Abstract
For single cell organisms, nutrient uptake and metabolism are at the crux of their most basic decision of whether to grow or divide. In metazoans, cell fate decisions are more complex: organismal homeostasis must be strictly maintained by balancing cell proliferation and death. Despite this increased complexity, cell fate within multicellular organisms is also influenced by metabolism; recent studies, triggered in part be an interest tumor metabolism, are beginning to illuminate the mechanisms through which proliferation, death, and metabolism are intertwined. In particular, work on Bcl-2 family proteins suggests that the signaling pathways governing metabolism and apoptosis are inextricably linked. Here, we review the crosstalk between these pathways, emphasizing recent work that illustrates the emerging dual nature of several core apoptotic proteins in regulating both metabolism and cell death.
Introduction
Eukaryotic metabolism encompasses a remarkably complex and tangled circuitry of catabolic and anabolic pathways. These pathways yield countless metabolic intermediates, many of which have potential signaling functions. Although our understanding of how metabolic pathways and metabolites integrate with cell fate signaling is still in its infancy, the interplay between metabolism and cell death has garnered new interest due to its emerging role in some of the most devastating human diseases: cancer, Alzheimer’s disease, and diabetes. Here we discuss how apoptotic signaling is linked to several metabolic pathways, including glycolysis, the tricarboxylic acid cycle (TCA cycle), and the pentose phosphate pathway (PPP) (summarized in figure 1).
Figure 1. A summary of metabolic and apoptotic pathways relevant to this review.
The left panel summarizes the fate of glucose through glycolysis, the TCA cycle, the pentose phosphate pathway, and lipid synthesis. The right panel outlines the key apoptotic steps addressed in this review. Abbreviations: Glut glucose transporter, HK hexokinase, CoA coenzyme A, NADPH nicotinamide adenine dinucleotide phosphate (reduced), PEP phosphoenolpyruvate, FBP fructose 1,6-bisphosphate, PFK phosphofructokinase, ACL ATP citrate lyase, TCA tricarboxylic acid, tBid truncated Bid, Apaf-1 apoptotic protease activating factor-1, Bcl-2 B cell lymphoma-2.
The fate of glucose
Glucose, a key source of both metabolic fuel and new cell mass, is taken up by the cell in a regulated manner via glucose transporters (GLUTs) (reviewed in (Thorens and Mueckler, 2010)). Once inside the cell, glucose is phosphorylated to glucose-6-phosphate (G6P) by hexokinases, at which point G6P can either continue through glycolysis to generate pyruvate for the TCA cycle, or be shunted into the PPP to generate ribose-5-phosphate and NADPH. PPP-mediated production of NADPH protects cells from oxidative damage by generating reduced glutathione, which neutralizes hydroperoxides. NADPH is also an essential cofactor for the reductive biosynthesis of nucleotides, amino acids and fatty acids. In addition, PPP-generated ribose-5-phosphate is further metabolized through the non-oxidative arm of the PPP and is ultimately used for the synthesis of nucleic acids. In this regard, the biosynthetic capacity of a cell is highly dependent on the PPP. As such, the upregulation of PPP activity is a hallmark of rapidly proliferating cells like those found in tumors or embryonic tissues.
The glycolytic end product, pyruvate, enters the mitochondria and undergoes a series of oxidative reactions via the TCA cycle. Pyruvate processing within the TCA cycle ultimately yields two ATP and six NADH molecules per molecule of glucose. TCA cycle-generated NADH is then used as a reducing agent in oxidative phosphorylation to generate a high yield of ATP. In addition to glucose, the TCA cycle also receives input from cytosolic amino acids that are transported into the mitochondria to generate TCA cycle intermediates (a-ketoglutarate, succinyl-CoA, fumarate, oxaloacetate, and acetyl-CoA). For example, a key energy source for tumor cells, glutamine, is converted to glutamate in the cytosol by glutaminase. Glutamate is then transported into the mitochondria where it is converted to α-ketoglutarate. One product of the TCA cycle particularly relevant to this review is acetyl-CoA. Transported out of the mitochondria and into the cytosol in the form of citrate (a combination of oxaloacetate and acetyl-CoA), acetyl-CoA and oxaloacetate are produced through the action of ATP citrate lyase (ACL). Once cytosolic, acetyl-CoA is converted to malonyl-CoA, which is used as a building block for fatty acid biosynthesis (summarized in figure 1). Citrate-derived acetyl-CoA is also used as the primary source of the acetyl group in lysine acetylation and N-alpha acetylation (to be discussed later).
Mitochondria: the nexus of metabolic and apoptotic signaling
Numerous studies over the past 10–20 years have revealed a complex picture of mitochondria—far removed from the traditional textbook image of morphologically static ATP generators. For one, mitochondria are the focal point of apoptotic signaling. Death stimuli ranging from DNA damage to metabolic stress to immune cell-mediated death receptor ligation converge on the mitochondria to trigger the release of cytochrome c from the mitochondrial intermembrane space. The release of cytochrome c (generally considered a critical decision point in apoptosis (Llambi et al., 2011)) occurs through a process known as mitochondrial outer membrane permeabilization (MOMP), which involves activation of the Bcl-2 family members Bax and Bak at the surface of mitochondria. Once in the cytosol, cytochrome c triggers the olgomerization of apaf-1 with the cysteine-aspartic protease caspase-9 to form the apoptosome, which goes on to activate the executioner caspases, caspase-3 and -7. These terminal caspases cleave various cellular substrates and ultimately dismantle the cells in the tidy manner characteristic of apoptosis (summarized in figure 1).
Mitochondria are also highly dynamic organelles, continuously rearranging themselves through fission and fusion (reviewed in (Martinou and Youle, 2011)). Notably, the Bcl-2 family proteins, long regarded as apoptotic regulators, have been shown to affect rates of mitochondrial fission and fusion. While their precise functions in controlling cell viability is still hotly debated, fission and fusion have been shown in some systems to impact both metabolism and apoptosis (Martinou and Youle, 2011). Taken together, the fact that many of the cell’s key metabolic and cell fate signaling pathways are routed through mitochondria suggests that mitochondria are a likely site of crosstalk between these pathways.
To date, probably the most direct example of a metabolism-apoptosis link lies with the dual functionality of cytochrome c. Cytochrome c is a heme protein associated with the inner mitochondrial membrane (IMM). Its long-known metabolic role is to pass an electron from respiratory complex III to complex IV in order to promote ATP generation through oxidative phosphorylation. In 1996, through a series of elegant biochemical experiments published in their seminal Cell paper, Xiaodong Wang and colleagues found that cytochrome c was necessary for activation of caspase activity (Liu et al., 1996). While the factors that control mitochondrial cytochrome c release (ie., Bax/Bak and other Bcl-2 family proteins—to be discussed later) seem to be a primary locus of regulation, the apoptotic activity of cytochrome c itself is also affected by glucose metabolism.
