Abstract
Background
Several studies have investigated associations between the -174G>C polymorphism (rs1800795) of the IL6-gene, but presented inconsistent results.
Aims
This joint analysis aimed to clarify whether IL6 -174G>C was associated with type 2 diabetes mellitus (T2DM) related quantitative phenotypes.
Methods
Individual-level data from all studies of the IL6-T2DM consortium on Caucasian subjects with available BMI were collected. As study-specific estimates did not show heterogeneity (P>0.1), they were combined by using the inverse-variance fixed-effect model.
Results
The main analysis included 9440, 7398, 24,117, or 5659 nondiabetic and manifest T2DM subjects for fasting glucose, 2-hour glucose, BMI or circulating interleukin-6 levels, respectively. IL6 -174 C-allele carriers had significantly lower fasting glucose (−0.091mmol/L, P=0.014). There was no evidence for association between IL6 -174G>C and BMI or interleukin-6. In an additional analysis of 641 subjects known to develop T2DM later on, the IL6 -174 CC-genotype was associated with higher baseline interleukin-6 (+0.75pg/mL, P=0.004), which was consistent with higher interleukin-6 in the 966 manifest T2DM subjects (+0.50pg/mL, P=0.044).
Conclusions
Our data suggest association between IL6 -174G>C and quantitative glucose, and exploratory analysis indicated modulated interleukin-6 levels in pre-diabetic subjects, being in-line with this SNP’s previously reported T2DM association and a role of circulating interleukin-6 as intermediate phenotype.
Keywords: blood glucose, body mass index, diabetes mellitus, type 2, epidemiology, molecular, genes, inflammation mediators, interleukin-6, intermediate phenotype, meta-analysis, polymorphism, single nucleotide
Introduction
Type 2 diabetes mellitus (T2DM) is a public health problem of pandemic proportions. Conservative estimates indicate that there are 171 million people in the world with symptomatic or asymptomatic diabetes mellitus and that this global prevalence will double by 2030 to 366 million people (1). The spread of the disease is also alarming in Europe, even though Caucasians have a low to moderate prevalence of T2DM compared with most other ethnic groups worldwide.
Type 2 diabetes mellitus is a multifactorial disease. While a lot is known about environmental risk factors for T2DM, identification of the genetic etiology of T2DM has proved to be challenging (2). An interesting candidate variant for T2DM is the -174G>C polymorphism (rs1800795) of the IL6 gene that codes for the cytokine interleukin-6 (IL-6). Interleukin-6 exerts pleiotropic biological functions in the regulation of the acute-phase reaction and immune responses and is associated with T2DM and related diseases (3–6). The functionally relevant (7;8) promoter polymorphism IL6 -174G>C was reported to be associated with circulating IL-6 levels (7;9–13), T2DM (14), insulin resistance (15), obesity (16–18), coronary heart disease (CHD) (19), cholesterol levels (5), and metabolic syndrome (20). However, association estimates pointed into inconclusive directions, while other studies of these traits did not find any association.
Two meta-analyses on association between IL6 -174G>C and two different outcomes have been reported to date: 1) Sie et al. did not find statistically significant evidence for an association with CHD in 19,798 individuals (21). 2) Recently, we established an international IL6-T2DM consortium showing an almost 9% reduced T2DM risk for C-allele carriers in 20,976 individuals (22). However, it was not clear yet whether the IL6 -174G>C polymorphism impacts IL-6 levels which together with the T2DM association would support a causal role of IL-6 levels in T2DM. Moreover, studies on genetic association with obesity pointed into a direction that was opposite to the T2DM association (16;17). Finally, analyzing the dichotomous trait T2DM is a substantial restriction of information, which can be overcome by analyzing the quantitative trait circulating plasma or serum glucose in the fasting state or after an oral glucose load. This is of special importance because the T2DM finding was borderline statistically significant (P=0.037) (22) and a consistent glucose association would greatly underscore this finding.
The objective of the present study was to clarify the epidemiological evidence for the association of IL6 -174G>C polymorphism with circulating glucose levels, body-mass index (BMI), and IL-6 levels as intermediate phenotypes on the way to T2DM. We used by far the largest dataset on this polymorphism in relation to quantitative phenotypes to date. It is important to note that earlier studies detected associations between -174G>C and IL-6 levels or obesity only in the presence of an inflammatory stimulus (9;10) or in subjects having a chronic subclinical (18), or an acute (11;13) inflammatory state. The inclusion of T2DM studies in our joint analysis was essential to investigate whether the impact of IL6 -174G>C indeed differed by the subclinical inflammatory state observed in type 2 diabetic individuals, as opposed to healthy subjects.
Subjects and Methods
All studies of the IL6-T2DM consortium on Caucasian subjects with available BMI were included in this joint analysis of quantitative traits. Information on the inclusion criteria, the search strategy and recruitment of the studies, data cleaning, and genotyping methods is provided in the online appendix.
