Abstract
Protein-based biomaterials are an important class of materials for applications in biotechnology and medicine. The exquisite control of their composition, stereochemistry, and chain length offers unique opportunities to engineer biofunctionality, biocompatibility, and biodegradability into these materials. Here, we report the synthesis of a thermally responsive peptide polymer-based hydrogel composed of a recombinant elastin-like polypeptide (ELP) that rapidly forms a reversibly cross-linked hydrogel by the formation of intermolecular disulfide cross-links. To do so, we designed and synthesized ELPs that incorporate periodic cysteine residues (cELPs), and show that cELPs are thermally responsive protein polymers that display rapid gelation under physiologically relevant, mild oxidative conditions. Gelation of cELPs, at concentrations as low as 2.5 wt%, occurs in ~2.5 min upon addition a low concentration of hydrogen peroxide (0.3 wt%). We show the utility of these hydrogels for the sustained release of a model protein in vitro, and demonstrate the ability of this injectable biomaterial to pervade tumors to maximize tumor coverage and retention time upon intratumoral injection. cELPs represent a new class of injectable reversibly cross-linked hydrogels with properties intermediate between ELP coacervates and chemically cross-linked ELP hydrogels that will find useful applications in drug delivery and tissue engineering.
Keywords: hydrogel, thermally responsive material, cross-linking, drug delivery
1. Introduction
Injectable hydrogels formed by in situ chemical polymerization or by sol-gel phase transition [1–5] are of increasing interest for drug delivery because they have the attractive feature of only requiring an injection to form a depot in vivo, which avoids the need for surgical implantation that is required for prefabricated sustained release implants [6–8]. An injectable biomaterial for the formation of an in situ depot should meet the following requirements: (1) the material should be soluble upon administration; (2) it should start to gel within minutes upon in vivo injection; (3) the gel should be non-cytotoxic and (4) bioresorbable; and (5) the degradation products should be non-toxic. In addition to these material requirements, the system should be able to (6) entrap a high enough concentration of a drug of interest and exhibit release kinetics that can be optimized at the material design level for the application of interest, which is dictated by the drug and its intended therapeutic function.
Recombinant peptide polymers provide an attractive route for the design of such materials as they are nontoxic, biodegradable, and bioresorbable. We are interested in the design of in situ depots using a class of recombinant peptide polymers called elastin-like polypeptides (ELPs). ELPs, a class of artificial peptide polymers inspired by the amino acid sequence of tropoelastin, are composed of oligomeric repeats of the pentapeptide sequence Val-Pro-Gly-Xaa-Gly — where Xaa is any amino acid except Pro. ELPs are attractive as injectable biomaterials because they undergo a soluble to insoluble phase transition when heated above a tunable transition temperature (Tt; also called lower critical solution temperature (LCST)).
To date, two approaches have been taken to form ELP-based depots for drug delivery. In the first approach, ELPs are designed to undergo their inverse phase transition under physiological conditions, so that a solution of ELP, upon injection in vivo, forms a viscous insoluble “coacervate”. We have shown that an ELP coacervate is retained for up to a week in vivo [9, 10] and can entrap and release drugs and entrap cells for regenerative medicine applications [11, 12]. While this approach is attractive due to its simplicity, ELP coacervates are not chemically cross-linked, and hence have poor structural stability and mechanical properties, features that may be necessary for some applications.
In a second, alternative approach, we and others have previously reported that ELPs can be engineered to form hydrogels by chemical, enzymatic, and photo/γ-irradiated cross-linking [13–16]. Some block co-polymers of ELPs have also been shown to form physically cross-linked hydrogels [17]. However, in all of these studies, heat, high polypeptide concentrations (over 20 wt%), or exposure to organic solvents to dissolve cross-linkers were required to drive gel formation, which limits the in vivo application of these biomaterials.
Herein, we report the design and synthesis of a third alternative, reversible hydrogels that are formed from disulfide cross-linked ELPs from low concentrations of the ELP in aqueous solution at physiological conditions. We decided to focus on the design of reversibly cross-linked hydrogels because we believe that they may offer useful features that are intermediate between coacervates and covalently cross-linked gels such as intermediate rates of degradation and clearance, useful mechanical properties and importantly, circumventing the toxicity associated with most forms of chemical cross-linking — especially in gels designed to cross-link in situ — due to the presence of residual cross-linker. An important consideration in our design of reversibly cross-linked ELP hydrogels was the need to ensure that the ELPs could be cross-linked from a low concentration of the polypeptide at physiological conditions in the span of a few minutes or less, conditions that are critical to their use in a clinical setting.
To explore the design of ELPs that would meet these needs, ELPs were recombinantly synthesized with different periodicity of cysteine residues — the primary variable that controls the extent of cross-linking and hence the structural properties of the disulfide cross-linked network— and different hydrophobicity — a secondary parameter that controls the Tt of the ELP and hence stabilizes the ELP hydrogel via coacervation mediated dehydration. The thermal properties of these Cys-containing ELPs (cELPs) were examined in their sol and gel states, and their ability to form hydrogels under mild oxidative conditions was assessed by the tube inversion test and rheological measurements.
To investigate their utility for drug delivery, we explored two different scenarios for their use. First, we investigated their potential utility for sustained release of a protein therapeutic for local or systemic therapy. We found that a model protein, bovine serum albumin (BSA), could be entrapped in the hydrogels in vitro and that cELP hydrogels exhibited first-order kinetics of release of the entrapped BSA, with an initial burst followed by a prolonged gradual release, and with different release rates between hydrogels. Next, we examined the potential use of the disulfide cross-linked cELP hydrogels for the local delivery of radionuclides within tumors, and showed that the disulfide cross-linked hydrogel had higher radionuclide retention within the tumor and more homogeneous spatial coverage across the entire tumor by near infrared (NIR) fluorescent real-time imaging as a function of time as compared to a soluble, control ELP.
