Abstract
Despite declines in heart failure morbidity and mortality with current therapies, re-hospitalization rates remain distressingly high, impacting substantially on individuals, society, and the economy. As a result, the need for new therapeutic advances and novel medical devices is urgent. Disease-related left ventricular remodeling is a complex process involving cardiac myocyte growth and death, vascular rarefaction, fibrosis, inflammation, and electrophysiological remodeling. As these events are highly inter-related, targeting one single molecule or process may not be sufficient. Here, we review molecular and cellular mechanisms governing pathological ventricular remodeling.
Keywords: remodeling, cell death, apoptosis, autophagy, hypertrophy, fibrosis, inflammation, electrophysiological remodeling, stem cells, progenitor cells
Introduction
It is predicted that as our population ages, the direct medical costs of all cardiovascular diseases (including hypertension, coronary heart disease, stroke, and heart failure) will triple, reaching $818 billion in 20301. Prominent within this population of patients are the five million Americans who suffer from chronic heart failure, the final common pathway of many forms of heart disease and the most common discharge diagnosis in Medicare for several years running. This syndrome carries a mortality of approximately 50% at 5 years, and its incidence and prevalence are expanding rapidly around the globe. Thus, not only is the problem of heart failure enormous and growing, it contributes importantly to runaway medical costs just as society is moving swiftly to contain those costs. As a result of these converging influences, we are at a crucial juncture where novel therapeutic approaches for heart failure are sorely needed. To accomplish this, comprehensive understanding of biological processes leading to heart disease and disease-related ventricular remodeling is required.
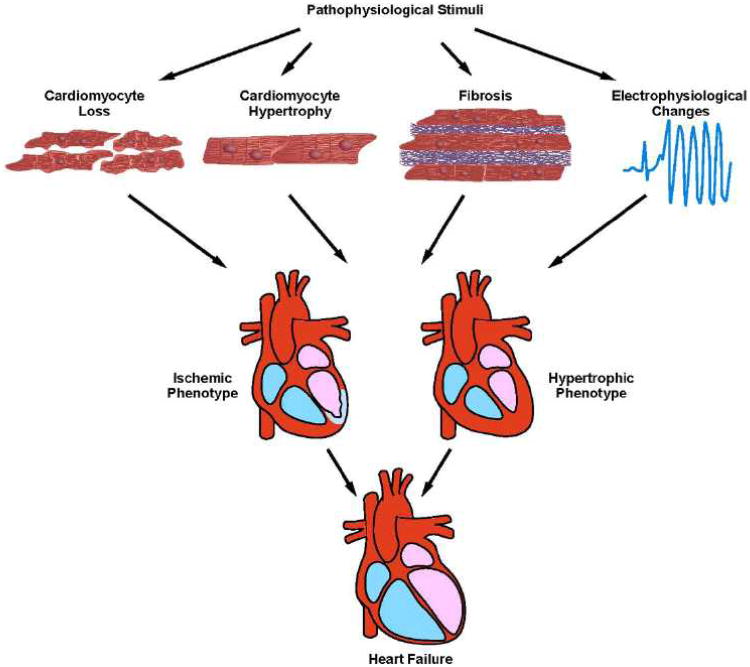
In the setting of disease, the left ventricle (LV) manifests a robust plasticity response which has been termed pathological remodeling2, 3. This process is the culmination of a complex series of transcriptional, signaling, structural, electrophysiological, and functional events occurring within the cardiac myocyte. In addition, other cellular elements within the ventricle participate, including fibroblasts (promoting fibrosis), vascular smooth muscle cells (promoting vascular stiffness), vascular endothelial cells (promoting endothelial dysfunction), and leukocytes (promoting inflammation) (Figure). Current thinking holds that these events – the heart's response to a variety of pathological insults – confer short-term benefit. However, left unchecked, these remodeling events are maladaptive and predispose to cardiovascular morbidity and mortality.
Figure.