Definition of Analyzed Samples and Data Collection
Because the majority of included studies were cross-sectional population-based or case-control studies, our main analysis focused on a combined sample of nondiabetic and prevalent T2DM subjects. We included the baseline examination of the two longitudinal cohort studies excluding subjects that developed T2DM during follow-up for this main analysis. All analyses were confined to Caucasian adults that were at least 18 years old with data on the genotype of the IL6 -174G>C polymorphism, age, sex, BMI and T2DM status. When known, type 1 diabetic individuals were excluded. In family studies, only the sibling generation was used. Data on circulating fasting and 2-hour plasma or serum glucose (measured two hours after consumption of 75 g glucose) and plasma or serum IL-6 levels were collected where available.
All participating studies have been conducted according to the principles expressed in the Declaration of Helsinki. Individual studies had either written informed consent for all subjects for genetic analyses or approval from their institutional review committee for genetic analyses. Further details on design of individual studies and use in this joint analysis are presented in the online appendix Table A2.
Statistical Analyses
Study-specific β-coefficients for the association between IL6 -174G>C and the quantitative traits were estimated by linear regression, using SAS PROC GLM for studies with unrelated individuals and SAS PROC MIXED for family studies. All analyses were adjusted for age and sex. Primary analyses were performed including all subjects (full data analyses) additionally adjusting for T2DM status when analyzing BMI or IL-6 levels. Secondary analyses were conducted separately for the nondiabetic and the prevalent T2DM subjects (T2DM status-specific analyses), additionally adjusting for BMI when analyzing fasting, 2-hour glucose or IL-6 levels. Studies with fasting glucose only on either T2DM cases or controls (EDSC, EPIC-POTSDAM_nCC-T2DM and MONICA-S3) were excluded from the full data glucose analysis to assure a full range of glucose levels.
IL6 -174G>C genotypes were analyzed model-free, comparing CC- or GC-genotype with the wild-type GG, and additionally by applying a dominant genetic model for the C-allele, which was the model reported previously for the T2DM association (22). Heterogeneity between study-specific β-coefficients was tested by the χ2-based Q-statistic and its impact was quantified by I2 (23). As the heterogeneity between study-specific β-coefficients was nonsignificant in all analyses (P>0.10), the β-coefficients were combined using the inverse-variance fixed-effect model (24). As recommended, summary association estimates of all studies with the genotype frequencies of nondiabetic subjects being in Hardy-Weinberg equilibrium (HWE) were reported as main results (25).
A conservative Bonferroni-corrected significance level of 0.017 (=0.05/3) was applied to account for the testing of the three phenotypes (glucose, BMI, and IL-6) in the primary (full data) analyses. Note that the phenotypes fasting and 2-hour postprandial glucose levels were highly correlated and thus not counted as two independent phenotypes here. In the secondary analyses, the significance level was further corrected for the two investigated subgroups yielding a significance level of 0.008 (0.05/6). More detailed information on statistical procedures is given in the online appendix.
Analysis in incident T2DM patients
In an additional exploratory analysis, we combined the linear regression estimates of baseline data from the incident T2DM cases of the two cohort studies to obtain estimates of the IL6 -174G>C association with BMI and IL-6 levels (glucose not available) among future T2DM subjects before they became cases (“pre-diabetic subjects”).
Results
Study Recruitment
Seventeen studies with 25,635 participants met the study and subject inclusion criteria and were included in the joint analyses (see Table 1 for an overview). Online appendix Table A3 shows for which outcome the respective study qualified for analysis; online appendix Table A4 presents the number of participants per study included in the analyses of the respective quantitative trait. All 17 studies with 25,635 subjects were analyzed for the outcome BMI. Association analyses with BMI as main outcome had been unpublished in 12 studies at the time of study recruitment (called “unpublished for BMI” in the following). Eight studies with 10,725 participants were analyzed for fasting glucose, seven of them unpublished for this trait; seven studies with 8399 participants were analyzed for 2-hour glucose (all unpublished). For the outcome circulating IL-6 levels, data from seven studies with 5659 participants were analyzed, six of them unpublished for IL-6 levels.
Table 1.