2. Materials and methods
2.1. Materials
The pET-25b(+) and pET-24a(+) expression vectors were purchased from Novagen Inc. (Madison, WI). Restriction enzymes, calf intestinal alkaline phosphatase (CIP), and pUC19 cloning vector were purchased from New England Biolabs (Beverly, MA). The DNA ligation kit (Mighty Mix) was obtained from Takara Bio Co. Ltd. Custom oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The DNA miniprep, gel purification, and PCR purification kits were purchased from Qiagen Inc. (Germantown, MD). Escherichia coli EB5alpha and BL21 (DE3) cells were purchased from EdgeBio (Gaithersburg, MD). All cultures were grown in Terrific Broth (TB) Dry medium from Mo Bio Laboratories, Inc. (Carlsbad, CA). Tris(2-carboxyethyl)phosphine (TCEP) hydrochloride, pre-coated Iodogen tubes, Slide-A-Lyzer dialysis cassette, and prepacked desalting columns (D-Salt dextran desalting column) were from Pierce Biotechnology, Inc. (Rockford, IL). [125I]Sodium iodide ([125I]NaI, ~17 Ci mg−1) was obtained from PerkinElmer Inc. (Boston, MA). Hydrogen peroxide (30 wt% solution) was purchased from EMD Millipore (Darmstadt, Germany). FITC-BSA was from Invitrogen (Carlsbad, CA). The IRDye800CW NHS ester infrared dye was from LI-COR Biosciences (Lincoln, NE).
2.2. ELP gene construction
The synthetic genes encoding six different ELP sequences were constructed as follows (Fig. S1). ELPs are described by the nomenclature ELP[XiYjZk-n], where n refers to the number of pentapeptide repeats, the capital letters in brackets are the single-letter amino acid code of guest residues along the length of the ELP, and their corresponding subscripts refer to the relative fraction of those guest residues. Genes encoding cELP1 and ELP6 were constructed using the RDL method [18]. In brief, short gene segments (ELP[A14VC-16] and ELP[A8G7V-16]) were assembled by annealing chemically synthesized oligonucleotides encoding the sense and antisense strands to form a gene cassette, which was then subcloned into the EcoRI and HindIII sites of pUC19. These DNA segments were oligomerized by RDL. The final oligomerized genes encoded 160 pentapeptides, which were then excised from the pUC19 by digestion with PflMI and BglI, and the genes were subcloned into the SfiI site of a modified pET-25b(+) vector. Genes encoding cELPs 2–4 and ELP5 were constructed using Pre-RDL method [19]. Briefly, short gene segments (ELP[V15C-16], ELP[V9C-10], ELP[V5C-6], and ELP[V-10]) were assembled by annealing oligonucleotides encoding the sense and antisense strands, which were then subcloned into the BseRI and AcuI sites of modified pET-24a(+). These genes were oligomerized by Pre-RDL in the expression vectors itself to obtain ELP genes encoding 160 and/or 168 pentapeptides. The expression vectors also contributed a short N-terminal sequence (SKGPG) and a C-terminal sequence to the ELP (WP or YGYGYGYGWP or YGYGYGYG) to facilitate radioiodination.
2.3. ELP expression and purification
The synthesis of ELPs was based on constitutive expression from the leaky T7 promoter in the expression host Escherichia coli BL21(DE3) cells. Typically, 1 L of TB media, supplemented with 100 μg mL−1 ampicillin or 50 μg mL−1 kanamycin, was inoculated with 50 mL of an overnight culture, and cultured for overnight at 37°C and 160 rpm. The resulting cultures were centrifuged, resuspended in 10 mL phosphate-buffered saline (PBS) and lysed by ultrasonication (Misonix, Farmingdale, NY). To precipitate genomic DNA with the insoluble cell debris, the lysate was supplemented with polyethyleneimine (at 1.2 wt%) and centrifuged at 16,000 g at 4°C. The supernatant containing ELPs was then purified by inverse transition cycling (ITC) as previously described [20]. Purification of cELPs by ITC was performed in 20 mM TCEP (pH 7.0) to prevent undesired disulfide bond formation. The purified ELPs were then dialyzed at 4°C for 16 h against PBS or water using 10-kDa molecular weight cut-off Slide-A-Lyzer dialysis cassettes, followed by lyophilization. The purity and molecular weight (MW) were characterized using SDS-PAGE stained with 0.5 M copper chloride. The concentration of ELPs was determined spectrophotometrically using the molar extinction coefficient (5,630 M−1cm−1 at 280 nm) for the sole tryptophan present in each ELP. The content of thiol moieties in the cELPs was quantified by 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) with L-cysteine as a standard. Briefly, the reactions were carried out in a total volume of 1 mL containing 1.5 μM cELP, 20 mM Tris-HCl (pH 8.0), and 0.2 mg mL−1 of DTNB. The reaction mixture was incubated at room temperature for 30 min, and the absorbance at 405 nm was then measured using a VICTOR3 multilabel counter (PerkinElmer Inc.).
2.4. Radioiodination and fluorescent labeling of ELPs
ELPs with a C-terminal YGYGYGYGWP sequence were radiolabeled with 125I by the IODO-Gen method, as previously reported [10, 21]. Briefly, 100 μL of ELP at ~700 μM in PBS was added to a pre-coated IODO-Gen tube containing 1.4 mCi [125I]NaI on ice, which was further incubated for 15 min. The reaction mixture was purified by gel filtration using a prepacked D-Salt dextran desalting column and eluted with PBS. An aliquot of the radioactive product was counted on a γ-counter (LKB-Wallac, Turku, Finland). After mixing with unlabeled ELP, the final radioactive dose of 125I-labelled ELPs ([125I]ELPs) for injection were 0.25 μCi μL−1 at an ELP concentration of 2.78 wt% ELP, with a specific activity of 9 μCi mg−1.