A common feature of neurons, like cancer cells, is their heavy use of glucose as a metabolic fuel. In addition, both neurons and cancer cells are refractory to cytochrome c-induced caspase activation, even when cytochrome c is directly microinjected into the cytosol (Schafer et al., 2006; Vaughn and Deshmukh, 2008). Vaughn and Deshmukh linked these observations by showing that glucose-stimulated production of intracellular glutathione, as a result of NADPH production through the PPP, led to inactivation of cytochrome c by keeping it in its reduced state (Vaughn and Deshmukh, 2008). As they further showed, induction of apoptosis led to an increase in reactive oxygen species (ROS) that subsequently triggered oxidation of cytochrome c, thus rendering it capable of caspase activation. This study and others (Brown and Borutaite, 2008; Li et al., 2008) affirmed that oxidation of cytochrome c is indeed important for apoptosis. An implication of these studies is that the native, steady state redox status of different tissues/cells will affect their sensitivity to cytochrome c. As NADPH levels are both predominately controlled by the PPP and a key determinant of cellular redox status due to the role of NADPH in GSH production, these studies suggest that cells with high PPP activity will be resistant to caspase activation downstream of MOMP. In support of this idea, in tumor cells with high endogenous PPP activity, inhibition of the PPP by dihydroepiandrosterone (DHEA) is sufficient to generate the oxidative cytosolic environment necessary to sensitize cells to caspase activation induced by micro-injected cytochrome c (Vaughn and Deshmukh, 2008).
The dual nature of Bcl-2 family proteins
Pro-apoptotic Bcl-2 proteins
Bcl-2 family proteins are the chief controllers of MOMP in the cell. The Bcl-2 family is divided into pro- and anti-apoptotic members, but all share combinations of alpha-helical regions, known as Bcl-2 homology (BH) domains. These domains largely determine each Bcl-2 member’s function in the apoptotic cascade. The pro-apoptotic Bcl-2 family proteins (e.g., Bax, Bak, Bid, Bim, Bad, Puma, Noxa) all contain a single amphipathic BH segment, known as the BH3 domain. Bax and Bak, which are the gateway controllers of MOMP, possess multiple BH domains, while the rest of the pro-apoptotic family members (e.g., Bad, Bim, Bid, Puma, Noxa) only possess the BH3 domain (hence their common name, “BH3 only proteins”). The precise mechanism by which the BH3 only proteins promote apoptosis can differ. Some, like Bid and Bim, are thought to interact with and promote the activation of Bax and Bak directly. In contrast, Bad, Puma, and Noxa can act as “derepressors”, neutralizing the anti-apoptotic activity of Bcl-xL and Bcl-2 to facilitate Bax/Bak activation.
While Bax is traditionally considered a tumor suppressor due to its role in triggering MOMP, early studies of Bax deficient mice revealed the presence of both hyperplasia and hypoplasia, depending on tissue type (Knudson et al., 1995). This study and a limited number of studies that followed have provided hints that Bax may have functions unrelated to MOMP. For one, Bax has been shown to regulate the levels of reactive oxygen species (ROS) in healthy neurons (Kirkland and Franklin, 2007). Bax and Bak together also regulate ER calcium homeostasis in lymphocytes (Jones et al., 2007). In addition, Bax has been observed to form foci at mitochondrial fission sites in healthy cells (Karbowski et al., 2002) and deletion of Bax and Bak alters the normal rate of mitochondrial fusion (Karbowski et al., 2006). The impact of Bax on mitochondrial fission/fusion in particular may, by extension, impact mitochondrial metabolism (Zorzano et al., 2010). In this regard, a recent study by Boohaker et al. (2011) showed the Bax- deficient cells display decreased oxygen consumption/ATP levels and increased glycolysis, all of which could be rescued by Bax overexpression. Importantly, the rescue of these metabolic defects was observed with a Bax mutant lacking the C terminal helix required for MOMP, suggesting that the metabolic and apoptotic functions of Bax are mechanistically distinct (Boohaker et al., 2011). However, whether this metabolic function of Bax is related to its effect on mitochondrial dynamics is still unclear.
Bax activation during MOMP has also been mechanistically linked to the metabolism of ceramide, a lipid metabolite long suspected to play a role in apoptosis. Ceramide is upregulated by numerous cellular stresses. Early cell-free experiments (in Xenopus egg extract, for example) implicated ceramide in the activation of the apoptotic cascade (Reviewed in (Woodcock, 2006)). Ceramide has even been proposed to induce MOMP directly by forming pores in the outer mitochondrial membrane (OMM) (Colombini, 2010). Ceramide can be produced through multiple mechanisms: de novo through the combination of serine and palmitoyl-CoA (products of glycolysis and fatty acid synthesis, respectively), through salvage pathways, or by sphingomyelinase (SMase)-mediated hydrolysis of the common membrane phospholipid sphingomyelin (also a product of de novo ceramide synthesis). A role for the latter mechanism in promoting Bax/Bak-mediated MOMP has been reported (Chipuk et al., 2012). Specifically, Chipuk and colleagues found that an SMase both co-fractionated with mitochondrial associated membranes (MAM) and generated a mitochondrial pool of ceramide. This pool of ceramide can be broken down into sphingosine-1-phosphate (S1P) and the fatty aldehyde hexadecenal (hex). The authors found that hex interacts directly with Bax to facilitate Bax activation in response to truncated Bid (tBid), while S1P seems to promote Bak activation (Chipuk et al., 2012). Although little is known about the cellular activates of hex, S1P has numerous cell signaling functions. In apparent contrast to the mechanism described above, S1P is commonly considered the pro-survival counterbalance to the pro-apoptotic activity of ceramide (reviewed in (Van Brocklyn and Williams, 2012)), possibly due to its role in the activation of ERK survival kinase signaling (Cuvillier et al., 1996). Resolving these apparently disparate functions will likely require a better understanding of S1P’s mitochondrial function. Indeed, despite its numerous cytosolic signaling roles, our understanding of S1P’s mitochondrial function is relatively limited (Strub et al., 2011). Further studies will be required to parse the mechanistic details of hex- and S1P-mediated Bax/Bak activation and to understand how this mechanism fits into the larger metabolic picture.