Characteristics of Included Studies
| Study* | Full study name | Country† | n T2DM / nondiabetic subjects‡ |
|---|---|---|---|
| BOTNIA | Botnia Study | SF | 731/557 |
| CAPPP | Captopril Prevention Project | S | 42/424 |
| DANISH | Danish Study | DK | 1212/4399 |
| EDSC | Ealing Diabetes Study of Coagulation | UK | 299/0 |
| EPIC- POTSDAM | European Prospective Investigation into Cancer and Nutrition Potsdam (EPIC-Potsdam) | D | 0/348 |
| FUSION 1 | The Finland-United States Investigation of NIDDM Genetics, 1st sampling wave | SF | 508/367 |
| FUSION 2 | The Finland-United States Investigation of NIDDM Genetics, 2nd sampling wave | SF | 437/201 |
| GIRONA | Girona Genetics of Diabetes Study | E | 42/123 |
| KORA- MIFAM | KORA MI Family Study | D | 95/881 |
| KORA-S4 | KORA Survey S4 | D | 225/1190 |
| KORA- T2DMFAM | KORA T2DM Family Study | D | 776/513 |
| MONICA/ KORA-BASE | MONICA/KORA Case Cohort Study S123(MONICA/KORA-S123) | D | 101/1744 |
| MONICA-S3 | MONICA/KORA Survey S3 | D | 151/3551 |
| NPHS II | Second Northwick Park Heart Study | UK | 0/2652 |
| RMIFAM | Regensburg Ml Family Study | D | 662/2614 |
| TGN | Tarraco Study | E | 166/64 |
| UDACS | University College Diabetes and Cardiovascular Study | UK | 560/0 |
MI = Myocardial Infarction
Abbreviated study name used in the present publication
Country of recruitment: D = Germany, DK = Denmark, E = Spain, SF = Finland, S = Sweden, UK = United Kingdom
Number of type 2 diabetic / nondiabetic subjects included in analyses of the outcome BMI
Study-Specific Statistics
Detailed characteristics of included studies and participants are summarized in online appendix Table A5. All studies had been conducted in European populations. Genotype frequencies of nondiabetic subjects were in HWE for all studies, except for the BOTNIA and the TGN study, which were thus excluded from the main analyses. This left 9440, 7398, 24,117, or 5659 subjects for the analysis of fasting glucose, 2-hour glucose, BMI or circulating IL-6 (see online appendix Table A4). The IL6 -174 C-allele frequency of nondiabetic subjects with genotype frequencies in HWE ranged from 41.1% [95%CI=38.3, 43.8] in the KORA-MIFAM study to 55.0% [95%CI=51.4, 58.6] in the FUSION 1 study.
IL6 -174G>C and Circulating Glucose
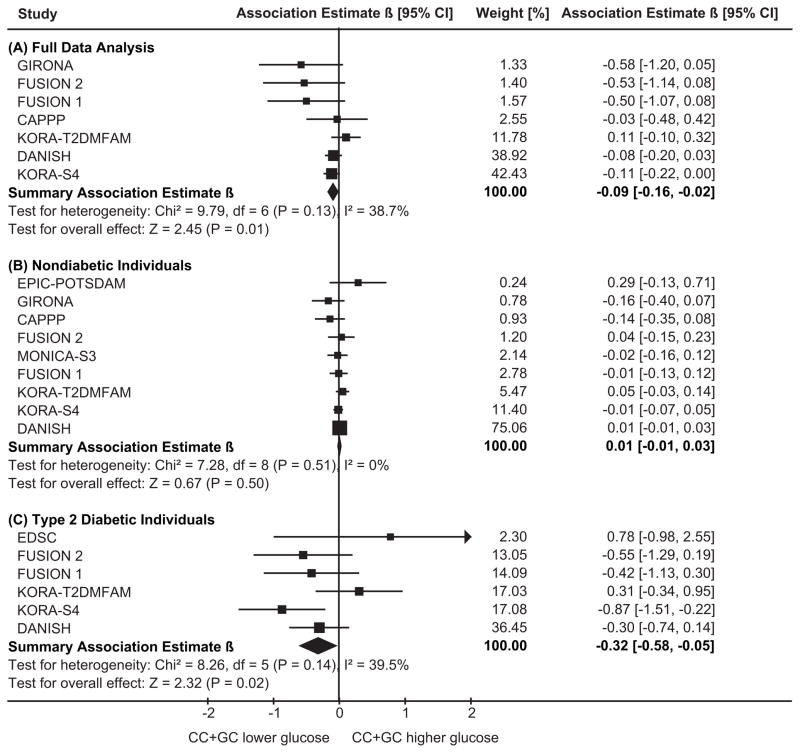
The outcome fasting glucose was investigated as full data main analysis for the seven studies where the genotype frequencies of nondiabetic subjects were in HWE, including 9,440 subjects. Figure 1 shows the study-specific and the summary β-coefficients, using a dominant model for the C-allele. Most studies exhibited a reduction of fasting glucose levels, though not significant in each study separately. The summary estimate provided a statistically significant decrease of −0.091mmol/L (95%CI=[−0.163, −0.018], P=0.014), corresponding to about 1.5% decrease, for subjects with GC- or CC-genotypes compared to GG. T2DM status-specific analyses showed a more pronounced reduction of −0.317mmol/L (95%CI=[−0.584, −0.049], P=0.020) among T2DM subjects.
Figure 1.
Table 2 provides model-free summary estimates, indicating that the dominant genetic model was consistent with the data.
Table 2.