For in vivo imaging, the amine groups of the cELP at their N-terminus and in the Lys (K) residue in the N-terminal leader sequence (SKGPG) were reacted with the IRDye800CW NHS ester. cELP1 (54 mg, 0.86 μmol) was dissolved in 1.2 mL of dimethyl sulfoxide (DMSO) containing 8 μL of triethylamine. A solution of IRDye800CW NHS ester (1 mg, 0.86 μmol) in 0.3 mL of DMSO was added, and the reaction mixture was stirred for 1.5 h at room temperature. The product was then purified by dialysis against water using Slide-A dialyzer at 4°C for 1 h, followed by size-exclusion chromatography with a PD-10 column (GE Healthcare, Piscataway, NJ). The labeling ratio, determined spectrophotometrically using an extinction coefficient of 270,000 M−1cm−1 at 780 nm for the IRDye800CW in a 1:1 mixture of PBS: methanol, was 0.11. The final concentration for in vivo imaging experiments was adjusted to 3.75 wt% (600 μM) cELP1 and 1 μM IRDye800CW by mixing with unlabeled cELP1.
2.5. Thermally responsive behavior
The phase transition behavior of ELPs was characterized by monitoring the absorbance of 2.5 wt% ELP in PBS at 350 nm as a function of temperature (1°C min−1) on a temperature-controlled UV-Vis spectrophotometer (Cary 300 Bio, Varian instruments, Palo Alto, CA). The Tt is defined as the solution temperature at which the first derivative of the optical density (OD) at 350 nm as a function of temperature (d(OD) dT−1) was the maximum.
2.6. Hydrogel formation under oxidative conditions
Hydrogels were prepared by cross-linking cELPs via disulfide bond formation. Freshly prepared solutions of each oxidant (3 wt% H2O2; 10 mM sodium periodate (NaIO4); 500 mM potassium permanganate (KMnO4); 500 mM potassium ferricyanide (K3Fe(CN)6)) were added to a PBS solution of 2.78 wt% cELP at a 10:1 volume ratio of ELP:oxidant, and the mixture was gently vortexed. The final concentration of cELP was 2.5 wt%. Hydrogel formation was determined by the tube inversion test [22]. The formation of a gel was defined by the absence of fluidity upon inverting the vial after a defined time.
2.7. Rheological characterization
All rheological data were obtained using an AR G2 rheometer (TA Instrument, New Castle, DE) with an 8 mm parallel plate. cELP solutions in PBS (2.5 wt%) and H2O2 (0.3 wt%) were mixed in by pipetting in a test tube. The mixtures were loaded into the plate at 25°C, and the mixtures were then compressed to a height of 750 μm, and excess material was scraped from the edge of the parallel plate. The mixtures were then compressed to 720–740 μm and allowed to equilibrate for 5 min. Oscillatory frequency sweeps from 0.1 to 10 rad s−1 were carried out at a fixed strain (γ = 2.5%) within the linear region, which had been determined by strain sweep experiments.
2.8. In vitro release study
The release of FITC-BSA from cELP hydrogels was measured at 37°C. FITC-BSA (1 μM) was added to an aqueous cELP solution (2.5 wt%), and 0.3 wt% H2O2 was then added at 25°C to trigger hydrogel formation. After incubation for 30 min, BSA (4 mg mL−1) was added on top of the hydrogels, and the mixture was further incubated at 37°C. After 4, 8, 24, 48, 72, 96, 120, and 168 h, 10 μL of medium was withdrawn, and 10 μL of fresh BSA solution was added. The amount of released FITC-BSA was estimated using a NanoDrop 3300 fluorescence spectrometer (Thermo Fisher Scientific, Wilmington, DE).
2.9. Intratumoral injection of ELPs
Human pharynx squamous cells carcinoma (FaDu, ATCC) were cultured in minimal essential medium (MEM) containing 10% heat-inactivated fetal bovine serum and 1% antibiotics (streptomycin and penicillin) (Invitrogen). The cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C.
Female nude mice (Balb/C nu/nu) with an average body weight of about 20 g were purchased from NCI (Frederick, MD). Tumor leg xenografts were established by inoculation of 1×106 FaDu cells in 30 μL of DMEM medium without serum/phenol red, into the subcutaneous tissue using a 27-gauge needle. Tumors were allowed to grow to 150 ± 20 mm3 prior to intratumoral injection of ELPs. Mice were monitored for general well-being, weight, and tumor volume. All animal experiments were performed in accordance with the Duke University Institutional Animal Care and Use Committee.
Tumor retention of ELPs following intratumoral infusion was examined in nude mice bearing FaDu tumor xenografts using [125I]ELPs. Groups of 8–10 mice anesthetized with sodium pentobarbital (80 mg kg−1, i.p.) received intratumoral infusion of 2.5 wt% ELPs, 0.3 wt% H2O2 and 10 μCi per 150 mm3 of tumor volume of [125I]ELPs through a 27-gauge needle in a volume such that the ratio of tumor volume to injection volume was 3.75:1. At 4, 8, 24, 48, 72, 96, and 168 h post injection, the mice were placed in an ATOMLAB 400 Dose Calibrator (Biodex Medical Systems, Inc., Shirley, NY), and radioactivity levels were measured with the calibrator. The radioactivity level of [125I]ELP in the tumor was expressed as the % injected dose (ID) per tumor for each mouse at different time points.
2.10. In vivo NIR fluorescence imaging
Using two syringes connecting to a syringe pump (Harvard Apparatus, Holliston, MA; New Era Pump Systems Inc, Farmingdale, NY), IRDye800CW-labelled ELP was co-infused with H2O2 into FaDu tumors at a 2:1 ratio of tumor volume to the injected volume of 2.5 wt% ELP and 0.3 wt% H2O2 in saline. All mice were anesthetized with 2% isoflurane throughout the procedure. Images were collected at 4, 8, and 24 hour post-injection. NIR fluorescence imaging in live animals was performed with a Pearl Impulse Small Animal Imaging System (LI-COR Biosciences) and the images were acquired and analyzed with Pearl Impulse Software.