Mechanisms of pathological ventricular remodeling. In response to pathophysiological stimuli, such as ischemia/reperfusion or excessive mechanical load, multiple molecular and cellular processes contribute to ventricular remodeling. These include cardiomyocyte loss through cell death pathways, such as necrosis, apoptosis, or possibly excessive autophagy. Cardiomyocytes become hypertrophic in response to both mechanical and neurohumoral triggers. Accumulation of excess extracellular matrix leads to fibrosis. Metabolic derangements, insulin resistance, and lipotoxicity can occur. Finally, structural changes and alterations in ion transporting processes culminate in a pro-arrhythmic phenotype.
Current therapies, including angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), aldosterone antagonists, and β-adrenergic receptor blockers (β-blockers), manifest significant efficacy in reducing morbidity and mortality in patients with chronic systolic heart failure4. However, in many instances disease progression continues unabated. Further, less is known about the substantial proportion of disease where systolic performance of the LV is preserved. Also, whereas novel disease targets are continually being discovered, most therapeutics do not demonstrate consistent efficacy in patients; indeed, many prove to be ineffective, even deleterious, before reaching Phase III clinical trials. Here, we review many of the major molecular and cellular pathways governing LV remodeling in the two broad types of heart failure, that with reduced (HFrEF) or preserved (HFpEF) systolic function. In an accompanying article, we review relevant therapies5.
Classification of heart failure
Most current therapies, and clinical trials to evaluate novel therapies, target HFrEF, previously termed systolic heart failure. However, it is estimated that 50% of heart failure patients have a preserved left ventricular ejection fraction, or HFpEF6. Initial studies attributed HFpEF to dysfunction of the myocardium during the filling phase of the cardiac cycle; diastolic stiffness, prolonged isovolumic LV relaxation, and slow LV filling were attributed to pathological dysfunction of the ventricular myocyte during diastole7. However, it is clear that in some instances, the left ventricular myocardium is an innocent bystander, manifesting dysfunctional filling due to volume overload, insufficiency of perfusion, or inadequate filling times8. In many instances, it is likely that a combination of perturbed diastolic relaxation9 and excessive volume due to extrinsic factors8 combine to perturb ventricular filling.
Vascular stiffening and generalized systemic vascular dysfunction are observed in patients with HFpEF10, 11. Reduced aortic distensibility and increased end-systolic elastance lead to exaggerated fluctuations in blood pressure for the same change in afterload and preload6. Indeed, therapeutic strategies that specifically target ventricular-arterial stiffening improve exercise tolerance in elderly, hypertensive individuals12. In addition, impaired flow-mediated vasodilation has been observed, implicating endothelial dysfunction in HFpEF pathophysiology and suggesting the possibility of benefit with therapies targeting nitric oxide bioavailability13. Pulmonary hypertension is also associated with HFpEF, and elevated pulmonary artery pressures predict mortality in HFpEF patients14.
Whether HFrEF and HFpEF are truly distinct disorders, or rather represent a syndrome that exists across a spectrum, is unknown. Also, within each of the two broad categories of HFrEF and HFpEF, a wide variety of disease etiologies dictate pathogenesis. In other words, heart failure, a syndrome defined on clinical terms, derives from numerous different diseases, such as myocardial infarction, hypertension, cytokine or neuroendocrine dyscrasias, genetic disorders, and more. One prominent example where “personalized medicine” has emerged to parse these elements is hypertrophic cardiomyopathy (HCM), where distinct genetic variants have been identified and which are informative in predicting phenotype and outcome15. Classically, familial HCM is caused by mutations in sarcomeric genes that control cardiac myocyte myofilament movement and calcium handling. At least one-third of patients presenting with HFpEF have normal extracellular matrix proteins (e.g. collagen) suggesting that cardiomyocyte stiffness due to sarcomeric aberrations also contributes to pathogenesis. It is conceivable that genetic testing for familial HCM may aid in accurately diagnosing HFpEF early on.