Summary Results of Association Between IL6 -174G>C and Fasting Glucose, 2-h Glucose, Body-Mass Index (BMI), or Interleukin-6 (IL-6) Levels. The Fixed-Effect Results are Presented for the Full Data Analysis* and for Analyses Stratified for Type 2 Diabetes Status.
| Outcome | Full data analysis* | Nondiabetic subjects | Type 2 diabetic subjects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | β-coefficient (P Value)† | N | β-coefficient (P value)† | N | β-coefficient (P value)† | ||||||||||
| Studies | Subjects | CCvsGG | GCvsGG | Dominant‡ | Studies | Sub jects | CCvsGG | GCvsGG | Dominant† | Studies | Subjects | CCvsGG | GCvsGG | Dominant† | |
| Fasting glucose[mmol/L] | 7 | 9440 | −0.081 (0.108) | −0.099 (0.011) | −0.091 (0.014) | 9 | 7420 | 0.007 (0.626) | 0.007 (0.523) | 0.007 (0.501) | 6 | 2412 | −0.322 (0.076) | −0.354 (0.013) | −0.317 (0.020) |
| 2-hour glucose[mmol/L] | 6 | 7398 | −0.127 (0.127) | −0.053 (0.441) | −0.075 (0.243) | 6 | 6731 | −0.022 (0.592) | −0.003 (0.920) | −0.009 (0.775) | 4 | 618 | −0.043 (0.927) | −0.114 (0.760) | −0.135 (0.703) |
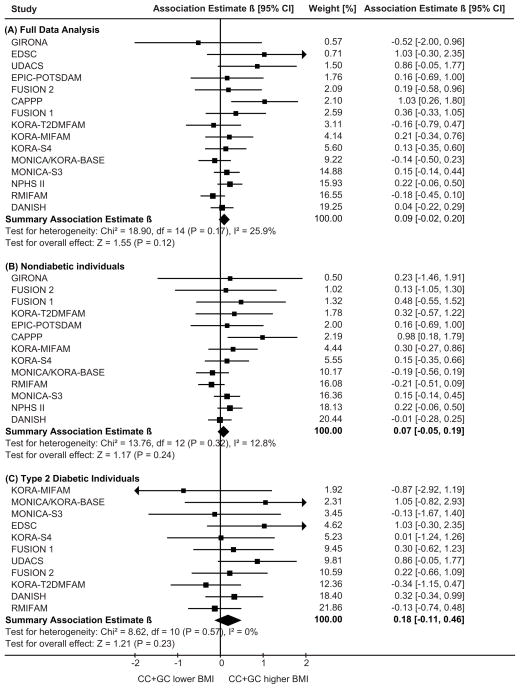
| BMI[kg/m2] | 15 | 24117 | 0.097 (0.197) | 0.086 (0.151) | 0.088 (0.120) | 13 | 19007 | 0.057 (0.478) | 0.080 (0.213) | 0.071 (0.242) | 11 | 5026 | 0.216 (0.255) | 0.154 (0.321) | 0.176 (0.226) |
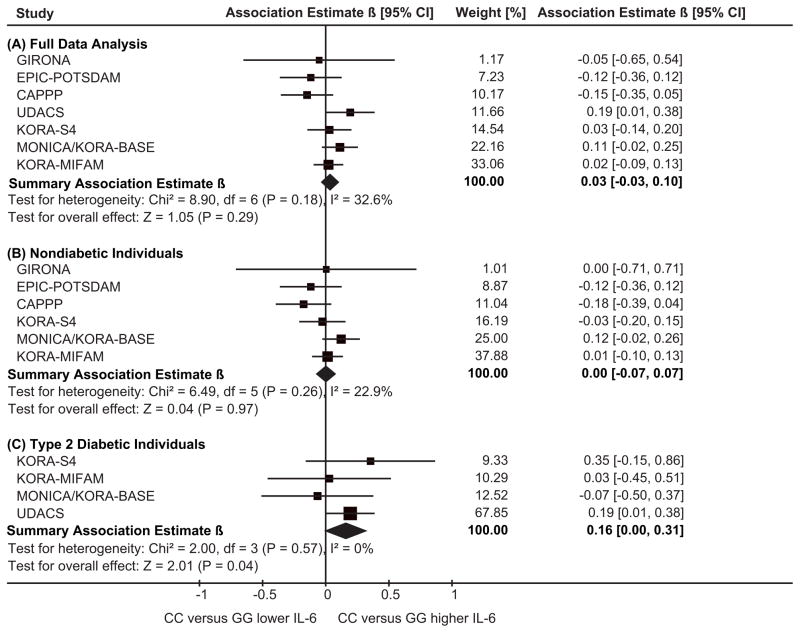
| Circulati ng IL-6|| | 7 | 5659 | 0.034 (0.292) | 0.018 (0.467) | 0.024 (0.304) | 6 | 4621 | 0.001 (0.969) | 0.022 (0.441) | 0.018 (0.504) | 4 | 966 | 0.159 (0.044) | 0.004 (0.938) | 0.042 (0.415) |
Analysis of nondiabetic and prevalent T2DM subjects together. The sum of the number of nondiabetic and T2DM subjects in stratified analyses does not yield the number of subjects in the full data analysis, because stratified analyses were performed in individual studies only if there were at least 50 nondiabetic or T2DM subjects available.
Summary estimates from generalized linear models adjusted for age and sex (for BMI and IL-6 levels: additionally adjusted for T2DM status; for glucose and IL-6 levels: additionally adjusted for BMI). Main findings are printed in bold letters.