3. Results
3.1. Synthesis and characterization of cELPs
We designed and synthesized six ELPs with a molecular weight (MW) of 60–70 kDa (Table 1). Cys-containing ELPs (cELPs) 1–4 contain periodically interspersed cysteine residues along the ELP sequence, whereas ELPs 5 and 6 lack cysteine and served as negative controls. The different numbers of cysteine residues allowed the effect of cross-linking density on hydrogel properties to be examined. cELP1 was designed to be hydrophilic with a higher Tt than body temperature and cELPs 2–4 are hydrophobic and were designed to have a lower Tt than body temperature. Hydrophobic and hydrophilic ELPs without Cys residues were also synthesized as negative controls, and are termed ELPs to distinguish them from the cELPs.
Table 1.
Cys-containing ELPs
| ELP# | na | MW (kDa) | [XiYjZk] b | Ratio of VPGCG |
|---|---|---|---|---|
| 1 | 160 | 62.5 | A14VC | 1/16 |
| 2 | 160 | 66.5 | V15C | 1/16 |
| 3 | 160 | 66.5 | V9C | 1/10 |
| 4 | 168 | 69.3 | V5C | 1/6 |
|
| ||||
| 5 | 160 | 66.4 | V | 0 |
| 6 | 160 | 61.2 | A8G5 | 0 |
ELP[XiYjZk-n], where the capital letters in bracket are single-letter amino acid codes of a guest residue, their corresponding subscripts denote the ratios of that guest residues in the monomer, and n indicates the number of pentapeptides in the ELP.
Cysteine (C) residue available for cross-linking in each ELP is underlined. ELPs devoid of C are separated by a dashed line form the cELPs in the Table.
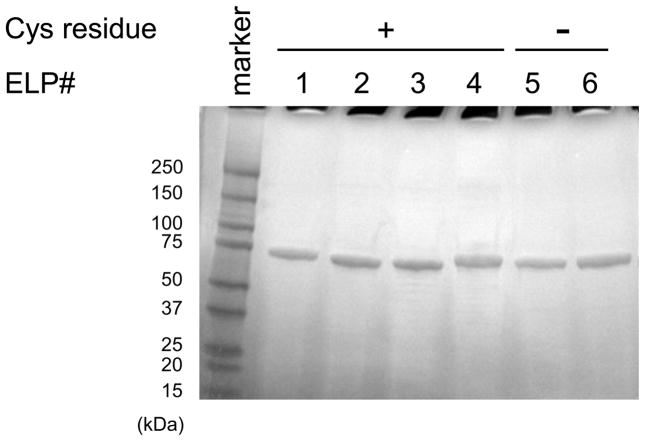
The overexpression of cELPs and ELPs without IPTG induction resulted in the efficient expression levels with typical purified yields of 100–200 mg L−1. All cELPs and ELP controls could be purified to greater than 95% purity with high yields by three rounds of ITC in the presence of TCEP (20 mM, pH7.0) and without undesired oligomerization due to disulfide formation (Fig. 1). Yields of purified cELPs 1–4 and ELPs 5 and 6 were 120, 150, 200, 160, 400, and 80 mg L−1 culture, respectively (Table 1). As shown in Table 2, the number of free thiol groups in all cELPs were close to their theoretical values (over about 80%), indicating that cELPs can be synthesized and purified from a bacterial host with high purity and yield, and without significant disulfide bond formation or irreversible oxidation of the thiols [23].
Fig. 1.
Copper-stained SDS-PAGE gel of cELPs. All cELPs were purified successfully by inverse transition cycling (ITC) using 20 mM tris(2-carboxyethyl)phosphine. The symbols + and − indicate the presence and absence of cysteine in the ELPs, respectively.
Table 2.
Theoretical and experimentally determined molar amounts of free thiol groups in 1 mol of cELP
| cELP# | Free thiol (mol)/1 mol CysELP
|
|
|---|---|---|
| Found | Theoretical | |
| 1 | 9.9 | 10 |
| 2 | 9.5 | 10 |
| 3 | 16.0 | 16 |
| 4 | 22.0 | 28 |
The content of thiol moieties of each Cys-containing ELP was quantified by 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) with L-cysteine served as a standard.
As shown in Fig. 2A, cELPs exhibited an inverse phase transition typical of single segment ELPs, although the reversibility of the transition is diminished (data not shown) at high temperatures as compared to ELPs due to accelerated disulfide bond formation upon coacervation of the cELPs. Fig. 2B summarizes the Tt of uncross-linked cELPs in PBS at 2.5 wt%. cELPs 2–4 and ELP5 have a Tt below body temperature (37°C), whereas cELP1 and ELP6 have a Tt well above body temperature.
Fig. 2.
Thermally responsive behavior of cELPs at 2.5 wt% in PBS. (A) Absorbance at 350 nm for all ELPs was measured under a temperature gradient of 1°C min−1 to determine the ELP inverse transition temperature (Tt). (B) Tt of uncross-linked cELPs in PBS at 2.5 wt%. The Tt was defined as the solution temperature that corresponded to the maximum in the first derivative of the absorbance (350 nm).
3.2. Hydrogel formation of cELPs by disulfide cross-linking
Hydrogel formation of the cELPs was judged by the tube inversion test. cELP1 (2.5 wt%) formed hydrogels at 37°C in the absence of an external oxidizer but gelation was slow, requiring over 30 hours to form a gel in the absence of an oxidant. This slow cross-linking process could be followed by SDS-PAGE (Fig. S2) for up to 8 h, after which time the high viscosity of the samples precluded further SDS-PAGE analysis. When the thiol groups were quantified by the DTNB method based on the formation of inter- and/or intramolecular disulfide bonds, the free thiol moieties in the hydrogel were significantly lower in comparison to their initial content in the uncross-linked, soluble cELP (less than 10% after 30 h incubation at 37°C; data not shown). These results confirmed that gelation proceeded via disulfide bond formation of thiol moieties, but that the gelation kinetics were very slow in the absence of an externally added oxidant.