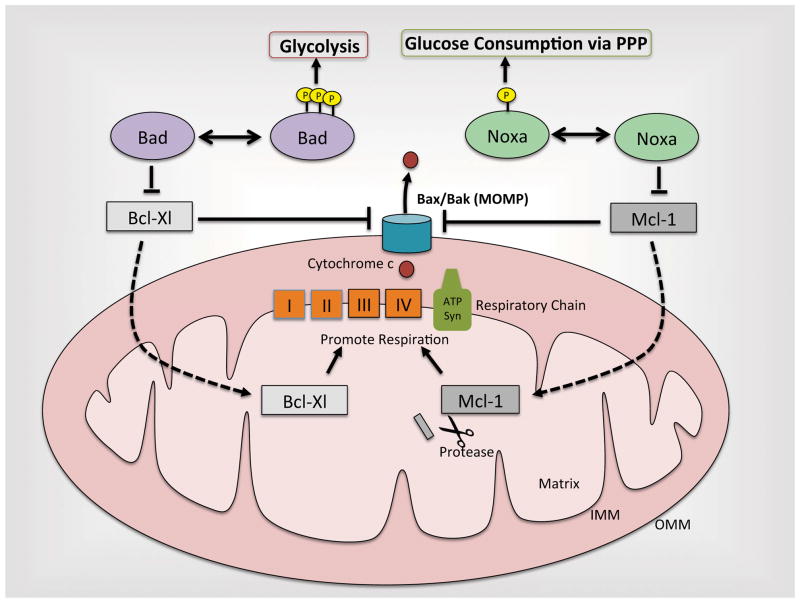
A link between nutrient pathways and the BH3 only protein Bad was first observed in the 1990s and has since been corroborated and extended by multiple groups (Datta et al., 2002; del Peso et al., 1997; Zha et al., 1996). In 1996, the Korsmeyer group found that supplementation of cells with the survival factor interleukin-3 (IL-3), which promotes glucose uptake and metabolism, induced the phosphorylation of Bad at S112 and S136 (mouse BadL numbering). This phosphorylation consequently led to an interaction between Bad and 14-3-3, resulting in the suppression of Bad’s proapoptotic activity (Zha et al., 1996). Later, Danial et al. showed that a pool of Bad resides at the mitochondria in a multiprotein complex with glucokinase (GK) (Danial et al., 2003), a hexokinase whose expression is limited to β-islet cells, hypothalamic neurons and hepatocytes (Matschinsky et al. 2006). By showing that Bad serves as a scaffold for this complex and is required for glucokinase activity and normal glycolytic function, Danial and colleagues implicated Bad as a key regulator of glucose metabolism (Danial et al., 2003). Toggling the function of Bad between metabolism and apoptosis, and working in concert with the phosphorylations at S112 and S136, is a third phosphorylation site at S155 (within the BH3 domain). When S155 is phosphorylated, Bad loses its ability to antagonize the anti-apoptotic Bcl-xL and begins to promote GK-mediated glucose metabolism (figure 2) (Danial, 2008; Datta et al., 2000; Tan et al., 2000). Conversely, dephosphorylation at all three sites increases apoptotic sensitivity by lowering the threshold for MOMP (Danial, 2008). Together, these studies help to set the paradigm for how Bcl-2 family proteins might coordinately regulate apoptosis and metabolism.
Figure 2. The dual roles of Bcl-2 family proteins.
Aside from their traditional roles in suppressing Bcl-Xl and Mcl-1, Bad and Noxa (when phosphorylated) modulate metabolism by enhancing glucose consumption through glycolysis and the pentose phosphate pathway, respectively. Additionally, Bcl-XL and an N-terminally cleaved form of Mcl-1 localize to the matrix to promote respiration. It is still unclear how these novel metabolic functions of Bcl-2 family proteins may intertwine with their long-studied roles in modulating MOMP.
While no other pro-apoptotic Bcl-2 family protein to date has been tied to metabolism with the same mechanistic detail as Bad, others are clearly responsive to metabolism. The BH3 only protein Puma, which can bind and inhibit all anti-apoptotic Bcl-2 family members as well as induce Bax activation directly, is modulated by glycolytic activity (Zhao et al., 2007). In a hematopoietic precursor cell model, IL-3 withdrawal causes a reduction in glycolysis and an increase in Puma expression. Using this system, Zhao et al. demonstrated that maintaining glycolytic activity (by forced expression of Glut1 and hexokinase) after IL-3 withdrawal was sufficient to attenuate Puma induction and block cell death (Zhao et al., 2007). Additionally, glucose deprivation, even in the presence of IL-3, induced Puma expression and Puma-dependent cell death; therefore a reduction in glycolysis is likely a key apoptotic stimulus in response to IL-3 withdrawal (Zhao et al., 2007). In this system, the link between metabolic stress and Puma was traced to p53. p53 is suppressed by high glycolytic activity and activated by IL-3 withdrawal through a protein kinase Cδ-dependent pathway, leading to the transcriptional upregulation of Puma (Mason and Rathmell, 2011; Zhao et al., 2007).
In addition to Puma, the BH3 only protein Noxa has also been linked to glucose deprivation-induced apoptosis. TCR-mediated activation of T cells leads to a PKC-dependent (and p53-independent) induction of Noxa (Alves et al., 2006). Induction of Noxa sensitized cells specifically to nutrient deprivation, but failed to sensitize against other stresses (e.g., DNA damage, ER stress, oxidative stress). Additionally, RNAi-mediated depletion of Noxa rendered leukemic and proliferating primary T cells resistant to apoptosis induced by glucose-limiting conditions. Interestingly, in addition to TCR engagement, Noxa upregulation was also observed in response to other mitogens (e.g., IL-4, IL-15), suggesting that Noxa induction is linked to proliferation and may serve as a safeguard against oncogenic transformation (Alves et al., 2006).
Expanding on the link between Noxa and glucose metabolism, Lowman et al. found that Noxa is phosphorylated at Ser13 (a residue outside of Noxa’s BH3 domain) by Cdk5 only when cells are in the presence of glucose (Lowman et al., 2010). This phosphorylation causes sequestration of Noxa in the cytosol and suppression of its apoptotic function. Despite not having apoptotic activity, phosphorylated Noxa still associates with the anti-apoptotic Bcl-2 family member, Mcl-1 (this observation corroborates earlier observations that the Noxa/Mcl-1 interaction persists in proliferating T cells with high glycolytic activity (Alves et al., 2006)). In addition, glucose stimulates a marked change in the association of Noxa with high molecular weight complexes that also harbor Mcl-1 (Lowman et al., 2010). The traditional view of Noxa’s pro-apoptotic effect is that Noxa, when induced/activated, binds Mcl-1 and promotes its degradation, thus sensitizing cells to MOMP. However, under high glucose conditions, Noxa binds, but does not promote Mcl-1 degradation or sensitization to MOMP. Furthermore, Jurkat cells overexpressing Noxa showed inordinately high rates of glucose metabolism, and while prolonged Noxa expression ultimately resulted in Jurkat cell death in stagnant media (in which glucose is depleted over time), the continual supplementation of cells with excess glucose abrogated Noxa-induced death (Lowman et al., 2010). The latter result presumably occurred due to enforcement of continued phosphorylation at Ser13. Together, these observations suggested that the change in Noxa observed by gel filtration might represent its shift from a pro-death to a glycolysis-promoting protein complex, in a scenario very reminiscent of its BH3 only relative Bad. However, while Bad promotes glycolysis when phosphorylated at Ser155, phosphorylated Noxa appears to decrease glycolysis despite increasing glucose consumption. This counterintuitive observation is likely explained by a mechanism wherein Noxa diverts glucose away from glycolysis and into the PPP, as suggested by experiments in which Noxa promoted the conversion of glucose to ribose-5-phosphate (Lowman et al., 2010). This observation is particularly striking, as this mode of metabolism, which generates NADPH and reduced glutathione, promotes suppression of oxidative stress (Ralser et al., 2007)—a far cry from Noxa’s classical role as an apoptosis inducer.
