Summary
Huntington's disease (HD) is an autosomal dominant neurodegenerative disease caused by expansion of polyglutamine repeats in the gene for huntingtin (Htt). In HD the corpus striatum selectively degenerates despite uniform expression of mutant huntingtin (mHtt) throughout the brain and body. Striatal selectivity reflects the binding of the striatal-selective protein Rhes to mHtt to augment cytotoxicity, but molecular mechanisms underlying the toxicity have been elusive. Here we report that the Golgi protein ACBD3 (Acyl-CoA binding Domain Containing 3) mediates mHtt cytotoxicity via a Rhes/mHtt/ACBD3 complex. ACBD3 levels are markedly elevated in the striatum of HD patients, in a striatal cell line harboring polyglutamine repeats, and in the brains of HD mice. Moreover, ACBD3 deletion abolishes HD neurotoxicity, which is increased by ACBD3 overexpression. Enhanced levels of ACBD3 elicited by ER, mitochondrial and Golgi stresses may account for HD associated augmentation of ACBD3 and neurodegeneration.
Huntington's Disease (HD) is an autosomal dominant neurodegenerative condition associated with mutations in the gene encoding the protein huntingtin (Htt) leading to expansion of glutamine repeats in mutant Htt (mHtt) (The Huntington's Disease Collaborative Research Group, 1993). HD patients display major disruption of motor behavior reflecting massive and highly selective destruction of the corpus striatum. Surprisingly, Htt and mHtt are expressed uniformly throughout the brain and the rest of the body despite the striatal selectivity of the disease. The striatal selectivity of HD may be explained by the binding of the striatal-selective small G protein Rhes (Ras Homologue Enriched in Striatum) to mHtt, enhancing mHtt-elicited neurotoxicity (Subramaniam et al., 2009). Reduced Rhes expression is neuroprotective in HD models (Seredenina et al., 2011), and mice with Rhes deletion are protected from motor disturbances in genetic models of HD (Baiamonte et al., 2013) and from striatal-selective neurotoxicity elicited by 3-nitropropionic acid (Mealer et al., 2013).
Within the large family of Ras proteins, Rhes most closely resembles Dexras1, first reported as a protein induced by the glucocorticoid dexamethasone (Kemppainen and Behrend, 1998). Because of its homology to Dexras1, Rhes is also designated RASD2. Dexras1 is linked via the carrier protein CAPON to neuronal NO synthase (nNOS) with NO acting as a guanine nucleotide exchange factor for Dexras1 (Fang et al., 2000). The nNOS-Dexras1 complex is also associated with the Golgi-specific protein Acyl-CoA binding domain containing 3 [ACBD3; previously known as PAP7 or GCP60] (Cheah et al., 2006); (Fan et al., 2010). ACBD3, in turn, binds to the Divalent Metal Transporter-1 (DMT1) in a cascade whereby NMDA neurotransmission, acting via nNOS, CAPON, Dexras1, and ACBD3, regulates neuronal iron influx (Cheah et al., 2006) and neurotoxicity (Chen et al., 2013). ACBD3 also binds other components of the Golgi such as giantin, Golgin-160 and phosphatidylinositol-4-kinase IIIβ (Sohda et al., 2001); (Sbodio et al., 2006); (Sasaki et al., 2012); (Greninger et al., 2012). ACBD3 interacts with non-Golgi proteins such as the Translocator Protein of 18 kDa [TSPO; previously known as Peripheral Benzodiazepine Receptor; PBR, (Li et al., 2001)] and PKA regulatory subunit Iα (Li et al., 2001). In most of these linkages ACBD3 appears to act as a scaffolding protein. The multifunctional role of ACBD3 acting as a signaling molecule through protein-protein interactions has been reviewed by Papadopoulos and associates (Fan et al., 2010).