To accelerate the gelation kinetics, so as to make this material potentially useful as an injectable gel-forming biomaterial, we screened a number of different oxidants such as potassium permanganate (KMnO4), potassium ferricyanide (K3Fe(CN)6), sodium periodate (NaIO4), and H2O2. These oxidants accelerated hydrogel formation to varying degrees as judged by the time required to form a cross-linked network by the tube inversion test: 3 seconds for 50 mM KMnO4, 3 hours for 50 mM K3Fe(CN)6, and 3 minutes for 1 mM NaIO4 and H2O2 (0.3 wt%). We selected H2O2 for all subsequent gelation studies, as it is a common, mild oxidant that is not toxic at low concentrations [24], and is produced in the body [25]. As expected, few residual thiols were detected in cELPs (2.5 wt%) after 1 min of incubation with 0.3 wt% H2O2, indicating that the thiol moieties in the cELPs were almost completely oxidized for all four cELPs.
Next, the effect of H2O2 on the gelation of cELPs was examined in detail. cELPs and H2O2 were mixed at 2.5 wt% and 0.3 wt%, respectively. The mixture was incubated on ice for 3 min, and then incubated at the indicated temperature for 10 min (Fig. 3A). All cELPs formed transparent hydrogels within the first 3 min on ice. We investigated the phase transition behavior of H2O2-triggered cELPs hydrogels as a function of temperature by turbidimetry (Fig. 3B). Hydrogels of 2.5 wt% cELPs with 0.3 wt% H2O2 were prepared in a cuvette on ice, and the hydrogels were then equilibrated at 15°C for 30 min, and the absorbance at 350 nm was then monitored at a heating rate of 1°C min−1. Interestingly, the thermally triggered phase transition behavior of cELPs was maintained in their gel state, although the polydispersity of the cross-linked network decreased the sharpness of the phase transition. Noteworthy, cELP1 displays phase transition at near body temperature only in the gel state. ELPs 5 and 6, which lack cysteine residues, did not form hydrogels in the presence of H2O2.
Fig. 3.
Effect of hydrogen peroxide (H2O2) on gelation. The final concentrations of cELP and H2O2 were 2.5 wt% and 0.3 wt%, respectively. (A) A mixture of cELP and H2O2 was incubated on ice for 3 min, and further incubated at 4°C and/or 37°C for 10 min, and the vials were then inverted to assess the fluidity of the ELP. The symbols + and − indicate the presence and absence of H2O2, respectively. (B) Thermally responsive behavior cELPs in the hydrogel state. The hydrogels were prepared in a cuvette on ice, and the resulting hydrogels were equilibrated at 15°C for 30 min before determining their turbidity profile. Data represent a typical result from three independent experiments.
3.3. Rheological properties of cELP hydrogels
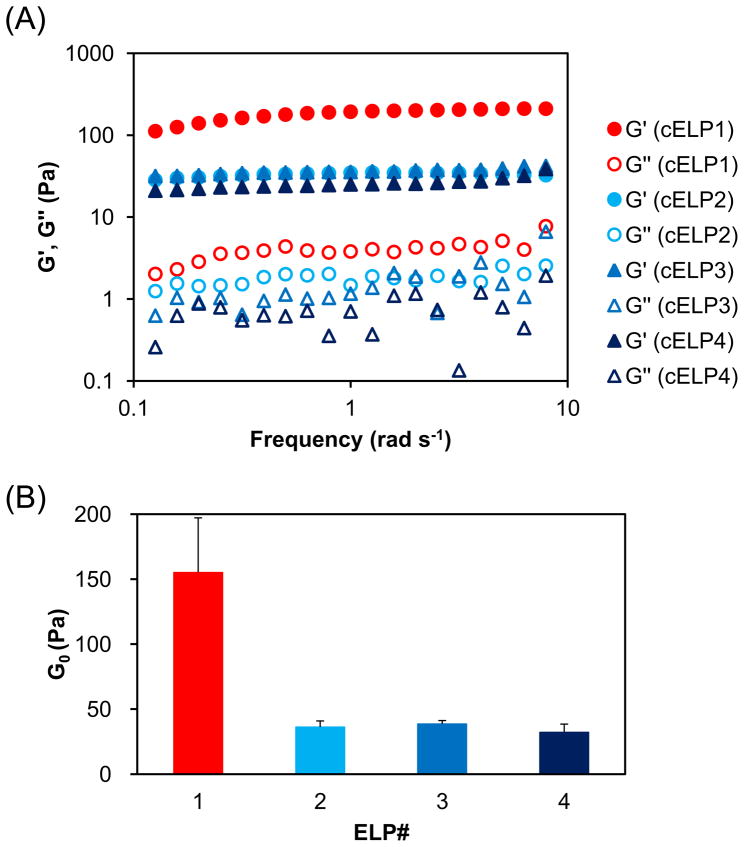
Hydrogels were prepared for rheology by mixing cELPs and H2O2 (2.5 wt% and 0.3 wt% final concentrations, respectively) and then linear oscillatory frequency sweep experiments were performed at 25°C. The storage modulus (G′) and loss modulus (G″) are plotted as a function of oscillation frequency in Fig. 4A. The dynamic mechanical spectra showed a frequency-independent G′ (frequencies ranging from 0.1 to 10 rad s−1), the magnitude of which greatly exceeded that of G″ across the same frequency range for all cross-linked cELP hydrogels, indicating the formation of a cross-linked elastic network [26]. In contrast, previous studies with uncross-linked ELP coacervates consistently showed G″ values that were greater than G′, indicating the absence of a cross-linked elastic network [11]. Together, these data for the cELPs are consistent with the elastic response expected of a cross-linked hydrogel, and they implicate disulfide cross-links in the mechanical response of the cELP hydrogels.