Together, these studies illustrate the emerging dual nature of the BH3 only proteins in regulating both apoptosis and metabolism. For future studies, it will be important to consider whether the interplay between BH3 only proteins and anti-apoptotic Bcl-2 proteins (traditionally considered primarily in the context of apoptosis) might have unanticipated metabolic consequences. Indeed, several recent studies have described metabolic functions for Bcl-xL and Mcl-1.
Anti-apoptotic Bcl-2 proteins
The activation of Bax/Bak and the consequent release of cytochrome c through MOMP are held in check by the anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-xL, Bcl-W and Mcl-1. The traditional view is that, under stress, BH3 only proteins antagonize the anti-apoptotic Bcl-2 proteins, thereby allowing for Bax/Bak activation and MOMP (reviewed in (Youle and Strasser, 2008)). While this conceptually simple model has been supported by years of research, an expanded view of Bcl-2 protein function has come from recent studies on mitochondrial dynamics. Expression of CED-9, a Bcl-2-like protein from C. elegans, can induce mitochondrial fusion (Yamaguchi et al., 2008) and has been shown to interact with core mitochondrial fusion/fission machinery (Lu et al., 2011; Yamaguchi et al., 2008). Furthermore, expression of Mcl-1 and Bcl-xL in mammalian cells can induce mitochondrial fusion (Delivani et al., 2006; Perciavalle et al., 2012; Sheridan et al., 2008); and the Drosophila Bcl-2 homologue (Buffy) promotes mitochondrial remodeling during oogenesis (Tanner et al., 2011). These observations raise a question concerning the fundamental mechanism by which Bcl-2 proteins modulate apoptosis: Do Bcl-2 proteins modulate apoptosis by controlling mitochondrial fission/fusion? Although mitochondrial fission occurs concomitantly with MOMP, and it seems highly plausible that Bcl-2 stimulation of mitochondrial fusion might suppress MOMP, a consensus answer to this question has not emerged (see a recent review on this topic (Martinou and Youle, 2011)). That said, these changes in mitochondrial morphology suggest a metabolic role for anti-apoptotic Bcl-2 proteins that may be linked to the mechanism by which they suppress apoptosis.
Several recent studies have implicated anti-apoptotic Bcl-2 proteins in the regulation of metabolism. An NMR- and mass spectrometry-based comparison of Bcl-xL-overexpressing and control cells revealed profound differences in metabolite levels (Yi et al., 2011). Notably, Bcl-xL expression reduced the levels of glucose-derived citrate, which consequently led to lower cytosolic acetyl-CoA levels. In turn, low acetyl-CoA levels were linked to a decrease in the N-alpha-acetylation of several-pro-apoptotic proteins, including Bax and caspase-2. For caspase-2, N-alpha-acetylation was shown to be important for its activation (Yi et al., 2011). While the anti-apoptotic effect of Bcl-xL is often attributed to its binding and suppression of Bax, mutants of Bcl-xL that are unable to bind Bax still retain up to 80% of their anti-apoptotic activity (Cheng et al., 1996). These same Bax-binding mutants still suppress acetyl-CoA levels, suggesting that modulation of metabolism and, in turn, acetyl-CoA levels may account for a significant portion of Bcl-xL’s antiapoptotic activity (Yiet al., 2011).
Bcl-xL has commonly been considered an OMM-associated protein. Numerous studies have characterized its suppression of Bax/Bak at this locale. Additional studies have even implicated Bcl-xL in controlling metabolite and ion permeability of the OMM (Basanez et al., 2002; Lam et al., 1998; Vander Heiden et al., 2001). Two recent studies expand this paradigm by describing a role for Bcl-xL at the inner mitochondrial membrane in promoting ATP generation (Alavian et al., 2011; Chen et al., 2011). Surprisingly, overexpression of Bcl-xL in neurons increases ATP generation but lowers mitochondrial oxygen consumption (Alavian et al., 2011). While this observation initially seemed paradoxical, an explanation appears to be found in the process of oxidative phosphorylation: H+ protons pumped out of the mitochondrial matrix can re-enter the matrix through ATP synthase (generating ATP) and through a non-productive H+ leak. Cristae-localized Bcl-xL appears to reduce the non-productive leakage of H+ into the matrix by directly modulating an ion channel at the inner mitochondrial membrane, thereby channeling more protons through ATP synthase to generate ATP. Bcl-xL also interacts with the β subunit of F1F0 ATP synthase and increases ATP synthase enzymatic activity both in cells and with purified recombinant enzymes in vitro (Alavian et al., 2011; Chen et al., 2011). Still, however, it is unclear whether the direct modulation of ATP synthase activity accounts for the reduction in H+ leakage.
Most relevant to this review is the question of whether the anti-apoptotic effect of Bcl-xL is linked to its role in modulating ATP production/metabolism. As mentioned above, the metabolic and anti-apoptotic properties of Bcl-xL co-segregate in Bcl-xL mutants deficient for Bax/Bak binding (Yi et al., 2011). Furthermore, two additional experiments suggest a role for metabolic modulation in Bcl-xL’s anti-apoptotic effect: Bcl-xL overexpression protected yeast from heat-induced death but failed to protect yeast deficient in the β subunit of F1F0 ATP synthase (Chen et al., 2011). Moreover, Bcl-xL overexpression protects Bax/Bak double knockout mouse embryonic fibroblasts from death induced by replacing media glucose with galactose, which forces cells to rely on oxidative phosphorylation (rather than glycolysis) for energy (Alavian et al., 2011). In this scenario, Bcl-xL-mediated enhancement of oxidative phosphorylation may help these cells keep pace, at least for a time, with energy demands.
Together, these observations indicate that Bcl-xl can promote survival in a non-traditional, Bax/Bak-independent manner. Indeed, it seems likely that in the face of increased energy demands on the mitochondria (i.e., in galactose-containing media) the Bcl-xL-induced enhancement of ATP production and suppression of a detrimental H+ ion leak, would maintain mitochondrial membrane potential. Conversely, in the absence of Bcl-xL, the non-productive leakage of H+ ions and weakened ATP synthase activity would likely prevent mitochondria from keeping pace with energy demand, potentially resulting in mitochondrial depolarization regardless of Bax/Bak status (Chen et al., 2011).
How might this view of Bcl-xL function jibe with the long-held view that Bcl-xL directly suppresses apoptosis by inhibiting Bax/Bak-mediated cytochrome c release? The relative contribution of either potential death-suppressing mechanism--Bcl-xL suppression of Bax/Bak versus modulation of metabolism--may depend on where Bcl-xL is predominately localized (OMM versus cristae) in a given cell type. Alternatively, these two functions of Bcl-xL may be related: ATP synthase activity has been linked to Bax activation (Matsuyama et al., 1998). In any case, the observation that mutants of Bcl-xL deficient for Bax/Bak binding still suppress apoptosis (albeit to a slightly lesser degree than WT) yet maintain their effect on metabolism clearly argues for an expansion of the Bcl-xL paradigm to include modulation of metabolism as part of its anti-apoptotic function.