Because of the close similarity of Rhes to Dexras1, we wondered whether Rhes also interacts with ACBD3. In the present study we demonstrate that ACBD3 participates in a ternary complex together with Rhes and mHtt. In this complex ACBD3 is a major determinant of neurotoxicity, as its overexpression is cytotoxic and its deletion abolishes Rhes neurotoxicity. A functional role for ACBD3 in HD is implied by the strikingly elevated levels of ACBD3 in the brains of patients with HD as well as in neuronal cell lines with extended glutamine repeats and in the brains of mice with genetic models of HD. ACBD3 levels are upregulated by diverse cellular stresses and in neuronal cells overexpressing mHtt. Thus ACBD3 is a major mediator of HD neurotoxicity with attendant therapeutic implications.
RESULTS
ACBD3 physiologically binds Rhes and mHtt
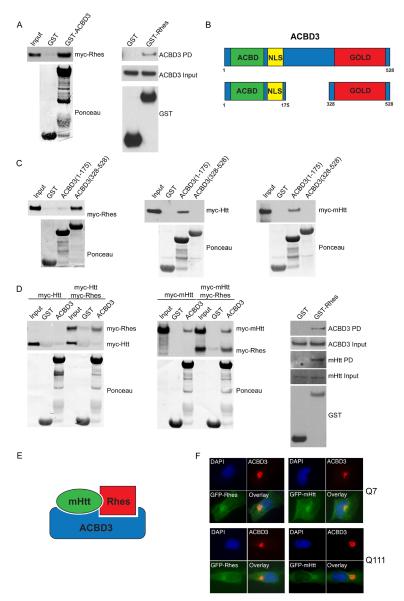
In HEK293 cells, GST-ACBD3 binds overexpressed Rhes (Figure 1A, left panel), while overexpressed GST-Rhes binds endogenous ACBD3 (Figure 1A, right panel). ACBD3 comprises an acyl-CoA binding domain (ACBD) at the N-terminus followed by a nuclear localization signal (NLS), while the C-terminus incorporates a Golgi Dynamics domain (GOLD) (Figure 1B). Most of the known ACBD3-interacting proteins, such as giantin, TSPO, PKARIα and Golgin-160, bind to the C-terminal GOLD domain (Sohda et al., 2001); (Li et al., 2001); (Sbodio et al., 2006). Mapping studies reveal that Rhes, like these other proteins, binds selectively to the C-terminal portion of ACBD3 (Figure 1C, left panel).
Figure 1.
ACBD3 binds Rhes and mutant huntingtin. A, HEK293 cells were transfected with a plasmid encoding myc-Rhes. Aliquots of cells were incubated with purified GST, or GST fused to ACBD3. Bound proteins were separated by SDS-PAGE and immunoblotted using myc antibodies (left panel). HEK293 cells transfected with a plasmid encoding GST or GST-Rhes were harvested and the GST proteins were allowed to bind the glutathione sepharose beads. Purified proteins were treated as above and immunoblotted with ACBD3 antibodies (right panel). B, Schematic representation of ACBD3 showing the Acyl-CoA binding domain (ACBD), Nuclear localization signal (NLS), and the Golgi-Dynamic domain (Gold). C, Purified GST or GST fused to an N-terminal fragment of ACBD3 (1-175) or a C-terminal fragment of ACBD3 (328-528) were incubated with lysates of cells expressing myc-huntingtin (Htt), myc-mutant-huntingtin (mHtt), or myc-Rhes, and bound proteins were detected using myc antibodies. D, HEK293 cells were transfected with plasmids encoding myc-Rhes or myc-Rhes with either myc-Htt (left panel) or myc-mHtt (middle panel) and incubated with purified GST or GST fused to ACBD3. Bound proteins were detected using myc antibodies. Binding of Rhes to endogenous ACBD3 and mHtt was detected by transfecting striatal Q111 cells with plasmids encoding GST or GST-Rhes for 16 h (right panel). GST proteins were allowed to bind the glutathione sepharose beads. Bound proteins were detected using ACBD3 or mHtt antibodies. E, Schematic representation of ACBD3 binding to Rhes and mHtt. F, Striatal Q7 and Q111 cells were transfected with plasmid encoding GFP-mHtt(103Q) or GFP-Rhes. 16 h after transfection, cells were fixed, permeabilized, and stained for endogenous ACBD3 and DNA (DAPI).