Fig. 4.
Rheological properties of cELP hydrogels. A mixture of 2.5 wt% cELP and 0.3 wt% H2O2 was loaded into the rheometer platen at 25°C, compressed to 720–740 μm, equilibrated for 5 min, and the rheological parameters were obtained by linear oscillatory frequency sweep experiments. (A) Storage modulus (G′) and loss modulus (G″) versus frequency for of cELP hydrogels. (B) Plateau modulus (G0) obtained from the plateau value of G′. Data shown are the mean ± SEM and are representative of three independent experiments.
3.4. In vitro BSA release from cELP hydrogels
The release profiles of a FITC conjugate of bovine serum albumin (FITC-BSA) encapsulated in cELP hydrogels was studied in vitro to explore the utility of these hydrogels as protein delivery systems. Figure 5A shows that the in vitro release of FITC-BSA from the different cELPs ranged from 20–40% after 4 h incubation in buffer. In contrast, ELP5 and ELP6, the two control ELPs that do not contain any Cys residues, showed complete release of FITC-BSA in 4 h. These results demonstrate the advantage of hydrogel formation for stable entrapment of a protein payload, as the control ELPs form viscous coacervates instead of disulfide cross-linked hydrogels.
Fig. 5.
In vitro FITC-BSA release from hydrogels of cELPs. ELPs pre-mixed with FITC-BSA were mixed with H2O2 at 25°C. BSA (4 mg mL−1) was added to the resulting hydrogels, and the mixture was incubated at 37°C. The amount of released FITC-BSA was quantified by the fluorescence intensity in the supernatant. (A) Percent release of FITC-BSA after 4 h. (B) Percent cumulative release of FITC-BSA from cELP hydrogels. Each data point represents the mean value ± SEM (n = 4–7).
The kinetics of release for the disulfide-cross-linked cELP hydrogels are shown in Figure 5B, which tracks the cumulative FITC-BSA released (%) as a function of time. Sustained release with significant difference in release rate among cELP hydrogels was observed. Following a small initial burst release, the hydrophilic hydrogel of cELP1 slowly released 100% of the loaded FITC-BSA over 5 days (120 h). Interestingly, hydrophobic hydrogels of cELPs 2 and 3 showed no apparent release after an initial burst release of ~40% FITC-BSA in the first 2 h, whereas the hydrophobic cELP4 hydrogel had a sustained release analogous to that of the hydrophilic hydrogel. After 7 days (168 h), the remaining FITC-BSA was still trapped in the hydrogel matrices of cELPs 2 and 3 and could not be completely released until the hydrogels were physically fractured (data not shown).
3.5. In situ gelation of cELPs
We carried out two pilot experiments to investigate the in vivo gelation of a cELP with the ultimate goal of developing an injectable depot for drug release. In the first experiment, we co-injected intramuscularly cELP1 (2.5 wt%) and H2O2 (0.3 wt%) into mice under anesthesia, together with bromophenol blue (0.005 wt%) to facilitate visualization of the gel. After 3 minutes, the mice were incised at the injection site to confirm hydrogel formation. The hydrogel at the injection site was easy to remove and manipulate (Fig. 6A), which demonstrated the rapid in situ gelation of intramuscularly administered cELP in vivo.
Fig. 6.
Rapid in situ-gelation of cELPs in vivo. The final concentrations of cELP and H2O2 were 2.5 wt% and 0.3 wt%, respectively for all in vivo studies. (A) Intramuscular co-injection of ELP1 with H2O2 into mice was carried out. Bromophenol blue was pre-mixed with ELP1 for visualization of the hydrogel. Three minutes after injection, the mice were incised to confirm hydrogel formation, and the hydrogel was excised from the injection site. (B) Tumor retention of ELP1 after intratumoral infusion of [125I]ELPs in a tumor-bearing mouse model. The ID%/tumor at 0, 4, 8, 24, 48, 72, 96 hour, and 1 week after administration are expressed as mean value ± SEM (n = 8–10; *, p<0.01). (C) Near-infrared in vivo imaging following intratumoral administration. The images at 8 hours after infusion are shown as pseudo-color-enhanced fluorescence intensity superimposed on the white light images. (D) Magnified images of mice 2 and 5 are shown in Fig. 6C. (E) Clearance of IRDye800CW-labelled cELP1. Fluorescence intensity in the tumor (mean pixel fluorescence) at 0, 4, 8, and 24 hour post-injection; data was expressed as mean value ± SEM (n = 4–5). *, p<0.02.
Next, we studied tumor retention of H2O2-triggered cELP1 hydrogels by quantifying the radioactivity of radiolabeled cELP in FaDu tumor-bearing mice. There was a significant difference (p < 0.01, t-test) between the levels of radioactivity in the tumor infused with or without H2O2 as a function of time (Fig. 6B). At 72 h after administration, greater than 40% of ID of the cELP1 hydrogel remained in the tumor, in contrast to less than 10% in the control groups (cELP1 without H2O2 and ELP6 negative control). These results demonstrated that hydrogel formation by oxidation-triggered disulfide cross-linking of cELPs significantly enhances their radionuclide retention.
We also examined the spatiotemporal distribution of cELP1 after intratumoral injection using in vivo NIR imaging. Fig. 6C shows NIR in vivo images of mice (at 8 h) following intratumoral administration of IRDye800CW-labelled cELP1. The intratumoral distribution of IRDye800CW-labelled cELP1 was remarkably uniform, and retention across the entire tumor was only observed for disulfide cross-linked cELP1 hydrogels, whereas uncross-linked cELP1 mostly accumulated at the injection site (Fig 6D). Clearance of IRDye800CW-labelled cELP1 was also delayed in the presence of H2O2 (Fig. 6E), consistent with the results obtained with radiolabeled cELP.
