates of fatality risks have fairly wide CIs that will narrow as illnesses resolve. Second, although we could estimate age-specific fatality risks, we could not estimate age-specific symptomatic-case risks in our analyses; further work is needed in this area. Third, our estimates of the number of symptomatic cases by two methods were based on extrapolation from the sentinel ILI network, and necessitated several simplifying assumptions, such as no geographical differences in ascertainment of patients admitted to hospital and no changes in health-care-seeking behaviour in late March, and early April, 2013. Our analysis could be biased if there were additional undetected hospital admissions associated with avian influenza A H7N9—eg, because of poor access to laboratory testing in some areas—or if health-care attendance was increased in view of the perceived severity of this novel infection. Fourth, without data for the proportion of subclinical or asymptomatic infections, we cannot estimate the fatality risk for all infected individuals (ie, not just the risk for those admitted to hospital). Such information might be available from serological studies in future.40 Finally, clinical information about some laboratory-confirmed cases was not available, and standardised collection and sharing of clinical data would assist risk assessment and treatment.41
In conclusion, our estimate of a symptomatic case fatality risk suggests that avian influenza A H7N9 is not as severe as influenza A H5N1,42 but more severe than 2009 influenza A H1N1 pandemic virus (panel ).51 We are not aware of comparative data for the symptomatic case fatality risks of seasonal influenza viruses, but we speculate that they are similar in size to the 2009 pandemic virus. As with seasonal influenza, the severity of avian influenza A H7N9 virus infection increases with age. Our findings will inform risk assessment and health policy during the present H7N9 outbreak, and will assist preparations for a potential resurgence in human infections towards the end of 2013.11 Our framework could be used for pandemic risk assessment of future avian influenza viruses that cause disease in people.
Panel. Research in context.
Systematic review
We searched PubMed on May 28, 2013, with the terms “A(H7N9)” or “H7N9” for articles published in English since Jan 1, 2013. We also searched articles available online from international medical and infectious disease journals. We did not identify any reports of human infections with avian influenza A H7N9 virus before 2013. Some reports of the present H7N9 outbreak in people in China divided the number of deaths by the number of laboratory-confirmed cases,9, 10, 28, 29, 30 and one of these studies clarified that this approach may underestimate the confirmed-case fatality risk, because some patients remain in hospital.10 Other studies reported the number of laboratory-confirmed cases and the number of deaths without attempts to infer the case fatality risk.1, 13, 18, 24, 43, 44, 45, 46, 47, 48, 49, 50 One published report suggested that at least 210–550 symptomatic infections with the avian influenza A H7N9 virus in China had occurred by April 21, 2013, on the basis of patterns in incidence compared with presumed patterns in exposure.17 We did not identify any other report in which estimation of the number of symptomatic cases of avian influenza A H7N9 virus infection or the symptomatic case fatality risk was attempted.
Interpretation
We have shown that human infections with avian influenza A H7N9 virus might be less serious than has been previously reported. Although most patients with laboratory-confirmed infection needed to be admitted to hospital and most of these required admission to intensive care units, the fatality risk for patients with avian influenza A H7N9 infection who were admitted to hospital of 36% seems to be lower than that for influenza A H5N1 in China (65%)31 and worldwide (60%),32 but higher than that for the 2009 influenza A H1N1 pandemic virus (21%). Identification of five laboratory-confirmed cases in a network of 554 sentinel hospitals conducting surveillance of influenza-like illness in outpatients in China is indicative of a much larger number of mild cases. Our findings will inform risk assessment and health policy during the present H7N9 outbreak, and will assist preparations for a potential resurgence in human infections towards the end of 2013.11 Our framework could be used for pandemic risk assessment of future avian influenza viruses that cause disease in people.
Acknowledgments
Acknowledgments
This study was funded by the US National Institutes of Health (Comprehensive International Program for Research on AIDS grant U19 AI51915); the China–US Collaborative Program on Emerging and Re-emerging Infectious Diseases; the Chinese Ministry of Science and Technology (2012 ZX10004-201); the National Ministry of Science and Technology Emergency Research Project on human infection with avian influenza H7N9 virus (KJYJ-2013-01-02); the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant U54 GM088558); the National Institute of Allergy and Infectious Diseases (under contract number HHSN266200700005C; ADB No N01-AI-70005; Centers for Excellence in Influenza Research and Surveillance); the Research Fund for the Control of Infectious Disease, Food and Health Bureau, Government of the Hong Kong Special Administrative Region (grant HK-12-07-01); and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant AoE/M-12/06). We thank staff members of the Bureau of Disease Control and Prevention and Health Emergency Response Office of the National Health and Family Planning Commission and provincial and local departments of health for providing assistance with administration and data collection; and staff members at county, prefecture, and provincial CDCs in the provinces where human cases occurred for providing assistance with field investigation, administration, and data collection. The views expressed are those of the authors and do not necessarily represent the policy of the China CDC.
Contributors
HY, BJC, EHYL, JTW, YW, and GML designed the study. HY, LF, QLia, ZP, FL, HZ, ML, LZ, ZX, ZL, HL, QLi, ZF, BC, WY, and YW collected data. HY, BJC, LF, EHYL, QLia, TKT, PW, VJF, and JTW analysed data. BJC wrote the first draft and all authors contributed to review and revision and have seen and approved the final version.