Familial dilated cardiomyopathy (DCM) can manifest mendelian patterns of inheritance, and mutations in at least 50 genes have been identified and linked to familial DCM16. These include sarcomeric genes, including those coding for proteins localized to the Z disk, nuclear membrane proteins, and proteins involved with connections to the plasma membrane. As with HCM, not all patients with DCM manifest the same phenotype. Importantly, some genetic variants, even within families, can cause either HCM or DCM, which renders diagnosis and risk prediction difficult based on genetic testing. Recently, however, mutations in the gene coding for the giant, sarcomeric protein, titin (TTN) have been identified in 25% of patients with familial idiopathic DCM, whereas only 3 of 231 patients with HCM harbor these mutations17. Mutations in this gene can also promote cardiomyocyte stiffness18 which can contribute to HFpEF. Therefore, testing for mutations in TTN may aid in differentiation of disease etiology and early diagnosis.
As research continues, more genetic mutations and polymorphisms will be identified, such as race-driven genetic predispositions19 that lead to cardiomyocyte stiffness or fibrosis. However, predicting disease based on genotype is further complicated by the fact that modifying genes, epigenetic factors, and environmental influences contribute to the complexity of the disparate phenotypes.
HFpEF is observed commonly in older women with a history of hypertension. Difficulty treating HFpEF derives, at least in part, from its segregation with multiple co-morbidities and a lack of standard definition20. In fact, trials using ACE inhibitors21, ARBs22, and β-blockers23 have failed to demonstrate efficacy in patients with HFpEF. Aldosterone antagonists are currently being tested in an NIH-funded trial called TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with Aldosterone Antagonist) (clinicaltrials.gov, NCT00094302).
Although current therapies have decreased overall morbidity and mortality in patients with HFrEF, individual responses are not uniform. For example, some heart failure patients on ACE inhibitor therapy harbor increased plasma Ang II levels, suggesting that ACE inhibition is incomplete24. Patients also respond variably to mineralocorticoid receptor antagonists (MRAs)25, 26. Inhibition of β-adrenergic signaling is standard-of-care for HFrEF patients27. However, beta-adrenergic receptor polymorphisms can render antagonist treatment ineffective28. New therapies targeting beta-receptor downstream effectors are being developed29.
Hypertensive ventricular remodeling
High blood pressure is the single most important risk factor for heart failure; approximately 75% of heart failure cases have antecedent hypertension30. As terminally differentiated cardiac myocytes are inefficient at reentering the cell cycle, these cells respond to pressure-overload stress by enlarging. This response, termed hypertrophy, ultimately leads to ventricular wall thickening and stiffening.
Based on Laplace's law, ventricular wall stress is proportional to both ventricular pressure and cavity radius and inversely proportional to wall thickness31. Thus, increases in wall thickness tend to diminish wall stress, decrease oxygen demand, and hence are adaptive. When the pressure stress is persistent, however, the myocardium slowly transitions to a state of decompensation and clinical heart failure. Our understanding of mechanisms underlying this transition from adaptive hypertrophy to maladaptive failure remains incomplete.
In recent years, a large number of preclinical studies have demonstrated that blunting load-induced hypertrophic growth of the LV is possible, even in the presence of persistent afterload stress, without compromising contractile performance32-34. These studies, then, have uncovered a potentially new target of anti-remodeling therapy, the hypertrophic phenotype itself. This strategy is based on the notion that while short-term hypertrophic remodeling may be adaptive, serving to normalize wall stress and oxygen demand, persistent, long-term activation of this response is detrimental. If true, suppressing pathological hypertrophy may be key to impeding progression to heart failure32. Suggestive evidence in humans supports therapeutic targeting of the hypertrophic process35.