4. Discussion
We have developed an injectable reversibly cross-linked hydrogel consisting of ELPs incorporating periodic cysteine residues to enable rapid, disulfide-mediated cross-linking in situ. Hydrophobic and hydrophilic ELPs containing different numbers of cysteine residues interspersed periodically along the ELP sequence were produced recombinantly in good yields and without undesired oligomerization (Fig. 1). The turbidity of cELP hydrogels significantly increased with the number of cysteine residues, despite the small difference in the Tt at the sol state among the hydrophobic cELPs 2–4 (20.5–23.0°C) (Figs. 2 and 3). These results suggest that intermolecular cross-linking was favored by the increased frequency of cysteine residues. The rheological properties of cELPs confirmed their rapid gelation under mild oxidative conditions (Fig. 4). The release profiles of the model protein, FITC-BSA, from cELP hydrogels indicated a sustained release pattern with different release rates among hydrogels (Fig. 5). For reasons that are as yet unclear to us, the release rate from the hydrogel having the possible highest cross-linking density (cELP4) was faster among the hydrophobic cELPs (cELPs 2–4) at 37°C. These release patterns were not observed at 4°C, and the profiles at 4°C indicated a sustained release rate depending on the hydrogel density (Fig. S3), revealing an interesting interplay between cross-linking density and the intrinsic phase behavior of ELP that may affect the microstructure of hydrogel. These results suggest that disulfide cross-linked ELP hydrogels may be a promising materials to design injectable depots for sustained release of protein and peptide therapeutics for systemic therapy, as the ELP depot shows favorable release kinetics of a model protein, and over time, the ELP depot is resorbed and degraded into amino acids in vivo [10, 27].
Another potential application of this injectable self-gelling ELP is as a radionuclide depot for local cancer therapy of tumors refractory to surgical removal. We confirmed that cELPs are useful injectable biomaterials in vivo by studying their intratumoral retention and homogeneity of tumor delivery (Fig. 6). cELP1 showed higher and more prolonged tumor retention and remarkably homogenous tissue distribution in the presence of H2O2 compared with cELP1 without H2O2, and a control ELP devoid of cysteine residues. This ideal homogenous distribution across the entire tumor, which we have only observed for soluble ELPs that are rapidly cleared from the tumor, contrasts with the localized depots obtained when exclusively relying on the intrinsic ultra-fast phase transition of ELPs to form an insoluble coacervate, while achieving comparable tumor retention over time [10]. Moreover, the gelation of cELPs is fast enough (< 2.5 min) to be useful in a clinical setting and offers an interesting alternative to the ultra-fast phase separation of LCST polymers to create an injectable depot.
We propose that the disulfide cross-linked ELP hydrogels presented herein could offer useful features as drug delivery systems as well as tissue engineering scaffolds. First, cELPs are injectable and capable of rapid, in-situ self-gelation at low concentrations. In contrast, mono- and multi-block Lys-containing ELPs, previously reported as potential injectable hydrogels, required a concentration of over 20 wt%, chemical cross-linkers, and heating for hydrogel formation [13, 28]. The sol should be of sufficiently low viscosity before administration to allow injection with minimal pain to the patient, a condition met by the cELPs. Second, the gelation of cELPs does not rely on exogenous cross-linkers; instead, the location and density of the cross-linking sites can be encoded into the ELP at the genetic level. Third, while thiolated polymers that are disulfide cross-linked to form hydrogels have been previously studied [29–33], cELP hydrogels offer the following advantages: a) they are genetically engineered protein-biomaterials that allow modification and precise control of the polymer chain composition, length, and stereochemistry; b) they allow the genetic fusion with any recombinant therapeutic protein or peptide of interest; and c) they permit tunable biodegradation, yielding non-toxic amino acid breakdown products.
The mild oxidative conditions required for the rapid hydrogelation of cELPs as formulated in this study are not expected to pose difficulties to the clinical translation of these injectable biomaterials. For instance, H2O2 is commonly used as an antiseptic for wound cleaning at concentrations that are 10 fold those used in our studies (i.e., 3 wt%). Additionally, it is well-known that H2O2 is rapidly metabolized by enzymes to oxygen and water that is non-toxic to cells, and is also a naturally occurring compound in animals [25]. Hence, we believe that a concentration of 0.3 wt% H2O2 is likely to be non-toxic, though further studies in an animal model are clearly needed to verify this claim in the context of a potential therapeutic application.
5. Conclusion
We have developed a thermally responsive, injectable biomaterial capable of in situ self-gelation. This biomaterial is composed of an ELP that contains multiple periodic cysteine residues that provide a means of chemical cross-linking through disulfide bond formation. The main advantages of this in situ self-gelling system over other injectable systems are the genetically engineered nature of the biomaterial that results in a polymer with a well-defined composition and MW, tunable cross-linking and biodegradability; the lack of organic solvent in hydrogel formation; the absence of unnatural degradation products; straightforward drug loading through disulfide mediated reversible attachment of drugs to the ELP or through direct fusion of protein-drugs at the genetic level. We anticipate that such cELP hydrogels will find applications in drug delivery, tissue engineering and regenerative medicine.
Supplementary Material
Gene and corresponding protein polymer sequences for all ELPs in this study. The genes were synthesized by oligomerization of a gene segment shown in blue. The polymerized gene region was flanked by short leader (SKGPG) and trailer (WP) peptide sequences that are needed for good expression yield and quantification of the ELP, respectively.
cELPs multimerize prior to hydrogel formation. The solution of cELP1 at 2.5 wt% was incubated at 37°C and analyzed by SDS-PAGE. Thirty μg of the protein was loaded on a lane. Higher molecular weight products appeared as a function of incubation time, as indicated by a square. The symbols + and − indicate the presence and absence of β-mercaptoethanol (β-ME), respectively.