Conflicts of interest
BJC has received research funding from MedImmune and is a paid consultant for Crucell NV. GML has received speaker honoraria from HSBC and CLSA. The other authors declare that they have no conflicts of interest.
Supplementary Material
References
- 1.Nicoll A, Danielsson N. A novel reassortant avian influenza A(H7N9) virus in China—what are the implications for Europe. Euro Surveill. 2013;18:15. [PubMed] [Google Scholar]
- 2.Trock SC, Burke SA, Cox NJ. Development of an influenza virologic risk assessment tool. Avian Dis. 2012;56(suppl 4):1058–1061. doi: 10.1637/10204-041412-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Implementation of the International Health Regulations (2005): report of the review committee on the functioning of the International Health Regulations (2005) in relation to pandemic (H1N1) 2009. World Health Organization; Geneva: 2011. [Google Scholar]
- 4.Centers for Disease Control and Prevention Influenza Risk Assessment Tool (IRAT) http://www.cdc.gov/flu/pandemic-resources/tools/risk-assessment.htm (accessed May 2, 2013).
- 5.Kelly H, Cowling BJ. Case fatality: rate, ratio, or risk? Epidemiology. 2013;24:622–623. doi: 10.1097/EDE.0b013e318296c2b6. [DOI] [PubMed] [Google Scholar]
- 6.Garske T, Legrand J, Donnelly CA. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 7.Gao R, Cao B, Hu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Liang W, Yang S. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arima Y, Zu R, Murhekar M, Vong S, Shimadaa T, World Health Organization Regional Office for the Western Pacific Event Management Team Human infections with avian influenza A(H7N9) virus in China: preliminary assessments of the age and sex distribution. Western Pac Surveill Response J. 2013;4:1–4. doi: 10.5365/WPSAR.2013.4.2.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Zhou L, Zhou M. Preliminary report: epidemiology of the avian influenza A (H7N9) outbreak in China. N Engl J Med. 2013 doi: 10.1056/NEJMoa1304617. published online April 24. [DOI] [Google Scholar]
- 11.Cowling BJ, Jin L, Lau EHY. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013 doi: 10.1016/S0140-6736(13)61171-X. published online June 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J, Jin M, Zhang P. Epidemiological link between exposure to poultry and all influenza A(H7N9) confirmed cases in Huzhou city, China, March to May 2013. Euro Surveill. 2013;18:2. [PubMed] [Google Scholar]
- 13.Xu J, Lu S, Wang H, Chen C. Reducing exposure to avian influenza H7N9. Lancet. 2013;381:1815–1816. doi: 10.1016/S0140-6736(13)60950-2. [DOI] [PubMed] [Google Scholar]
- 14.Murhekar M, Arima Y, Horby P. Avian influenza A(H7N9) and the closure of live bird markets. Western Pac Surveill Response J. 2013;4:1–4. doi: 10.5365/WPSAR.2013.4.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boni MF, Chau NVV, Dong N. Population-level antibody estimates to novel influenza A/H7N9. J Infect Dis. 2013 doi: 10.1093/infdis/jit224. published online May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koopmans M, de Jong MD. Avian influenza A H7N9 in Zhejiang, China. Lancet. 2013;381:1882–1883. doi: 10.1016/S0140-6736(13)60936-8. [DOI] [PubMed] [Google Scholar]
- 17.Cowling BJ, Freeman G, Wong JY. Preliminary inferences on the age-specific seriousness of human disease caused by avian influenza A(H7N9) infections in China, March to April 2013. Euro Surveill. 2013;18:1. [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Havers F, Wang L. Monitoring avian influenza A(H7N9) virus through national influenza-like illness surveillance, China. 2013. http://wwwnc.cdc.gov/eid/article/19/8/13-0662_article.htm#top (accessed June 11, 2013). [DOI] [PMC free article] [PubMed]
- 19.Shu YL, Fang LQ, de Vlas SJ, Gao Y, Richardus JH, Cao WC. Dual seasonal patterns for influenza, China. Emerg Infect Dis. 2010;16:725–726. doi: 10.3201/eid1604.091578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewell NP, Lei X, Ghani AC. Non-parametric estimation of the case fatality ratio with competing risks data: an application to Severe Acute Respiratory Syndrome (SARS) Stat Med. 2007;26:1982–1998. doi: 10.1002/sim.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation for nonresponse in surveys. J Wiley and Sons; New York, NY: 1987. [Google Scholar]
- 23.Xu C, Bai T, Iuliano AD. The seroprevalence of pandemic influenza H1N1 (2009) virus in China. PLoS One. 2011;6:e17919. doi: 10.1371/journal.pone.0017919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao HN, Lu HZ, Cao B. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013 doi: 10.1056/NEJMoa1305584. published online May 22. [DOI] [PubMed] [Google Scholar]
- 25.Wong JY, Kelly H, Ip DK, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza A(H1N1pdm09): a systematic review. Epidemiology (in press). [DOI] [PMC free article] [PubMed]
- 26.Lipsitch M, Finelli L, Heffernan RT, Leung GM, Redd SC. Improving the evidence base for decision making during a pandemic: the example of 2009 influenza A/H1N1. Biosecur Bioterror. 2011;9:89–115. doi: 10.1089/bsp.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Presanis AM, De Angelis D, The New York City Swine Flu Investigation Team The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6:e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Wang L, Hu W. Epidemiological characteristics of cases for influenza A (H7N9) virus infections in China. Clin Infect Dis. 2013 doi: 10.1093/cid/cit277. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skowronski D, Janjua N, Kwindt T, De Serres G. Virus-host interactions and the unusual age and sex distribution of human cases of influenza A(H7N9) in China, April 2013. Euro Surveill. 2013;18:4. [PubMed] [Google Scholar]
- 30.Kahn RE, Richt JA. The novel H7N9 influenza A virus: its present impact and indeterminate future. Vector Borne Zoonotic Dis. 2013;13:347–348. doi: 10.1089/vbz.2013.999.ceezad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Gao Z, Feng Z. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One. 2008;3:e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2013. June 4, 2013. http://www.who.int/influenza/human_animal_interface/EN_GIP_20130604CumulativeNumberH5N1cases.pdf (accessed June 11, 2013).