Atrophic remodeling
One goal of antihypertensive therapy is to slow, arrest, or possibly even reverse the progression of cardiomyocyte growth. Indeed, the cardiac myocyte is capable of significant shrinkage or atrophy36. This shrinkage leads to reductions in LV mass and occurs under conditions of mechanical unloading (prolonged bedrest, mechanical support with a left ventricular assist device, weightlessness during space travel) or increased catabolic state (e.g. cancer)37-39. Atrophy is an energy-consuming process that involves changes in both anabolic and catabolic processes40.
Whether atrophy is associated with changes in cardiac function may depend on its magnitude, duration, and inciting factors. In a small number of patients with cachexia, significant loss of LV mass was not associated with specific cardiac abnormalities as compared with non-cachectic patients41. However, short-term mechanical unloading in animals by heterotopic heart transplantation can reverse hypertrophy, whereas long-term unloading was associated with decreased function and increased fibrosis42. Current investigations are ongoing to determine whether cardiac atrophy causes diastolic dysfunction during long-duration space flight37.
Ventricular remodeling in ischemic heart disease
Coronary artery disease is a leading cause of HFrEF43. In fact, most of our knowledge regarding LV remodeling is derived from patients and animal models of myocardial infarction. The extent of myocardial damage, as well as its location within the LV, directly impacts the magnitude of LV remodeling44. Underlying mechanisms derive directly from the infarction itself, including cell death and loss of contractile activity in the affected zone, as well as secondary ventricular dilation and remodeling in infarct-remote zones due to enhanced hemodynamic burden43. Over time, a process termed “infarct expansion” occurs, wherein unremitting mechanical forces stretch the abnormally stressed tissue. The end-result is a dilated LV with abnormal levels of wall stress and distorted and ineffective contractile performance.
Reperfusion of the occluded, infarct-related artery is key to minimizing infarct size and maintaining ventricular performance45. Significant advances in our understanding of the biology of ischemic heart disease, including the critical importance of restoration (percutaneous angioplasty) and maintenance (drug-eluting stents, anti-thrombotic agents) of arterial perfusion to the at-risk zone have culminated in robust improvements in clinical outcomes45. Although these advances have provided significant declines in mortality, the LV will inexorably, over subsequent months and years, remodel in response to abnormally elevated load and demand, leading to ventricular dilation and ultimately dysfunction43.
Remodeling of the LV following myocardial infarction has been divided into stages44. Following interruption of arterial perfusion from occlusion of a coronary vessel, death of cardiac myocytes immediately ensues. These cells die via necrosis, apoptosis, or possibly autophagy. Although cardiac stem cells have been identified in the adult heart, and cardiac myocytes themselves are capable of re-entering the cell cycle under only limited circumstances46-48, myocyte proliferation does not contribute significantly to the response to infarct-related wave of cell death. In the next stage of infarct healing, dying cardiac myocytes release intracellular proteins into the circulation and trigger an inflammatory response. Inflammatory cells, including neutrophils, monocytes, macrophages, and lymphocytes, infiltrate the tissue. These immune cells remove dead myocytes and pave the way for healing. After the resolution of the inflammatory response, cardiac fibroblasts proliferate and secrete extracellular matrix proteins, such as collagen I, to form a fibrotic scar that replaces dead myocytes. The resulting tightly cross-linked, fibrotic scar with significant tensile strength serves to prevent rupture. This remodeling of the LV continues progressively in response to increases in wall stress, provoking cardiac myocyte hypertrophy in the infarct border zone, wall thinning, and chamber dilation. This global adverse remodeling response leads to increases in both LV end-diastolic and end-systolic volumes and reduced ejection fraction43.