Dominant model comparing CC- and GC-subjects versus GG.
IL-6 β-coefficients were computed and are displayed on the logarithm of the original scale [pg/mL].
The full data main analysis of 2-hour glucose included six studies with 7398 subjects. There was no statistically significant association, but the β-coefficients pointed into the same direction as for fasting glucose (C-allele dominant model for full data analysis: β= −0.075mmol/L, 95%CI=[−0.201, 0.051], P=0.243, Table 2).
IL6 -174G>C and Body-Mass Index
The full data main analysis of the outcome BMI included 15 studies with 24,117 subjects. Figure 2 shows the study-specific and the summary β-coefficients applying the dominant model for the C-allele. There was no statistically significant evidence for an association between IL6 -174G>C and BMI in the full data (β=0.088, 95%CI=[−0.023, 0.200], P=0.120) or the T2DM status-specific analyses (P>0.10, Table 2).
Figure 2.
IL6 -174G>C and Circulating Interleukin-6 Levels
There was no evidence in the full data joint analysis including 5659 subjects of seven studies for an association between IL6 -174G>C and circulating IL-6 levels (Table 2 and Figure 3). Likewise, there was no statistically significant IL-6 level association among the 4621 nondiabetic subjects of six studies or among the 966 T2DM subjects of four studies. Interestingly, the analysis among T2DM subjects pointed towards higher IL-6 levels in subjects with the IL6 -174 CC-genotype (βCCvsGG=0.159 [95%CI=0.004, 0.314], P=0.044) compared to subjects with the GG-genotype. The size of the β-estimate on the log-scale corresponded to a difference of 0.50pg/mL on the original scale (+17%).
Figure 3.
Analysis of BMI and IL-6 levels in incident T2DM cases before they became cases
The results from the additional analysis of the baseline data from the 641 incident T2DM subjects before they became type 2 diabetic are summarized in Table 3. There was no association with BMI (P>0.10).
Table 3.
Summary Results of Association Between IL6 -174G>C and Pre-diabetic Baseline Values on Body-Mass Index (BMI) or Interleukin-6 (IL-6) Levels of Incident T2DM Subjects*.
| Outcome | Incident type 2 diabetic subjects* | ||||
|---|---|---|---|---|---|
| N | β-coefficient (P value)† | ||||
| Studies | Subjects | CCvsGG | GCvsGG | Dominant† | |
| BMI [kg/m2] | 2 | 658 | 0.777 (0.129) | −0.215 (0.583) | 0.044 (0.905) |
| Circulating IL-6 levels† | 2 | 641 | 0.266 (0.004) | 0.060 (0.394) | 0.116 (0.084) |
From the two cohort studies EPIC-POTSDAM_nCC-T2DM and MONICA/KORA-S123.
Explanations are analogous to Table 2.
Regarding circulating IL-6, the IL6 -174G>C showed higher (but not statistically significantly higher) levels in the C-allele dominant model (β=0.116, 95%CI=[−0.016, 0.248], P=0.08) and a strong association of the homozygous CC-genotype with higher IL-6 levels (βCCvsGG=0.266, 95%CI=[0.085, 0.448], P=0.004) compared to the GG-genotype, which was consistent with and even more pronounced than the association in the prevalent T2DM cases (see above). The size of the β-estimate on the log-scale corresponded to a difference of 0.75pg/mL on the original scale (+30%).
Sensitivity Analyses
Regarding fasting glucose, 2-hour glucose or IL-6 levels, there was no evidence for publication bias: the Egger’s regression test was nonsignificant (P>0.10) and excluding published studies would not change the main findings (online appendix Table A6). Regarding BMI, there was some evidence for publication bias. In the five published studies with 10,704 subjects, the IL6 -174 C-allele was statistically significantly associated with higher BMI (β=0.190, 95%CI=[0.023, 0.358]). In contrast, there was no association in the ten unpublished studies with 13,413 subjects (β=0.007, 95%CI=[−0.142, 0.157]). Moreover, the funnel plot (online appendix Figure A1) and the Egger’s regression test (P=0.06) showed some evidence for publication bias of the summary BMI estimate when including all 15 studies, but none when restricting to the ten unpublished studies (P=0.27).
Including studies with genotype HWE violation (online appendix Table A7) or omitting adjustment for BMI or T2DM status (data not shown) would not change our main findings. Furthermore, there were no large differences between association estimates of men and women in sex-stratified analyses (data not shown).
Discussion
Major findings
In this joint analysis of individual-level data from 9440 study participants, C-allele carriers of the IL6 -174G>C polymorphism had lower fasting glucose levels (−0.091mmol/L, P=0.014) independently of BMI. No association was found with BMI in 24,117 subjects or with IL-6 levels in 5659 subjects. Exploratory analysis of baseline IL-6 levels in 641 future T2DM subjects elucidated that pre-diabetic subjects with the IL6 -174 CC-genotype had significantly higher circulating IL-6 levels (+0.75pg/mL, P=0.004), which was consistent with higher IL-6 levels in the 966 manifest T2DM subjects (+0.50pg/mL, P=0.044).