We also observe substantial binding of overexpressed wild-type and mutant Htt (mHtt) to ACBD3, but unlike Rhes, Htt and mHtt binds selectively to the N-terminal portion of ACBD3 (Figure 1C, middle and right panel). Co-precipitation studies indicate a ternary complex of ACBD3 with Rhes and wild-type Htt (wtHtt) or mHtt (Figure 1D, E).
If Htt, Rhes and ACBD3 are physiologically associated, they should have similar localizations. Immunofluorescence experiments reveal virtually exclusive localization of ACBD3 to Golgi (Figure 1F), fitting with earlier studies (Sohda et al., 2001); (Sbodio et al., 2006). Rhes is more widely distributed but with an intense focus of immunoreactivity in the Golgi, colocalizing with ACBD3. Though mHtt occurs throughout the cell, it is most concentrated in Golgi together with ACBD3. Rhes, a farnesylated protein, is also associated with the plasma membrane (Vargiu et al., 2004). Diverse localizations have been reported for Htt and mHtt with nuclear translocation of mHtt fragments during neurotoxicity (Velier et al., 1998); (DiFiglia et al., 1995) (Saudou et al., 1998).
ACBD3 is required for Rhes/mHtt cytotoxicity
In our earlier study (Subramaniam et al., 2009), overexpressing Rhes or mHtt alone was not cytotoxic, while the combination elicited substantial cell death. In HEK293 cells we observe modest but significant cell death associated with Rhes or mHtt overexpression, while co-expression of Rhes and mHtt elicits greater cytotoxicity (Figure 2A). Overexpressing ACBD3 by itself leads to cell death comparable to the combination of ACBD3/mHtt or ACBD3/Rhes. The greatest cytotoxicity occurs when ACBD3 is co-expressed with Rhes and mHtt.
Figure 2.
ACBD3 is required for Rhes/mHtt toxicity. A, HEK293 cells were transfected with plasmids encoding myc-vector (myc), myc-Rhes (R), myc-mHtt (mH), myc-ACBD3 (A) or in combination R/mH, R/mH/A, A/mH, or A/R. Cell viability was assessed by the MTT assay 48 h after transfection. * P < 0.005, **p < 0.02, ***p < 0.01. B, Striatal Q7 were transfected plasmids encoding myc-vector, myc-Rhes, myc-Rhes plus myc-ACBD3, or myc-ACBD3. Cell viability was assessed as in A. C, Striatal Q111 cells were transfected with plasmids encoding myc-vector, myc-Rhes, myc-Rhes plus myc-ACBD3, or myc-ACBD3. Cell viability was assessed as in A. * p < 0.02. D, HEK293 cells were cotransfected with plasmids encoding human ACBD3 shRNA or control shRNA, myc-Rhes plus myc-mHtt, or myc vector. Cell viability was assessed by MTT assay. * p < 0.01. E, Striatal Q111 were transfected with mouse ACBD3 shRNA or control shRNA; the next day cells were transfected again with shRNA together with myc or myc-Rhes plus myc-mHtt. * p < 0.002. All the data in these figures are presented as mean +/− SEM.
A striatal neuronal cell line stably expressing mHtt with 111 glutamines (Q111) models HD cellular dynamics and is routinely compared with a line of the same cells expressing Htt with 7 glutamines (Q7) (Trettel et al., 2000). In Q7 cells, overexpression of Rhes, ACBD3 or their combination does not alter cell survival (Figure 2B). By contrast, in Q111 cells overexpression of either Rhes or ACBD3 decreases cell survival, while co-expression produces further cell death (Figure 2C). To assess whether endogenous ACBD3 is required for Rhes and/or mHtt cytotoxicity, we depleted ACBD3 by about 70% from HEK293 cells and Q111 cells using RNA interference (Figure 2D, E). In HEK293 cells, depleting ACBD3 completely prevents cytotoxicity elicited by Rhes/mHtt (Figure 2D). Similarly, depletion of ACBD3 in Q111 cells abolishes Rhes-associated neurotoxicity (Figure 2E). As reported earlier, mHtt fails to elicit cytotoxicity in these cells in the absence of Rhes (Subramaniam et al., 2009).