Contributing cellular events
Cardiac Myocyte Death
Biology
Cardiac myocytes carry out the contractile function of the myocardium, and they are largely incapable of replication; hence, their survival is crucial. Following myocardial injury, cardiac myocytes undergoing necrosis lyse, releasing intracellular contents, some of which can be detected in the blood and used as markers of injury (e.g. creatine kinase, cardiac troponins). Apoptosis, an energy-dependent, programmed cell death response, does not entail release of intracellular contents and does not trigger an inflammatory response; it is reversible up to a “point of no return”. An emerging literature suggests that necrosis may itself be a programmed cellular process, rather than uncontrolled disintegration of the cell49. Further, recent evidence suggests that necrosis and apoptosis are integrally linked and may be different faces of a single process (“necroptosis”)49.
Often, dying cells manifest evidence of up-regulated autophagy, an evolutionarily ancient process of ordered recycling of intracellular contents50, 51. Considerable debate has centered around whether this autophagic cascade reflects the cellular response to stress, serving to promote cell survival, or represents a process which, itself, contributes to cell death52. Consensus has emerged recently, however, that at least in some instances, autophagic cell death (programmed cell death type II) exists53, including in heart muscle54. That said, divergent views exist52. Irrespective of whether autophagy can trigger cardiomyocyte death, considerable evidence supports a model where cardiomyocyte autophagy can be adaptive or maladaptive, depending on the context55-59.
As a Therapeutic Target
Although all three types of cell death/intracellular remodeling occur within the heart, it is not entirely clear whether these are truly distinct and discrete events or represent a continuum of overlapping biochemical and molecular processes. Nevertheless, selective inhibitors targeting apoptosis (caspase inhibitors), necrosis (inhibitors of mitochondrial permeability transition pore [MPTP] opening), and necroptosis (necrostatin 1) have been employed in the heart60. Suppression of apoptosis decreases adverse remodeling and subsequent progression to heart failure in models of ischemia/reperfusion61, MI-induced heart failure62, and nonischemic cardiomyopathy63. However, optimal timing of therapy, targets for inhibition within apoptotic signaling cascades, precise mechanisms of inhibition, and even the cell types involved, remain unresolved. Of note, several pharmacological therapies in current clinical use may suppress cell death. For example, Ang II and norepinephrine can each trigger cardiomyocyte apoptosis, and their respective blockers antagonize these responses64, 65.
Cardiac Myocyte Hypertrophic Growth
Biology
A central tenet in cardiac biology is the notion that most adult cardiac myocytes are terminally differentiated cells and therefore do not proliferate; rather, they respond to stress by growing, shrinking, or dying. Recent work has revealed that a fraction of cells within the ventricle are, in fact, capable of re-entering the cell cycle and proliferating46-48, 66, 67, although the size of this fraction is the subject of intense debate. Nevertheless, the preponderance of evidence indicates that the majority of cardiomyocytes are incapable of dividing and respond to stress by eliciting a hypertrophic growth response. As part of this, a wide range of transcriptional and post-translational events occurs, including activation of a pattern of gene expression reminiscent of that observed during fetal development (“fetal gene program”).
Besides mechanical loading, cardiac myocytes respond to a variety of other growth cues, including cytokines, growth factors, catecholamines, vasoactive peptides, and hormones. Some evidence suggests that cell size is regulated by shared signaling pathways, whereas cell shape and sarcomeric organization are regulated by distinct pathways68. If borne out by additional studies, this observation might facilitate precise definitions of cellular phenotype-specific regulatory mechanisms.
As a Therapeutic Target
Although no therapeutic agents target hypertrophic growth directly, some strategies in current use alter the hypertrophic response secondarily, including suppression of neurohormones (catecholamines, Ang II, aldosterone), calcium (e.g. L-type Ca2+ channel blockers), or preload (e.g. vasodilators or diuretics). However, efficacy of these strategies varies and is dependent on the pathway that is modulated. Further, as there is redundancy among these pathways, downstream points of convergence may be more suitable to inhibit or reverse cardiac hypertrophy. Potential targets include oxidative stress, serine/threonine phosphatases, non-gated Ca2+ influx/Ca2+ signaling, downstream effectors of rapamycin or G-protein-coupled receptors, protein kinases, and chromatin remodeling agents (e.g. histone deacetylases)69.