In an intriguing parallel to Bcl-xL, Mcl-1 has recently been shown to reside in the mitochondrial matrix (Huang and Yang-Yen, 2010; Perciavalle et al., 2012) and have a role in promoting both ATP production and maintenance of mitochondrial membrane potential (Perciavalle et al., 2012). Perciavalle et al. found that inducible deletion of Mcl-1 results in gross defects in both inner mitochondrial membrane (IMM) architecture and mitochondrial fusion. A low molecular weight isoform of Mcl-1, generated by an N-terminal cleavage, was found to reside in the mitochondrial matrix; its expression restored normal IMM morphology and mitochondrial dynamics. Furthermore, this Mcl-1 isoform is both necessary for normal mitochondrial respiration and ATP production, and its expression enhances the assembly of F1F0-ATP synthase oligomers (Perciavalle et al., 2012). The latter observation, in particular, is similar to the emerging picture of Bcl-xL. However, while the anti-apoptotic function of Bcl-xL appears to be tied to its role in promoting mitochondrial metabolism, the matrix-localized, metabolism-modulating species of Mcl-1 fails to protect cells from chemotherapy-induced apoptosis (Perciavalle et al., 2012). In contrast, a mutant of Mcl-1 that localizes to the OMM, but not the matrix, maintains its anti-apoptotic function (Perciavalle et al., 2012). Thus, Mcl-1, while performing metabolic functions inside the mitochondria, seems to conform to the canonical view of anti-apoptotic Bcl-2 proteins by inhibiting apoptosis at the level of Bax/Bak on the mitochondrial surface.
Prior to this recent work on Mcl-1’s function in the matrix, Mcl-1 had been characterized as a metabolically controlled protein: Mcl-1 protein stability is affected by the presence of glucose, such that in the absence of glucose, glycogen synthase kinase-3 (GSK3) phosphorylates and targets Mcl-1 for degradation via the proteasome. Conversely, the stimulation of glucose uptake by growth factors leads to the inhibition of GSK3 and consequent stabilization of Mcl-1 (Maurer et al., 2006; Zhao et al., 2007). While in the context of apoptosis, the stabilization of Mcl-1 had been considered to be a mechanism to protect growth factor-stimulated cells, the metabolic consequences of glucose-mediated Mcl-1 stabilization have yet to be explored. Given that growth factor stimulation induces cell proliferation, which carries with it the need for increased biosynthetic metabolism and efficient mitochondrial energy production, it seems plausible that glucose-mediated Mcl-1 stabilization might serve the metabolic needs of proliferating cells. In addition, determining whether the ratio of OMM- to matrix-localized Mcl-1 depends on circumstances (e.g., proliferation versus quiescence) may be illuminating, as this ratio may be dictated by the metabolic demands of a given cell.
Metabolic regulation of apoptosis upstream of the Bcl-2 family
The tumor suppressor p53
As one of the most studied proteins in the cell, the tumor suppressor p53 has been extensively characterized as a stress-induced, pro-apoptotic protein. Its most well characterized role in apoptosis is as a transcriptional activator of certain pro-apoptotic Bcl-2 family proteins, including Bax, Puma, and Noxa. In addition, p53 can act in the cytosol as a direct activator of MOMP by engaging directly with Bax/Bak (Chipuk et al., 2004). Adding new levels of complexity to p53, recent work suggests that p53’s role in cell stress is quite nuanced, such that the p53-dependent response to stress can vary from apoptosis, to senescence, autophagy, or metabolic adaption (reviewed in detail by (Gottlieb and Vousden, 2010)).
p53 is activated by metabolic stress. Under low nutrient conditions, the activation of AMP-activated protein kinase (AMPK) induces both p53 transcription and stabilization of p53 protein (Jones et al., 2005; Okoshi et al., 2008). p53 induction in this scenario appears to result in the general activation of catabolic pathways in order to sustain energy levels under low nutrient conditions. More specifically, there is accumulating evidence that p53 can impact several metabolic pathways to tailor a cell’s response to this metabolic stress (Gottlieb and Vousden, 2010): p53 inhibits glycolytic flux by suppressing the expression of the insulin receptor and glucose transporters (Kawauchi et al., 2008; Schwartzenberg-Bar-Yoseph et al., 2004). Additionally, activation of p53 results in the induction of TP53-induced glycolysis and apoptosis regulator (TIGAR), which functions as a fructose-2,6-bisphophatase. Thus, TIGAR antagonizes the 3rd step in glycolysis, the conversion of fructose-6-phosphate to fructose-2,6-bisphosphate (see figure 1). Blockage at this step causes an accumulation of fructose-6-phosphate, which can be isomerized back to G6P to promote pentose phosphate pathway activity. Indeed, the net metabolic effect of TIGAR induction seems to be the rerouting of glucose into the oxidative arm of the PPP, which generates reduced glutathione and neutralizes toxic reactive oxygen species (ROS). Consistent with this, TIGAR expression leads to suppression of ROS-induced cell death (Bensaad et al., 2006). In addition to its effects on glycolysis, p53 promotes oxidative phosphorylation (Matoba et al., 2006; Okamura et al., 1999; Stambolsky et al., 2006) and thereby seems to favor energy production through the TCA cycle, as opposed to glycolysis. Furthermore, p53 promotes the use of glutamine as a metabolic fuel (Hu et al., 2010; Suzuki et al., 2010) and promotes the oxidation of fatty acids (Buzzai et al., 2007; Ide et al., 2009); both potential adaptive responses to promote TCA cycle-mediated generation of energy during periods of nutrient scarcity.
These observations clearly expand the circle of p53 influence beyond traditional associations with DNA damage and apoptosis induction, and implicate it as a pivotal switch in cellular metabolism. Notably, the p53-induced pattern of metabolic regulation (suppression of glycolytic flux, upregulation of TCA cycle) is in stark contrast to the high levels of aerobic glycolysis (Warburg effect) commonly observed in cancerous tissue, suggesting that the deletion of p53 in tumors may serve a metabolic function (Gottlieb and Vousden, 2010). Additionally, the p53-regulated metabolic adaptations described above appear to cast p53, at least under certain circumstances, as a survival factor. In this regard, it is interesting to note that p53 has been shown to both promote and antagonize the induction of autophagy (Crighton et al., 2006; Scherz-Shouval et al., 2010; Tasdemir et al., 2008; Yee et al., 2009), a pro-survival process by which cells recycle their own organelles and protein to be used as fuel during periods of nutrient scarcity, again suggesting that p53 may toggle between promoting survival or cell death, likely depending on the intensity and/or duration of the stress (Maddocks and Vousden, 2011; Scherz-Shouval et al., 2010).
Sirtuins, metabolism and cell death
Sirtuins are a family of lysine deacetylases and mono-ADP ribosyltransferases (Sirt1-7 in mammals) that use NAD+ as a co-substrate to target numerous cellular proteins (for review, see (Guarente, 2011)). As a group, they have been implicated in several diseases including, cancer, metabolic syndrome, and Alzheimer’s disease, and Sirtuins are thought to mediate many of the metabolic effects of caloric restriction (Guarente, 2011). Given their dependence on NAD+, a metabolite and hydride-transferring cofactor in the redox reactions of metabolism, Sirtuins are an intriguing signaling link between cell fate and metabolism.