ACBD3 is upregulated in Huntington's Disease (HD)
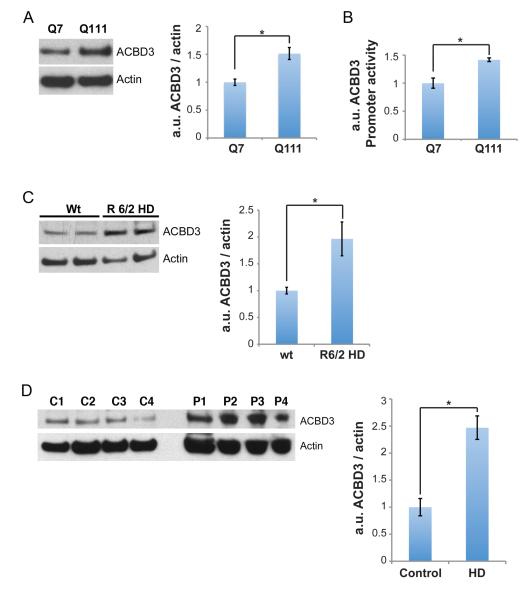
The abolition of Rhes/mHtt neurotoxicity by depleting ACBD3 implies a role for ACBD3 in neurotoxicity associated with HD. In Q111 cells ACBD3 protein levels are about 50% greater than in Q7 cells (Figure 3A). This increase appears to be mediated at the transcriptional level, as ACBD3 promoter activity, monitored by luciferase activity, is also substantially greater in Q111 than Q7 cells (Figure 3B).
Figure 3.
ACBD3 is upregulated in HD. A, Lysates from striatal Q7 and Q111 cells were resolved by SDS-PAGE and ACBD3 levels were quantified. The data are presented as mean +/− SEM. *p < 0.01. B, ACBD3 promoter activity was monitored in Q7 and Q111 cells as described in Experimental Procedures. The data are presented as mean +/− SEM. *p < 0.002. C, Striatal lysates from 12-week old R6/2 mice were resolved by SDS-PAGE and ACBD3 levels were quantified (n=4). The data are presented as mean +/− SEM. *p < 0.02. D, Striatal lysates from HD patients were resolved by SDS-PAGE and ACBD3 levels were quantified and presented as mean +/− SEM. *p <0.01.
Several murine genetic models of HD have been established with R6/2 mice most widely employed (Li et al., 2005). Brain levels of ACBD3 in R6/2 HD mice are twice control values (Figure 3C). Even more impressive is the 2.5 fold augmentation of ACBD3 levels in striatal samples of patients with HD (Figure 3D). The substantial increase of ACBD3 levels in the striatum of HD patients is especially notable, because in HD the striatum profoundly deteriorates with major decreases in levels of many proteins (Seredenina and Luthi-Carter, 2012); (Luthi-Carter et al., 2002). Presumably the increased levels of ACBD3 in HD enhance the cytotoxicity of striatal Rhes and mHtt.
ACBD3 is induced by cellular stress
In HD, damaged mitochondria and ER are dysfunctional (Roussel et al., 2013); (Johri and Beal, 2012). Thapsigargin, a calcium ATPase pump inhibitor that triggers ER stress, increases ACBD3 levels in Q7 cells and even more so in Q111 cells (Figure 4A). In the absence of thapsigargin ACBD3 levels in Q111 cells are slightly greater than in Q7 cells treated with thapsigargin (Figure 4A). The mitochondrial complex II inhibitor 3-nitropropionic acid (3NP), frequently used to model HD, also augments ACBD3 levels (Figure 4B).