Overlapping mechanisms exist in pathological (pressure overload) and physiological (exercise) hypertrophic growth, such as increased expression of genes responsible for cardiac myocyte structure, ion transport, and proteolysis70. However, genes associated with metabolic processes and muscle contraction may be up-regulated to a greater extent in response to exercise70. Furthermore, capillary growth does not keep pace with myocyte growth in disease models, which, in concert with fibrotic change, limits oxygen delivery to the myocardium71, 72.
Cardiac Myocyte Hyperplasia
Biology
Whereas growth of the adult heart has been classically held not to involve a significant hyperplastic response, recent evidence has demonstrated existence of progenitor cells resident within the myocardium, as well as cardiomyocytes capable of re-entering the cell cycle, findings which contradict the traditional idea that the heart is a strictly post-mitotic organ46-48, 66, 67. These dividing cells may participate in cardiac homeostasis at basal levels and potentially replace dying cardiac myocytes, albeit, at low levels. Cardiac progenitors include cells characterized by expression of cell surface markers including c-Kit73, Sca-174, or Islet-175, and cardiac “side population” (SP) cells76. Self-adherent clusters of cells termed “cardiospheres” have been developed from human biopsy specimens77, 78. The neonatal heart harbours cardiomyocytes capable of re-entering the cell cycle, promoting wound repair47, 79.
Cardiac progenitors have been localized to the epicardial surface of the heart, where they contribute to coronary vasculature formation during embryogenesis80. These epicardial cells are pluripotent and migrate into the myocardium, undergoing epithelial-to-mesenchymal transition to give rise to multiple cell types81, 82. Nevertheless, cardiac stem cells, regardless of their true identity, are unable to mount a proliferative response sufficient to replace dying myocardium in the setting of injury. One goal is to develop pharmacological strategies that enhance regenerative potential of resident progenitor cells sufficient to contribute to reversal of tissue loss83.
Fibrosis
Biology
A hallmark feature of ventricular remodeling is deposition of excessive extracellular matrix (ECM). This surplus ECM, which constitutes “scar” or fibrosis, promotes both contractile dysfunction and rhythm disturbances84. As a result, cardiac fibrosis contributes to morbidity and mortality in many forms of heart disease. Indeed, the amount of fibrotic scar in the myocardium correlates strongly with increased incidence of arrhythmias and sudden cardiac death85-87.
ECM deposition and fibrosis formation occur through the action of cardiac fibroblasts. In the setting of pathological stress, fibroblasts proliferate and differentiate into myofibroblasts, thereby gaining the capacity to contract and secrete collagen I, collagen III, and fibronectin84. Proliferation and activation of these cells, the most abundant cell type in the myocardium, derives from a variety of sources, including resident fibroblasts, adult epicardial cells undergoing endothelial to mesenchymal transition81, 88, and circulating, collagen-secreting bone marrow-derived cells89.
Scar formation following myocardial infarction arises from replacement fibrosis, where regions of myocyte “drop out” are replaced by scar. In contrast, fibrosis arising during hypertension-induced pressure overload and in remote regions following myocardial infarction is reactive (perivascular or interstitial), leading to decreased compliance and diminished oxygen diffusion capacity. Both individual myofibroblasts and collagenous septa within the left ventricle facilitate and propagate the arrhythmic phenotype of the remodeled heart90, 91.