Five of the seven Sirtuins (Sirt1, 3, 4, 5, 6) have been reported to modulate metabolic pathways (reviewed in (Li and Kazgan, 2011)). Sirt1, in particular, seems to have wide-ranging metabolic effects. In response to long term caloric restriction, Sirt1 increases hepatic glucose levels (via gluconeogenesis), stimulates fatty acid oxidation, and inhibits lipogenesis, while the mitochondria-localized Sirt3 enhances mitochondrial lipid catabolism and stimulates the urea cycle (reviewed in (Chalkiadaki and Guarente, 2012)). Additionally, Sirt1 has been heavily implicated in the regulation of cell death. Sirt1 suppresses cell death through the modulation of forkhead-dependent transcriptional pathways in response to oxidative stress and nutrient deprivation (Brunet et al., 2004; Motta et al., 2004) and a similar function was reported for Sirt2 (Wang et al., 2007). Sirt1 also promotes survival by deacetylating and suppressing the nuclear translocation of p53, which, in turn, prevents p53 from upregulating its pro-apoptotic target genes (Luo et al., 2001; Vaziri et al., 2001).
Like Sirt1, Sirt3 has been characterized as a modulator of metabolism. Among its metabolic functions, Sirt3 promotes oxidative phosphorylation by deacetylating complexes I and II (Ahn et al., 2008; Cimen et al., 2010), and promotes fatty acid oxidation, under fasting conditions, by deacetylating LCAD (Hirschey et al., 2010). Additionally, Sirt3 deacetylates and promotes the activity of Mn Superoxide dismutase (MnSOD), a mitochondrial ROS scavenger (Tao et al., 2010). Interestingly, stimulation of MnSOD may counterbalance the ROS produced as a result of the Sirt3-mediated increase in oxidative phosphorylation. In line with a role in suppressing oxidative stress, Sirt3 deacetylates and activates mitochondrial isocitrate dehydrogenase (IDH2), leading to the production of NADPH. This function of Sirt3 protects cells from oxidative damage in a mouse model of age-related hearing loss (Someya et al., 2010).
Taken alone, the antioxidant functions of Sirt3 cast it as a pro-survival enzyme, a characteristic associated with oncogenes. However, higher levels of ROS caused by Sirt3 deletion appear to be linked to genomic instability, which renders cells more susceptible to oncogenic transformation (Kim et al., 2010; Tao et al., 2010). In addition, Sirt3 expression is commonly lost in tumors (Finley et al., 2011) and deletion of Sirt3 appears to promote aspects of cancer metabolism, as Sirt3 −/− MEFs display increased glycolysis and decreased oxidative phosphorylation (Finley et al., 2011; Kim et al., 2010). Furthermore, depletion of Sirt3 in tissue culture cells is reported to increase the association of hexokinase II to mitochondria, and thereby block the activation of Bax-mediated MOMP (Verma et al., 2012). Together, the metabolic and apoptosis-regulating functions of Sirt3 have begun to frame this enzyme as a tumor suppressor.
While Sirtuin activity can be modulated by phosphorylation (North and Verdin, 2007; Sasaki et al., 2008), NAD+ levels seem to play a central role, such that turning NAD+ levels up or down is sufficient to trigger a corresponding rise or fall in Sirtuin activity (Imai, 2009; Revollo et al., 2004). Therefore, as varying nutrient levels cause fluctuations in NAD+, Sirtuins are poised to translate these changes to the molecular level by modifying the function of their substrates (Guarente, 2011), resulting in the modulation of cell survival and metabolism pathways described above. Thus, the connection between NAD+ metabolism and the cell fate-modulating functions of Sirtuins represent a clear example of metabolism-to-cell fate crosstalk.
In mammals, NAD+ can be generated de novo from L-tryptophan and/or through salvage pathways, one of which involves the rate-limiting nicotinamide phosphoribosyltransferase (NAMPT), an enzyme that has gained notoriety as a potential anti-cancer target (Bi and Che, 2010). The NAMPT-mediated salvage pathway is the primary source of NAD+ synthesis in mammals (Collins and Chaykin, 1972; Rongvaux et al., 2003), and NAMPT expression is increased by caloric restriction, resulting in an increase in mitochondrial NAD+ levels (Yang et al., 2007). Importantly, the NAMPT-mediated increase in mitochondrial NAD+ causes marked resistance to cell death, which requires the mitochondria-localized Sirtuins, Sirt3 and Sirt4 (Yang et al., 2007). The PTM that most Sirtuins antagonize, lysine acetylation, is also metabolite-derived, from acetyl-CoA, suggesting that different modes of metabolism may communicate to cell death effectors both via the regulation of enzymes (e.g., NAD+) and more directly through regulating the availability of donor substrates (e.g., acetyl-CoA). Indeed, simply increasing the levels of cellular acetyl-CoA (by the addition of citrate to cells, for example) is sufficient to modulate protein acetylation and affect cell survival (Wellen et al., 2009; Yi et al., 2011). In addition to acetylation, recent studies show that Sirt5 antagonizes lysine succinylation and malonylation (Du et al., 2011; Peng et al., 2011), which, like acetylation, are metabolite (acyl-CoA)-derived modifications. Furthermore, other metabolite-derived PTMs, such as glycosylation and lipidation, likely also influence core cellular pathways. Indeed, in response to tumor cell hypoxia, glycosylation was recently shown to suppress phosphofructokinase-1 (PFK1), leading to a redirection of glucose through the PPP and the consequent generation of NADPH. In this context, an increase in PPP-generated NADPH protected cells from oxidative stress-induced cell death (Yi et al., 2012). Although the precise functions and breadth of many of these PTMs are still emerging, it underscores how fluctuations in metabolite levels (e.g., acyl-CoA, glucose, N-acetyl-D-glucosamine) may directly communicate to cell fate pathways (for more detail, we recommend a recent review on this topic (Wellen and Thompson, 2012)).
Caspase-2
Despite being one of the most evolutionarily conserved caspases, caspase-2 continues to be somewhat enigmatic. Its precise function and the contexts in which it plays a central role in promoting apoptosis have been hard to pin down. These issues have been well documented in various reviews on the topic (Bouchier-Hayes, 2010; Krumschnabel et al., 2009a; Krumschnabel et al., 2009b), and recent work suggests that caspase-2 may have novel functions in promoting anti-oxidant defense (Shalini et al., 2012), and regulating cell cycle checkpoints (Ho et al., 2009; Kumar, 2009; Oliver et al., 2011). In the apoptotic cascade, Caspase-2 functions as an apical caspase, upstream of MOMP, that cleaves and activates Bid (generating tBid) to promote Bax/Bak activation (Nutt et al., 2005; (Bonzon et al., 2006; Bouchier-Hayes et al., 2009; Harvey et al., 1997). Strikingly, however, the only overt developmental defect observed in the caspase-2 knockout mouse was an overabundance of oocytes, suggesting that caspase-2 may play a prominent role in this tissue (Bergeron et al., 1998; Morita and Tilly, 1999).