Figure 4.
ACBD3 is induced by diverse cell stressors. Striatal cells Q7 and Q111 were incubated with 1 μM thapsigargin or DMSO (A), 1 mM 3-nitropropionic acid (3NP) or PBS (B), and 10 μM monensin (Mone) or DMSO (C) for 24 h. Cells were harvested, resolved by SDS-PAGE, and ACBD3 levels were quantified. *p < 0.2, **p < 0.05 (A), *p < 0.02, **p < 0.005 (B), *p < 0.02, **p < 0.0001 (C). D, Striatal Cells Q7 and Q111 were transfected with plasmids encoding GFP or GFP-Rhes. At 48 h following transfection cells were fixed, permeabilized and stained for endogenous ACBD3. The Golgi targeting of ACBD3 was analyzed in GFP or GFP-Rhes positive cells and classified as cytoplasmic or Golgi for ACBD3 localization. * p < 0.001. All the data in these figures are presented as mean +/− SEM.
The evident role of ACBD3 in HD neurotoxicity suggests that the Golgi apparatus participates in HD pathophysiology. Golgi stress has been proposed as a unique form of cell damage (Oku et al., 2011). The ionophore monensin selectively impacts the Golgi and is employed as a tool to elicit “Golgi stress” (Oku et al., 2011). During Golgi stress induced by monensin, ACBD3 is upregulated and appears to translocate to cytosol as the Golgi disperses (Oku et al., 2011). In Q7 striatal cells monensin increases ACBD3 levels 1.3 fold, almost to those of ACBD3 in control Q111 cells (Figure 4C). Upregulation of ACBD3 by monensin is greater in Q111 than Q7 cells (Figure 4C).
To examine Golgi stress associated with HD cytotoxicity in the context of Rhes, we monitored the intracellular localization of ACBD3 in striatal cell lines overexpressing Rhes (Figure 4D). These experiments were conducted 48 h following transfection with GFP-Rhes, a time when cytotoxicity is elicited. In the absence of Rhes, Q111 cells display low basal levels of ACBD3 in the cytoplasm, similar to Q7 cells containing GFP alone or GFP-Rhes (Figure 4D). By contrast, Rhes overexpression in Q111 cells elicits a 2.5 – 3 fold increase in ACBD3 cytoplasmic localization, reflecting Golgi stress (Figure 4D) reminiscent of ACBD3's cytosolic translocation elicited by monensin (Oku et al., 2011). Thus, the cytotoxicity of Rhes/mHtt appears linked to Golgi stress.
DISCUSSION
In the present study we demonstrate a major role for the Golgi protein ACBD3 in HD. ACBD3 levels are markedly increased in brains of patients with HD, in brains of genetic HD mice, and in Q111 striatal cell lines where the increase appears to involve augmented transcription. ACBD3 is physiologically associated with Rhes and Htt/mHtt in a ternary complex. In this complex mHtt and Rhes bind respectively to the N-terminus and C-terminus of ACBD3 so that their binding is not mutually exclusive, and ACBD3 may be functioning as a scaffolding protein, as occurs in other systems. Thus ACBD3 acts as a scaffold linking the divalent metal transporter DMT1 with the small G protein Dexras1 (Cheah et al., 2006). ACBD3 also displays scaffolding properties in mitochondria, facilitating the association of TSPO, which transports cholesterol into mitochondria (Midzak et al., 2011), with the regulatory subunit of protein kinase A (Liu et al., 2006).
Overexpression of ACBD3 enhances Rhes/mHtt cytotoxicity. More strikingly, depletion of ACBD3 by RNA interference abolishes Rhes/mHtt toxicity, establishing a pathophysiologic role for endogenous ACBD3. Thus, ACBD3 appears to be a critical element in Rhes/mHtt toxicity that has been implicated in the pathophysiology of HD (Subramaniam et al., 2009); (Mealer et al., 2013); (Baiamonte et al., 2013).