IL6 -174G>C and Circulating Glucose
The present analysis results of the IL6 -174G>C with fasting glucose was in-line with our previous joint analysis on T2DM, which had shown that individuals carrying the IL6 -174 C-allele had a 9% lower odds for T2DM compared to individuals with the GG-genotype (P=0.037) (22). We also confirmed the dominant genetic model. Our previous T2DM analysis had included a larger number of subjects; but when restricting to the seven studies with fasting glucose data, a T2DM summary odds ratio of 0.89 [95%CI=0.78, 1.02] with a nonsignificant P value of 0.09 would have been yielded, which impressively demonstrates the gain from additional information by using glucose as a quantitative variable.
The association with 2-hour postprandial glucose levels pointed into the same direction as the fasting glucose estimates but was statistically not significant, which might be due to the fewer subjects available for this phenotype.
IL6 -174G>C and Body-Mass Index
Several lines of evidence suggest that the cytokine IL-6 plays a role in the regulation of body composition, probably by acting in a catabolic manner (26–28). To date, association between IL6 -174G>C and obesity has been investigated by several comparably small studies with inconsistent results (16;17;19;29). In our quantitative analysis of nearly 25,000 subjects there was some evidence for publication bias and no statistically significant evidence for an association between IL6 -174G>C and BMI when including published and unpublished studies. Our power to detect a BMI difference of 0.2kg/m2 or 0.3kg/m2 between IL6 -174 C-allele carriers and non-carriers was more than 80% or nearly 100%, respectively, given the type 1 error probability of 0.050/3=0.017, a GG-genotype frequency of 31.2% and a BMI standard deviation of 4.2kg/m2.
IL6 -174G>C and Circulating Interleukin-6 Levels
There was no evidence for association between IL6 -174G>C and circulating IL-6 levels including nondiabetic and manifest T2DM subjects, which was consistent with the five largest hitherto published studies on healthy or population-based subjects with 641 to 1526 Caucasian subjects each (21;30–33).
Our exploratory analysis of baseline IL-6 levels of the 641 subjects, who became T2DM cases during the follow-up of two cohort studies, indicated a significant increase of IL-6 levels for subjects with the CC-genotypes compared to the GG. This is intriguing as these IL-6 levels of the subjects known to develop T2DM later on were not yet influenced by treatment or metabolic effects from manifest T2DM, which could have attenuated the genetic association in the prevalent cases. The 966 prevalent T2DM subjects had shown similar, but less pronounced, IL-6 level estimates. Based on these data, we derive the hypothesis that the IL6 -174G>C modulates IL-6 levels in pre-diabetic subjects, which needs to be proven in other samples. For the apparent dominant effect (CC or GC versus GG subjects) for glucose levels and the rather recessive effect (CC versus GC or GG subjects) of the IL-6 levels, we frankly lack an explanation other than the ever threatening assembly of small effects, measurement uncertainty in the variables, lack of power despite the large sample size here or latent effect modifiers.
There is previous functional evidence being in-line with the fact that the effect of the promoter polymorphism -174G>C on IL-6 concentrations differed between nondiabetic and T2DM subjects thus depending on a subject’s metabolic state: type 2 diabetic patients and subjects prone to become type 2 diabetic are characterized by a subclinical inflammatory state, and inflammatory stimuli were reported to influence the association of IL6 -174G>C with circulating IL-6 levels in vivo (9;10;12). This would also be supported by in vitro and ex vivo studies demonstrating that IL-6 expression was cell-type specific (8) and depended on the presence (11) and type of stimulation (34).
Circulating IL-6 Levels – The Intermediate Phenotype for the Association Between IL6 -174G>C and Type 2 Diabetes Mellitus?
We were able to analyze large-scale datasets on both the association of the IL6 -174G>C polymorphism with circulating glucose (and risk of T2DM) as well as with the most obvious intermediate phenotype, the gene product IL-6. Thus, we attempted to contribute to the understanding of the widely discussed link between circulating IL-6 levels and T2DM (35) by concomitantly considering our analyses results of both phenotypes (36).
Our data suggest that the IL6 -174 CC-genotype is associated with reduced risk of T2DM and increased circulating IL-6 levels in subjects with a pre- or manifest type 2 diabetic state, but the polymorphism explains only a small fraction of the IL-6 variance. Together with the assumption that IL-6 is the mediating factor between the IL6-polymorphism and T2DM, it could be speculated that the IL6 -174 CC-genotype modulates the cytokine IL-6 towards higher levels and consequently reduces the risk of T2DM. Several lines of functional evidence support a positive impact of elevated cytokine IL-6 levels on β-cell function or peripheral glucose disposal and thus on T2DM (4;26;28;37–40) The seemingly contradicting association of increased circulating IL-6 levels with higher risk of T2DM observed in most case-control and prospective cohort studies (6) might be caused by feed-back mechanisms such as increased IL-6 production and release from insulin-sensitive tissue (41), larger IL-6 producing adipose tissue characteristic of pre- and manifest T2DM subjects (3), an IL-6 resistant state of these subjects, or counter regulation of low-grade inflammation induced by proinflammatory mediators (35).