Building on the oocyte-caspase-2 connection, studies in the Xenopus egg/oocyte system have shown that caspase-2 activation is metabolically controlled. Depletion of metabolites in egg extracts over time leads to spontaneous “cell free” apoptosis, characterized by caspase-2 activation, MOMP, and downstream caspase activation. Supplementation of egg extracts with metabolites (e.g., glucose-6-phosphate) suppresses caspase-2 activation by triggering a CamKII-dependent phosphorylation within the caspase-2 prodomain (Ser135, Xenopus numbering) (Nutt et al., 2005). The Ser135 phosphorylation is protected from PP1-mediated removal by the binding of 14-3-3ζ, which thereby maintains caspase-2 suppression (Nutt et al., 2009). Thus the removal of 14-3-3ζ from caspase-2 appears to be a key step toward caspase-2 activation in the oocyte.
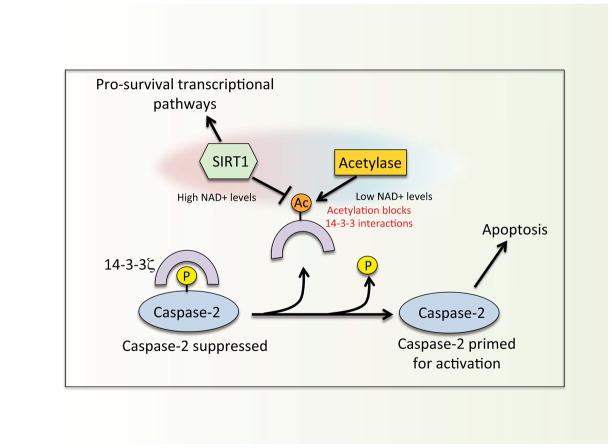
Recent experiments implicated NAD+ dependent Sirtuin deacetylase activity (see below for discussion on Sirtuins) in controlling 14-3-3ζ binding to caspase-2. Treatment of egg extract with nicotinamide, a pan Sirtuin inhibitor, accelerated 14-3-3ζ release from caspase-2, and a biotin switch-based approach later identified 14-3-3ζ as a target of Sirt1 deacetylase (Andersen et al., 2011). As the egg extract ages, 14-3-3ζ acetylation (on lysine residues) occurs concomitantly with its release from caspase-2 (Andersen et al., 2011). Correspondingly, 14-3-3ζ-directed deacetylase activity declines with time in the extract, and is stimulated by adding excess G6P, suggesting that a decrease in metabolically regulated Sirt1 activity leads to 14-3-3ζ acetylation and dissociation from caspase-2. Interestingly, the positive charges at specific lysine residues (e.g., Lys49) on 14-3-3ζ are critical for numerous 14-3-3ζ interactions (Fu et al., 2000), and neutralization of this charge by an acetyl group renders 14-3-3ζ less able to bind phospho-proteins (Choudhary et al., 2009). All together, these observations suggest a model in which the regulation of caspase-2 may integrate multiple metabolic signals: The production of NADPH via the PPP stimulates CaMKII-mediated phosphorylation of caspase-2, while NAD+ dependent Sirtuin activity prevents dephosphorylation by promoting the interaction between 14-3-3ζ/caspase-2 (figure 3).
Figure 3. Model for the metabolic regulation of caspase-2 in Xenopus eggs/oocytes.
High NAD+ levels stimulate Sirt1 deacetylase activity, which, in turn, activates pro-survival transcriptional pathways. Additionally, Sirtuin deacetylase activity maintains 14-3-3 in a deacetylated state, permitting binding between 14-3-3 and caspase-2. As NAD+ levels drop, Sirtuin activity diminishes and 14-3-3 becomes acetylated at lysine residues critical for protein-protein interactions. This disrupts 14-3-3/caspase-2 binding, and frees caspase-2 to become active in response to a stress stimulus.
Conclusion
The decision of a cell to live or die by apoptosis is clearly influenced by the availability of nutrients and the metabolic pathways active within the cell. In light of this, it may not be completely unexpected that Bcl-2 proteins and other regulators of apoptosis have dual metabolic and apoptotic functions and in some cases, such as Bcl-xL and Noxa, may exert their effect on cell death, at least in part, by regulating metabolism. Furthermore, this emerging theme of Bcl-2 protein duality may only represent a small fraction of crosstalk between metabolic and apoptotic pathways, as metabolism feeds back to regulate cell fate-controlling proteins through metabolite derived PTMs such as acetylation and glycosylation (areas that are still largely unexplored). Indeed, an understanding of how these metabolite-derived PTMs differ across the proteome between diseased and normal tissue and affect apoptotic-signaling pathways will be illuminating. Moreover, as this field is pushed forward, it will be critical to apply our understanding of the metabolism-apoptosis connection to the development of therapeutics for diseases in which metabolic alterations precede the apoptotic loss of critical tissues/cells, as seen in Alzheimer’s disease and diabetes. In this regard, the studies presented here suggest that therapeutic intervention to prevent metabolic alterations in these tissues may be an effective way to prevent downstream tissue degeneration via apoptosis (rather than therapeutically targeting apoptosis itself). Conversely, the development of metabolism-targeted therapies to enhance cell death in tumors is well under way. All together with the innumerable metabolites and complex circuitry of metabolism, the recent examples of molecular crosstalk between apoptosis and metabolism highlighted here illustrate what will be a very challenging and important field of study for years to come.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Thompson JW, Lindblom KR, Johnson ES, Yang C-S, Lilley LR, Freel CD, Moseley MA, Kornbluth S. A biotin-switch based approach identifies 14-3-3z as a target of Sirt1 in the metabolic regulation of caspase-2. Mol Cell. 2011 Sep 2;43(5):834–42. doi: 10.1016/j.molcel.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, et al. Defects in regulation of apoptosis in caspase-2- deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi TQ, Che XM. Nampt/PBEF/visfatin and cancer. Cancer biology & therapy. 2010;10:119–125. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD. Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol Biol Cell. 2006;17:2150–2157. doi: 10.1091/mbc.E05-12-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boohaker RJ, Zhang G, Carlson AL, Nemec KN, Khaled AR. BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells. American journal of physiology. Cell physiology. 2011;300:C1466–1478. doi: 10.1152/ajpcell.00325.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchier-Hayes L. The role of caspase-2 in stress-induced apoptosis. Journal of cellular and molecular medicine. 2010;14:1212–1224. doi: 10.1111/j.1582-4934.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchier-Hayes L, Oberst A, McStay GP, Connell S, Tait SW, Dillon CP, Flanagan JM, Beere HM, Green DR. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol Cell. 2009;35:830–840. doi: 10.1016/j.molcel.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochimica et biophysica acta. 2008;1777:877–881. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nature reviews. Endocrinology. 2012;8:287–296. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax- independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247:778–783. [PubMed] [Google Scholar]