In vitro FITC-BSA release from hydrogels of hydrophobic cELPs. ELPs pre-mixed with FITC-BSA were mixed with H2O2 on ice. BSA (4 mg mL−1) was added to the resulting hydrogels, and the mixture was incubated at 4°C. The amount of released FITC-BSA in the supernatant was quantified by the fluorescence intensity. The final concentrations of cELP and H2O2 are 2.5 wt% and 0.3 wt%, respectively. Each data point represents the mean value ± SEM (n = 5).
Acknowledgments
This work was financially supported by NIH grant R01-GM-061232 and R01-EB-000188 to A.C., NIH grant R01-CA-138784 to W.L., and NIH grant R01-EB-001037 and NSF grant CHE-0646670 to S.L.C. D.A. acknowledges a grant from the Science and Technology Foundation of Japan. We thank Dr. Takeshi Mori, Dr. Xinghai Li, Dr. Ganesan Vaidyanathan, and Mr. Jonathan R. McDaniel for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1–3):9–28. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58(2):445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MK, Lee DS. Injectable biodegradable hydrogels. Macromol Biosci. 2010;10(6):563–579. doi: 10.1002/mabi.200900402. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 7.Kopecek J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28(34):5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355(1–2):1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 9.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115(2):175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, MacKay JA, Dreher MR, Chen M, McDaniel JR, Simnick AJ, et al. Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model. J Control Release. 2010;144(1):2–9. doi: 10.1016/j.jconrel.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betre H, Setton LA, Meyer DE, Chilkoti A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3(5):910–916. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 12.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27(1):91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl)phosphines in aqueous solution. Biomacromolecules. 2007;8(5):1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11(11–12):1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 15.Nagapudi K, Brinkman WT, Leisen JE, Huang L, McMillan AR, Apkarian RP, et al. Photomediated solid-state cross-linking of an elastin-mimetic recombinant protein polymer. Macromolecules. 2002;35(5):1730–1737. [Google Scholar]
- 16.Lee J, Macosko CW, Urry DW. Mechanical properties of cross-linked synthetic elastomeric polypentapeptides. Macromolecules. 2001;34(17):5968–5974. [Google Scholar]
- 17.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliv Rev. 2002;54(8):1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 18.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3(2):357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel JR, Mackay JA, Quiroz FG, Chilkoti A. Recursive directional ligation by plasmid reconstruction allows rapid and seamless cloning of oligomeric genes. Biomacromolecules. 2010;11(4):944–952. doi: 10.1021/bm901387t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17(11):1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 21.Unak T, Akgun Z, Yildirim Y, Duman Y, Erenel G. Self-radioiodination of iodogen. Appl Radiat Isot. 2001;54(5):749–752. doi: 10.1016/s0969-8043(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 22.Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials. 2007;28(18):2791–2800. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Tan JT, Bardwell JC. Key players involved in bacterial disulfide-bond formation. Chembiochem. 2004;5(11):1479–1487. doi: 10.1002/cbic.200400036. [DOI] [PubMed] [Google Scholar]
- 24.Goth L. The hydrogen peroxide paradox. Orv Hetil. 2006;147(19):887–893. [PubMed] [Google Scholar]
- 25.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486(1):10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 26.Nishinari K. Some thoughts on the definition of a gel. Progr Colloid Polym Sci. 2009;136:87–94. [Google Scholar]
- 27.Liu W, Dreher MR, Chow DC, Zalutsky MR, Chilkoti A. Tracking the in vivo fate of recombinant polypeptides by isotopic labeling. J Control Release. 2006;114(2):184–192. doi: 10.1016/j.jconrel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Lim DW, Nettles DL, Setton LA, Chilkoti A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules. 2008;9(1):222–230. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu ZM, Zhang XG, Zheng C, Li CX, Zhang SM, Dong RN, et al. Disulfide-crosslinked chitosan hydrogel for cell viability and controlled protein release. Eur J Pharm Sci. 2009;37(3–4):198–206. doi: 10.1016/j.ejps.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Koo H, Jin GW, Kang H, Lee Y, Nam HY, Jang HS, et al. A new biodegradable crosslinked polyethylene oxide sulfide (PEOS) hydrogel for controlled drug release. Int J Pharm. 2009;374(1–2):58–65. doi: 10.1016/j.ijpharm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 32.Sakloetsakun D, Hombach JM, Bernkop-Schnurch A. In situ gelling properties of chitosan-thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials. 2009;30(31):6151–6157. doi: 10.1016/j.biomaterials.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 33.Anumolu SS, Menjoge AR, Deshmukh M, Gerecke D, Stein S, Laskin J, et al. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials. 2011;32(4):1204–1217. doi: 10.1016/j.biomaterials.2010.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene and corresponding protein polymer sequences for all ELPs in this study. The genes were synthesized by oligomerization of a gene segment shown in blue. The polymerized gene region was flanked by short leader (SKGPG) and trailer (WP) peptide sequences that are needed for good expression yield and quantification of the ELP, respectively.
cELPs multimerize prior to hydrogel formation. The solution of cELP1 at 2.5 wt% was incubated at 37°C and analyzed by SDS-PAGE. Thirty μg of the protein was loaded on a lane. Higher molecular weight products appeared as a function of incubation time, as indicated by a square. The symbols + and − indicate the presence and absence of β-mercaptoethanol (β-ME), respectively.
In vitro FITC-BSA release from hydrogels of hydrophobic cELPs. ELPs pre-mixed with FITC-BSA were mixed with H2O2 on ice. BSA (4 mg mL−1) was added to the resulting hydrogels, and the mixture was incubated at 4°C. The amount of released FITC-BSA in the supernatant was quantified by the fluorescence intensity. The final concentrations of cELP and H2O2 are 2.5 wt% and 0.3 wt%, respectively. Each data point represents the mean value ± SEM (n = 5).