ACBD3 is predominantly a Golgi protein. Rhes and mHtt also display prominent Golgi localizations. These findings suggest a role for Golgi in mediating cell damage in HD. Lipton and associates (Nakagomi et al., 2008) provided evidence for such an association, reporting that Golgi fragmentation and dispersal precede neuronal cell death following oxidative/nitrosative insults, excitotoxins and ER stress. Moreover, a dominant-negative fragment of a Golgi-associated protein, GRASP65, blocks Golgi fragmentation and cell death (Nakagomi et al., 2008). Yoshida and associates (Oku et al., 2011) proposed that Golgi stress reflects a novel pathway for cell death, analogous to ER stress and mitochondrial stress. The Golgi complex processes and sorts diverse proteins and lipids that traffic through the secretory pathway. DiFiglia and associates (Velier et al., 1998) characterized roles for secretory and processing pathways for mHtt that, in part, involve the Golgi. Post-Golgi trafficking of brain-derived neurotrophic factor (BDNF) is damaged by mHtt (del Toro et al., 2006).
The pronounced increase of ACBD3 levels elicited by agents that impact Golgi, ER, and mitochondria, coupled with the marked increase in ACBD3 cytoplasmic staining elicited by Rhes in Q111 cells, are consistent with a role for ACBD3 in the neurotoxicity mediated by Rhes/mHtt. The Golgi apparatus may impact the pathology of other neurodegenerative diseases, as its fragmentation has been reported in conditions as diverse as amyotrophic lateral sclerosis (ALS), Alzheimer's Disease, Jacob-Creutzfeldt Disease, corticobasal degeneration and spinocerebellar ataxia type 2 (Gonatas et al., 2006).
Our findings may have therapeutic implications. As Rhes appears responsible for selective striatal damage in HD, efforts to prevent Rhes-mHtt binding may delay/prevent the onset of HD. Consistent with this possibility, mice with genetic deletion of Rhes are resistant to the neuropathology and motor defects elicited by the striatal-selective toxin 3-nitropropionic acid, (Mealer et al., 2013); (Baiamonte et al., 2013). Efforts to decrease levels of mHtt have been undertaken as another therapeutic approach in HD (Carroll et al., 2011). Both Rhes and mHtt bind physiologically to ACBD3, which appears to function as a scaffold that promotes their toxicity. Accordingly, agents that block the binding of ACBD3 to Rhes and/or mHtt may be therapeutic.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HEK293 cells were maintained in normal Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 5 mM glutamine and antibiotics. STHdhQ7/Q7 (Q7) and STHdhQ111/Q111 (Q111) were a generous gift from Marcy MacDonald and were maintained in DMEM containing 400 μg/ml Geneticin (Invitrogen). R6/2 transgenic mice (B6CBA-Tg(HDexon1)62Gpb/1J) were from Jackson Laboratories. Post mortem human patients' striatal samples were a gift from Juan Troncoso and Olga Pletnikova (Division of Neuropathology, Department of Pathology, Johns Hopkins University).
Thapsigargin, 3-nitropropionic Acid, and monensin were from Sigma.
Plasmids and Antibodies
Myc (pCMV-myc, Clontech), myc-Rhes, myc-Htt (171-18Q), myc-mHtt(171-82Q) and pCMV-GST-Rhes were previously described (Subramaniam et al., 2009). GFP-Rhes was from RM Mealer (unpublished). GST-ACBD3(1-175), GST-ACBD3(328-528) were previously described (Sbodio et al., 2006). Myc-ACBD3 and full length GST-ACBD3 were cloned from GFP-ACBD3 (Sbodio et al., 2006). ACBD3 and actin-HRP antibodies were from Santa Cruz, myc from Roche, and mHtt (MAB2166) from Millipore. Secondary antibodies were from GE Healthcare.