- 33.Yu H, Feng Z, Uyeki TM. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52:457–465. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Kerkhove MD, Vandemaele KA, Shinde V. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, Chan PK, Lui GC. Complications and outcomes of pandemic 2009 influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis. 2011;203:1739–1747. doi: 10.1093/infdis/jir187. [DOI] [PubMed] [Google Scholar]
- 36.Fleming DM, Elliot AJ. Lessons from 40 years' surveillance of influenza in England and Wales. Epidemiol Infect. 2008;136:866–875. doi: 10.1017/S0950268807009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baguelin M, Hoschler K, Stanford E. Age-specific incidence of A/H1N1 2009 influenza infection in England from sequential antibody prevalence data using likelihood-based estimation. PLoS One. 2011;6:e17074. doi: 10.1371/journal.pone.0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghani AC, Donnelly CA, Cox DR. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. Am J Epidemiol. 2005;162:479–486. doi: 10.1093/aje/kwi230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip PS, Lau EH, Lam KF, Huggins RM. A chain multinomial model for estimating the real-time fatality rate of a disease, with an application to severe acute respiratory syndrome. Am J Epidemiol. 2005;161:700–706. doi: 10.1093/aje/kwi088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Kerkhove MD, Broberg E, Engelhardt OG, Wood J, Nicoll A. The consortium for the standardization of influenza seroepidemiology (CONSISE): a global partnership to standardize influenza seroepidemiology and develop influenza investigation protocols to inform public health policy. Influenza Other Respir Viruses. 2013;7:231–234. doi: 10.1111/irv.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horby P. H7N9 is a virus worth worrying about. Nature. 2013;496:399. doi: 10.1038/496399a. [DOI] [PubMed] [Google Scholar]
- 42.Van Kerkhove MD, Riley S, Lipsitch M. Comment on “Seroevidence for H5N1 influenza infections in humans: meta-analysis”. Science. 2012;336:1506. doi: 10.1126/science.1221434. [DOI] [PubMed] [Google Scholar]
- 43.Osterholm MT, Ballering KS, Kelley NS. Major challenges in providing an effective and timely pandemic vaccine for influenza A(H7N9) JAMA. 2013 doi: 10.1001/jama.2013.6589. published online May 9. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Wu F, He J. Emerging risk of H7N9 influenza in China. Lancet. 2013;381:1539–1540. doi: 10.1016/S0140-6736(13)60767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The Lancet Infectious Diseases A proportionate response to H7N9. Lancet Infect Dis. 2013;13:465. doi: 10.1016/S1473-3099(13)70134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hvistendahl M, Normile D, Cohen J. Despite large research effort, H7N9 continues to baffle. Science. 2013;340:414–415. doi: 10.1126/science.340.6131.414. [DOI] [PubMed] [Google Scholar]
- 47.Alcorn T. As H7N9 spreads in China, experts watch and wait. Lancet. 2013;381:1347. doi: 10.1016/s0140-6736(13)60868-5. [DOI] [PubMed] [Google Scholar]
- 48.Eurosurveillance editorial team Novel influenza A(H7N9) virus linked to human disease in China, April 2013. Euro Surveill. 2013;18:5. [Google Scholar]
- 49.Guan Y, Farooqui A, Zhu H, Dong W, Wang J, Kelvin DJ. H7N9 incident, immune status, the elderly and a warning of an influenza pandemic. J Infect Dev Ctries. 2013;7:302–307. doi: 10.3855/jidc.3675. [DOI] [PubMed] [Google Scholar]
- 50.Parry J. H7N9 avian flu kills seven and infects 23 in China. BMJ. 2013;346:f2222. doi: 10.1136/bmj.f2222. [DOI] [PubMed] [Google Scholar]
- 51.Wong JY, Wu P, Nishiura H. Infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013;177:834–840. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.