Strengths and Limitations of this Joint Analysis
The large number of subjects analyzed in our investigation was a definite strength. For all analyzed phenotypes, our study represents the largest study conducted to date to address the role of IL6 -174G>C. Thus, we were able to reveal small associations, which nevertheless are of importance due to the high prevalence of the genetic variant in the general population (41–55%) and their potential to help understand the pathogenesis of this severe disease. Our non-finding of an association with BMI in this highly powered study may also be of great importance to solve to some extent the puzzle from contrary reports.
Furthermore, it was a distinctive strength of our joint analysis that it was based on individual participants’ data allowing for standardized data cleaning and analysis, to which the low heterogeneity among study-specific estimates may be attributed.
Finally, our analysis included many unpublished studies, which guards against the greatest threats of meta-analyses, the publication or selective reporting bias. Stratifying for published or unpublished studies depicted a potential of some bias for the published association studies of IL6 -174G>C and BMI.
It may be considered a limitation that all T2DM status-specific analyses exhibited truncated glucose distributions, which consequently also affected the correlated BMI and IL-6 level distributions. In addition, no data were available on antidiabetic medication for T2DM subjects for the glucose analyses or on physical activity before blood extraction for the circulating IL-6 level analyses. This might have decreased the precision of the estimates but is unlikely to have caused false positive findings.
Conclusion
This joint analysis on 25,000 subjects represents by far the largest study to date on the association of the IL6 promoter polymorphism -174G>C with quantitative phenotypes relevant for T2DM. Our data suggest an association of the widely debated IL6 -174G>C polymorphism with quantitative glucose. Additional exploratory analysis on baseline IL-6 levels of future T2DM patients gave rise to hypothesize on an association of the -174G>C polymorphism with circulating IL-6 levels apparent only in pre-diabetic subjects, that may ultimately decrease the risk of T2DM. In general, we demonstrated that a consortium-based approach is well-suited to investigate genetic variants with small effects.
Supplementary Material
Key Messages.
Several studies have investigated associations between the -174G>C polymorphism (rs1800795) of the IL6-gene, but presented inconsistent results. For all analyzed phenotypes, our joint analysis on individual-level data represents the largest study conducted to date to address the role of IL6 -174G>C.
We were able to reveal lower fasting glucose levels in carriers of the IL6 -174 C-allele. There was no evidence for association between IL6 -174G>C and BMI or circulating interleukin-6 levels in nondiabetic subjects.
Exploratory analysis gave rise to hypothesize on an association between the IL6 -174 CC-genotype and higher circulating IL-6 levels of subjects known to develop T2DM later on.
Acknowledgments
Funding:
British Heart Foundation; Diabetes UK; European Foundation for the Study of Diabetes; German Diabetes Center; German Federal Ministry of Education, Science, Research and Technology / National Genome Research Network; German Federal Ministry of Health and Social Security; German Research Foundation; Helmholtz Zentrum München; Ministry of Science and Research of the State North Rhine-Westphalia; Munich Center of Health Sciences; National Institutes of Health.
We gratefully acknowledge the participation in the original studies of all individuals used in this joint analysis.
Abbreviations
- BMI
body-mass index
- CHD
coronary heart disease
- HWE
Hardy-Weinberg equilibrium
- IL-6
interleukin-6
- IL6
interleukin-6 gene
- T2DM
type 2 diabetes mellitus
- 2-h
2-hour
- 95%CI
95% confidence interval
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Elbein SC. The Genetic Basis of Type 2 Diabetes. Cellscience. 2006;2:100–131. doi: 10.1901/jaba.2006.2-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey AL, Bruce CR, Sacchetti M, et al. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia. 2004;47:1029–1037. doi: 10.1007/s00125-004-1403-x. [DOI] [PubMed] [Google Scholar]
- 4.Andreozzi F, Laratta E, Cardellini M, et al. Plasma interleukin-6 levels are independently associated with insulin secretion in a cohort of Italian-Caucasian nondiabetic subjects. Diabetes. 2006;55:2021–2024. doi: 10.2337/db06-0063. [DOI] [PubMed] [Google Scholar]
- 5.Henningsson S, Hakansson A, Westberg L, et al. Interleukin-6 gene polymorphism -174G/C influences plasma lipid levels in women. Obesity. 2006;14:1868–1873. doi: 10.1038/oby.2006.216. [DOI] [PubMed] [Google Scholar]
- 6.Thorand B, Baumert J, Kolb H, et al. Sex Differences in the Prediction of Type 2 Diabetes by Inflammatory Markers: Results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetes Care. 2007;30:854–860. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- 7.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 9.Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene -174g>c and -572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol. 2001;21:1458–1463. doi: 10.1161/hq0901.094280. [DOI] [PubMed] [Google Scholar]