Binding Assays
GST constructs expressed in Escherichia coli BL21 (DE3) (Invitrogen), GST-ACBD3, GST-ACBD3(1-175), GST-ACBD3(328-528) were purified on glutathione-sepharose 4B as recommended by the manufacturer (Amersham Biosciences). Binding assays were performed as described previously (Sbodio et al., 2006). Briefly, 10 μg of purified GST or GST fusion proteins bound to glutathione beads were incubated overnight with lysates of HEK293 cells that were previously transfected and harvested in detergent solution (62.5 mM EDTA, 50 mM Tris-HCl, pH 8, 0.4% deoxycholate, 1% Nonidet P-40, and protease inhibitors (Sbodio et al., 2006). The bound proteins were washed three times in detergent solution, eluted in sample buffer, boiled, resolved by SDS-PAGE, and detected by immunoblotting. For GST-Rhes binding, assays were performed as described above except that pCMV-GST-Rhes was transfected in HEK293 or in striatal Q111 cells and purified by binding it to glutathione beads overnight.
Depletion of ACBD3
ACBD3 was knocked down using shRNA (Santa Cruz). Briefly HEK293 cells were co-transfected with plasmids encoding human ACBD3 shRNA or control shRNA, myc-Rhes and myc-mHtt, or myc alone. Cell viability was detected by MTT 72 h after transfection. Striatal Q111 cells were transfected with plasmids encoding mouse ACBD3 shRNA or control (Santa Cruz). At 24 h post transfection, cells were transfected again using mouse ACBD3 shRNA or control, together with myc-Rhes or myc alone. Cell viability was measured using the MTT assay 48 h after the second transfection.
Reporter gene assays
Q7 and Q111 cells were transfected with the ACBD3 promoter reporter constructs (Renilla luciferase) and internal control, TK construct (Cypridina luciferase) from Switchgear Genomics, and cells were assayed 72 h post transfection using the dual luciferase kit as per the manufacturer's recommendations (Switchgear Genomics). The luciferase readings from the promoter construct were normalized to the values obtained from the TK construct.
Indirect Immunofluorescence Microscopy
Striatal cells were cultured on cover slips transfected and processed as described (Sbodio et al., 2006) 16 h after transfection for the colocalization experiments and 48 h post transfection for the ACBD3 Golgi targeting assay. The localization of ACBD3 (cytoplasmic or Golgi) in the presence or absence of Rhes was quantified using GFP-tagged Rhes and GFP alone as a control.
Cell viability assay
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as recommended by the manufacturer (Millipore). Briefly, cells were seeded in 24-well or 6-well plates, transfected using Polyfect (Qiagen) reagent for the HEK293 cells or lipofectamine (Invitrogen) for the striatal cells. At 48 h after transfection, MTT (5 mg/ml) was added to the cells for 45 min. The culture medium was centrifuged to collect any floating cells. The remaining cells in the well were lysed in DMSO. The pelleted cells from the culture medium were lysed in DMSO and added to the original well from which they were taken. Absorbance was measured at 570 nm and 630 nm to determine cell viability.
Highlights
ACBD3 is upregulated in Huntington's disease.
ACBD3 interacts with Rhes and mutant huntingtin.
ACBD3 augments Rhes-induced cytotoxicity in HD.
ACBD3 is induced during cellular stress such as ER, Golgi and Mitochondrial stress.