- Colombini M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochimica et biophysica acta. 2010;1797:1239–1244. doi: 10.1016/j.bbabio.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annual review of pharmacology and toxicology. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harbor perspectives in biology. 2010;2:a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. The New England journal of medicine. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Butt AJ, Kumar S. Functional activation of Nedd2/ICH-1 (caspase-2) is an early process in apoptosis. J Biol Chem. 1997;272:13134–13139. doi: 10.1074/jbc.272.20.13134. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci U S A. 2009;106:5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CR, Yang-Yen HF. The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity. FEBS Lett. 2010;584:3323–3330. doi: 10.1016/j.febslet.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Ide T, Brown-Endres L, Chu K, Ongusaha PP, Ohtsuka T, El-Deiry WS, Aaronson SA, Lee SW. GAMT, a p53-inducible modulator of apoptosis, is critical for the adaptive response to nutrient stress. Mol Cell. 2009;36:379–392. doi: 10.1016/j.molcel.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Imai S. The NAD World: a new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell biochemistry and biophysics. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Bui T, White C, Madesh M, Krawczyk CM, Lindsten T, Hawkins BJ, Kubek S, Frauwirth KA, Wang YL, et al. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity. 2007;27:268–280. doi: 10.1016/j.immuni.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. The Journal of cell biology. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Bax affects production of reactive oxygen by the mitochondria of non-apoptotic neurons. Experimental neurology. 2007;204:458–461. doi: 10.1016/j.expneurol.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Krumschnabel G, Manzl C, Villunger A. Caspase-2: killer, savior and safeguard-emerging versatile roles for an ill-defined caspase. Oncogene. 2009a doi: 10.1038/onc.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumschnabel G, Sohm B, Bock F, Manzl C, Villunger A. The enigma of caspase-2: the laymen’s view. Cell Death Differ. 2009b;16:195–207. doi: 10.1038/cdd.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more? Nat Rev Cancer. 2009;9:897–903. doi: 10.1038/nrc2745. [DOI] [PubMed] [Google Scholar]
- Lam M, Bhat MB, Nunez G, Ma J, Distelhorst CW. Regulation of Bcl-xl channel activity by calcium. J Biol Chem. 1998;273:17307–17310. doi: 10.1074/jbc.273.28.17307. [DOI] [PubMed] [Google Scholar]
- Li M, Wang AJ, Xu JX. Redox state of cytochrome c regulates cellular ROS and caspase cascade in permeablized cell model. Protein and peptide letters. 2008;15:200–205. doi: 10.2174/092986608783489490. [DOI] [PubMed] [Google Scholar]
- Li X, Kazgan N. Mammalian sirtuins and energy metabolism. Int J Biol Sci. 2011;7:575–587. doi: 10.7150/ijbs.7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman XH, McDonnell MA, Kosloske A, Odumade OA, Jenness C, Karim CB, Jemmerson R, Kelekar A. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol Cell. 2010;40:823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Lu Y, Rolland SG, Conradt B. A molecular switch that governs mitochondrial fusion and fission mediated by the BCL2-like protein CED-9 of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108:E813–822. doi: 10.1073/pnas.1103218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Maddocks OD, Vousden KH. Metabolic regulation by p53. J Mol Med (Berl) 2011;89:237–245. doi: 10.1007/s00109-011-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EF, Rathmell JC. Cell metabolism: an essential link between cell growth and apoptosis. Biochimica et biophysica acta. 2011;1813:645–654. doi: 10.1016/j.bbamcr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Xu Q, Velours J, Reed JC. The Mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17. doi: 10.1006/dbio.1999.9344. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. Mitotic regulation of SIRT2 by cyclin-dependent kinase 1- dependent phosphorylation. J Biol Chem. 2007;282:19546–19555. doi: 10.1074/jbc.M702990200. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Buchakjian MR, Gan E, Darbandi R, Yoon SY, Wu JQ, Miyamoto YJ, Gibbons JA, Andersen JL, Freel CD, et al. Metabolic control of oocyte apoptosis mediated by 14-3-3zeta-regulated dephosphorylation of caspase-2. Dev Cell. 2009;16:856–866. doi: 10.1016/j.devcel.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S, Ng CC, Koyama K, Takei Y, Arakawa H, Monden M, Nakamura Y. Identification of seven genes regulated by wild-type p53 in a colon cancer cell line carrying a well-controlled wild-type p53 expression system. Oncology research. 1999;11:281–285. [PubMed] [Google Scholar]
- Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283:3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- Oliver TG, Meylan E, Chang GP, Xue W, Burke JR, Humpton TJ, Hubbard D, Bhutkar A, Jacks T. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell. 2011;43:57–71. doi: 10.1016/j.molcel.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Molecular & cellular proteomics: MCP. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, Struys EA, Klipp E, Jakobs C, Breitenbach M, Lehrach H, et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. Journal of biology. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. BioEssays: news and reviews in molecular, cellular and developmental biology. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ZT, Parrish AB, Wright KM, Margolis SS, Marks JR, Deshmukh M, Kornbluth S. Enhanced sensitivity to cytochrome c-induced apoptosis mediated by PHAPI in breast cancer cells. Cancer Res. 2006;66:2210–2218. doi: 10.1158/0008-5472.CAN-05-3923. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Weidberg H, Gonen C, Wilder S, Elazar Z, Oren M. p53- dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc Natl Acad Sci U S A. 2010;107:18511–18516. doi: 10.1073/pnas.1006124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Wilson C, Puccini J, Ho L, Kumar S. Impaired antioxidant defence and accumulation of oxidative stress in caspase-2-deficient mice. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolsky P, Weisz L, Shats I, Klein Y, Goldfinger N, Oren M, Rotter V. Regulation of AIF expression by p53. Cell Death Differ. 2006;13:2140–2149. doi: 10.1038/sj.cdd.4401965. [DOI] [PubMed] [Google Scholar]
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- Tanner EA, Blute TA, Brachmann CB, McCall K. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development. 2011;138:327–338. doi: 10.1242/dev.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Mueckler M. Glucose transporters in the 21st Century. American journal of physiology. Endocrinology and metabolism. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology. 2012 doi: 10.1016/j.cbpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Verma M, Shulga N, Pastorino JG. Sirtuin-3 Modulates Bak/Bax Dependent Apoptosis. Journal of cell science. 2012 doi: 10.1242/jcs.115188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- Woodcock J. Sphingosine and ceramide signalling in apoptosis. IUBMB life. 2006;58:462–466. doi: 10.1080/15216540600871118. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax- induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135–1145. doi: 10.1038/cdd.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CH, Pan H, Seebacher J, Jang IH, Hyberts SG, Heffron GJ, Vander Heiden MG, Yang R, Li F, Locasale JW, et al. Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival. Cell. 2011;146:607–620. doi: 10.1016/j.cell.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, Wofford JA, Dimascio LN, Ilkayeva O, Kelekar A, et al. Glycogen synthase kinase 3alpha and 3beta mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol. 2010;21:566–574. doi: 10.1016/j.semcdb.2010.01.002. [DOI] [PubMed] [Google Scholar]