- 10.Bennermo M, Held C, Stemme S, et al. Genetic predisposition of the interleukin-6 response to inflammation: implications for a variety of major diseases? Clin Chem. 2004;50:2136–2140. doi: 10.1373/clinchem.2004.037531. [DOI] [PubMed] [Google Scholar]
- 11.Acalovschi D, Wiest T, Hartmann M, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–1869. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 12.Gaudino M, Andreotti F, Zamparelli R, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(Suppl 1):II195–II199. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 13.Kilpinen S, Hulkkonen J, Wang XY, Hurme M. The promoter polymorphism of the interleukin-6 gene regulates interleukin-6 production in neonates but not in adults. Eur Cytokine Netw. 2001;12:62–68. [PubMed] [Google Scholar]
- 14.Vozarova B, Fernandez-Real JM, Knowler WC, et al. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112:409–413. doi: 10.1007/s00439-003-0912-x. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Real JM, Broch M, Vendrell J, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517–520. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 16.Berthier MT, Paradis AM, Tchernof A, et al. The interleukin 6–174G/C polymorphism is associated with indices of obesity in men. J Hum Genet. 2003;48:14–19. doi: 10.1007/s100380300002. [DOI] [PubMed] [Google Scholar]
- 17.Klipstein-Grobusch K, Möhlig M, Spranger J, et al. Interleukin-6 g-174G>C Promoter Polymorphism Is Associated with Obesity in the EPIC-Potsdam Study. Obes Res. 2006;14:14–18. doi: 10.1038/oby.2006.3. [DOI] [PubMed] [Google Scholar]
- 18.Walch K, Grimm C, Zeillinger R, Huber JC, Nagele F, Hefler LA. A common interleukin-6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril. 2004;81:1638–1641. doi: 10.1016/j.fertnstert.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Georges JL, Loukaci V, Poirier O, et al. Interleukin-6 gene polymorphisms and susceptibility to myocardial infarction: the ECTIM study. Etude Cas-Temoin de l’ Infarctus du Myocarde. J Mol Med. 2001;79:300–305. doi: 10.1007/s001090100209. [DOI] [PubMed] [Google Scholar]
- 20.Hamid YH, Rose CS, Urhammer SA, et al. Variations of the interleukin-6 promoter are associated with features of the metabolic syndrome in Caucasian Danes. Diabetologia. 2005;48:251–260. doi: 10.1007/s00125-004-1623-0. [DOI] [PubMed] [Google Scholar]
- 21.Sie MP, Sayed-Tabatabaei FA, Oei HH, et al. Interleukin 6 -174 g/c promoter polymorphism and risk of coronary heart disease: results from the rotterdam study and a meta-analysis. Arterioscler Thromb Vasc Biol. 2006;26:212–217. doi: 10.1161/01.ATV.0000194099.65024.17. [DOI] [PubMed] [Google Scholar]
- 22.Huth C, Heid IM, Vollmert C, et al. IL6 Gene Promoter Polymorphisms and Type 2 Diabetes: Joint Analysis of Individual Participants’ Data From 21 Studies. Diabetes. 2006;55:2915–2921. doi: 10.2337/db06-0600. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2004;24:1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 26.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 27.van Hall G, Steensberg A, Sacchetti M, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- 28.Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol. 2002;543:379–386. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbieri M, Rizzo MR, Papa M, et al. Role of interaction between variants in the PPARG and interleukin-6 genes on obesity related metabolic risk factors. Exp Gerontol. 2005;40:599–604. doi: 10.1016/j.exger.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Bennet AM, Prince JA, Fei GZ, et al. Interleukin-6 serum levels and genotypes influence the risk for myocardial infarction. Atherosclerosis. 2003;171:359–367. doi: 10.1016/j.atherosclerosis.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 31.Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: Tagging-SNP haplotype analysis in large-scale case-control study and meta-analysis. Hum Mol Genet. 2006 doi: 10.1093/hmg/ddl113. [DOI] [PubMed] [Google Scholar]
- 32.Herbert A, Liu C, Karamohamed S, et al. BMI modifies associations of IL-6 genotypes with insulin resistance: the Framingham Study. Obesity. 2006;14:1454–1461. doi: 10.1038/oby.2006.165. [DOI] [PubMed] [Google Scholar]
- 33.van Oijen M, Arp PP, de Jong FJ, et al. Polymorphisms in the interleukin 6 and transforming growth factor beta1 gene and risk of dementia. The Rotterdam Study. Neurosci Lett. 2006;402:113–117. doi: 10.1016/j.neulet.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 34.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, O’Keefe GE. Interleukin-6 promoter haplotypes and interleukin-6 cytokine responses. Shock. 2003;20:218–223. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54 (Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 36.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 37.Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 38.Glund S, Deshmukh A, Long YC, et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56:1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- 39.Stouthard JM, Oude Elferink RP, Sauerwein HP. Interleukin-6 enhances glucose transport in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1996;220:241–245. doi: 10.1006/bbrc.1996.0389. [DOI] [PubMed] [Google Scholar]
- 40.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.