ACKNOWLEDGEMENTS
This work was supported by grants to S.H.S. from the US Public Health Service grant (MH18501) and the Cure Huntington's Disease Initiative (CHDI) and by National Institutes of Health grant R01 GM42522 to C.E.M. We thank Juan Troncoso and Olga Pletnikova for the post-mortem human patient striatal samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Baiamonte BA, Lee FA, Brewer ST, Spano D, LaHoste GJ. Attenuation of Rhes activity significantly delays the appearance of behavioral symptoms in a mouse model of Huntington's disease. PLoS One. 2013;8:e53606. doi: 10.1371/journal.pone.0053606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB, Warby SC, Southwell AL, Doty CN, Greenlee S, Skotte N, Hung G, Bennett CF, Freier SM, Hayden MR. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene / allele-specific silencing of mutant huntingtin. Mol Ther. 2011;19:2178–2185. doi: 10.1038/mt.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Khan RS, Cwanger A, Song Y, Steenstra C, Bang S, Cheah JH, Dunaief J, Shindler KS, Snyder SH, et al. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J Neurosci. 2013;33:3582–3587. doi: 10.1523/JNEUROSCI.1497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Toro D, Canals JM, Gines S, Kojima M, Egea G, Alberch J. Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism. J Neurosci. 2006;26:12748–12757. doi: 10.1523/JNEUROSCI.3873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Reeves SA, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Fan J, Liu J, Culty M, Papadopoulos V. Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog Lipid Res. 2010;49:218–234. doi: 10.1016/j.plipres.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Gonatas NK, Stieber A, Gonatas JO. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J Neurol Sci. 2006;246:21–30. doi: 10.1016/j.jns.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Greninger AL, Knudsen GM, Betegon M, Burlingame AL, Derisi JL. The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIbeta. J Virol. 2012;86:3605–3616. doi: 10.1128/JVI.06778-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- Li H, Degenhardt B, Tobin D, Yao ZX, Tasken K, Papadopoulos V. Identification, localization, and function in steroidogenesis of PAP7: a peripheral-type benzodiazepine receptor- and PKA (RIalpha)-associated protein. Mol Endocrinol. 2001;15:2211–2228. doi: 10.1210/mend.15.12.0736. [DOI] [PubMed] [Google Scholar]
- Li JY, Popovic N, Brundin P. The use of the R6 transgenic mouse models of Huntington's disease in attempts to develop novel therapeutic strategies. NeuroRx. 2005;2:447–464. doi: 10.1602/neurorx.2.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, et al. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- Mealer RG, Subramaniam S, Snyder SH. Rhes Deletion Is Neuroprotective in the 3-Nitropropionic Acid Model of Huntington's Disease. J Neurosci. 2013;33:4206–4210. doi: 10.1523/JNEUROSCI.3730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336:70–79. doi: 10.1016/j.mce.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi S, Barsoum MJ, Bossy-Wetzel E, Sutterlin C, Malhotra V, Lipton SA. A Golgi fragmentation pathway in neurodegeneration. Neurobiol Dis. 2008;29:221–231. doi: 10.1016/j.nbd.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku M, Tanakura S, Uemura A, Sohda M, Misumi Y, Taniguchi M, Wakabayashi S, Yoshida H. Novel cis-acting element GASE regulates transcriptional induction by the Golgi stress response. Cell Struct Funct. 2011;36:1–12. doi: 10.1247/csf.10014. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- Sasaki J, Ishikawa K, Arita M, Taniguchi K. ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites. EMBO J. 2012;31:754–766. [Google Scholar]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- Sbodio JI, Hicks SW, Simon D, Machamer CE. GCP60 preferentially interacts with a caspase-generated golgin-160 fragment. J Biol Chem. 2006;281:27924–27931. doi: 10.1074/jbc.M603276200. [DOI] [PubMed] [Google Scholar]
- Seredenina T, Gokce O, Luthi-Carter R. Decreased striatal RGS2 expression is neuroprotective in Huntington's disease (HD) and exemplifies a compensatory aspect of HD-induced gene regulation. PLoS One. 2011;6:e22231. doi: 10.1371/journal.pone.0022231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenina T, Luthi-Carter R. What have we learned from gene expression profiles in Huntington's disease? Neurobiol Dis. 2012;45:83–98. doi: 10.1016/j.nbd.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yamamoto A, Yano A, Nakamura N, Ikehara Y. Identification and characterization of a novel Golgi protein, GCP60, that interacts with the integral membrane protein giantin. J Biol Chem. 2001;276:45298–45306. doi: 10.1074/jbc.M108961200. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- Velier J, Kim M, Schwarz C, Kim TW, Sapp E, Chase K, Aronin N, DiFiglia M. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp Neurol. 1998;152:34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]