As a Therapeutic Target
Cardiac fibrosis is an independent and predictive risk factor for heart failure in both ischemic and non-ischemic cardiomyopathy92-94. Recent work has demonstrated that cardiac fibrosis, long held to be irreversible, may regress under certain circumstances81, 95. Some evidence suggests that modulation of cardiac fibrosis alters the arrhythmic phenotype in patients with heart disease96, 97. To date, no therapeutic strategy has been developed to specifically target fibrosis in the heart. Cardiac fibroblasts are unique and phenotypically distinct from fibroblasts isolated from other tissues (as reviewed elsewhere98); they also display phenotypic heterogeneity within the heart itself. In addition, the precise phenotypes of fibroblasts from normal, injured, and failing hearts are ill defined, and mechanisms underlying the transition from normal wound healing to maladaptive fibrotic remodeling remain unresolved. Interestingly, the abundance of newly formed, thin collagen fibers increases in the remote region of infarcted heart, but decreases with time in the infarct zone, suggesting collagen maturation in the infarct zone99. Furthermore, neurohormonal inhibition leads to an increase in scar maturation while diminishing remote, reactive fibrosis100. As infarct-associated scar is necessary to prevent ventricular rupture, it may be advantageous to target new collagen fiber formation to allow for scar maturation.
Irrespective of these challenges, there is reason to believe that therapies focusing on cardiac fibrosis may prove salutary in the treatment of ventricular remodeling. Some therapies in current use may target, at least in part, cardiac fibroblasts. Specifically, Ang II provokes cardiac fibroblast proliferation and net accumulation of collagen in vitro and cardiac fibrosis in vivo101, 102. Interestingly, expression of Ang II receptors in cardiac fibroblasts exceeds that in cardiac myocytes103, and ARBs appear to have antifibrotic actions104. In patients with hypertensive heart disease, losartan reduced cardiac fibrosis and serum collagen markers105. In addition, treatment with HMG Co-A reductase inhibitors (“statins”) resulted in reduced fibrosis and reduced collagen synthesis106, 107. Small molecule inhibitors of histone deacetylases (HDACs) attenuate fibrosis in a preclinical model of pressure overload108 via mechanisms involving transcriptional silencing of the gene coding for connective tissue growth factor (unpublished observations).
Inflammation
The immune system plays a significant role in ventricular remodeling, and its persistent activation may lead to long-term cardiac injury. Specifically, activation of a variety of inflammatory molecules and pathways, the complement system, T cells, and the formation of autoantibodies have been reported in heart failure patients109-111. Consequently, a number of strategies have been proposed to mitigate the harm caused by these inflammatory events; most have failed112, 113. In the 1970's, it became apparent that immunosuppression with glucocorticoids or nonsteroidal anti-inflammatory agents conferred risk in patients with ischemic heart disease114, 115. More recently, however, early results of studies seeking to decrease autoantibody titers are promising116. High doses of intravenous immunoglobulin therapy to neutralize autoantibodies and the complement system improve heart failure symptoms, but long-term use is required117, 118. The few trials using immunoadsorption therapy in patients with dilated cardiomyopathy, where autoantibodies are thought to play a role in pathogenesis, were promising119, 120. Therapeutic plasma exchange, where large amounts of plasma are removed from the circulation and replaced with 5% albumin, potassium chloride, calcium gluconate, and then terminally supplemented with immunoglobulins to replace the removed proteins, is being tested121.
Vascular Remodeling
Biology
A wide range of cardiovascular diseases are marked by vascular remodeling. For example, both hypertension and immunosuppressive treatment are associated with vessel wall thickening122, 123. In preclinical models of myocardial infarction, hypertrophy in the border zone of the infarct is associated with diminished coronary flow reserve, increases in the media/lumen ratio, and increases in medial thickness124.
Development of significant coronary collateral circulation is a major mechanism of vascular remodeling. A recent meta-analysis reported diminished mortality risk in patients with high collateralization compared to those with low collateralization125. Another study reported that while collaterals may be protective during early stages of infarct healing, after infarction is complete their presence is not an independent predictor of clinical outcome126. Some evidence suggests that promoting angiogenesis in the setting of pressure overload can protect the heart from injury127.
As a Therapeutic Target
A large number of clinical trials of therapeutic neovascularisation employing gene or protein therapies have failed. This failure may stem, at least in part, from single, high dose administration of therapy128. For example, short-term exposure to VEGF (vascular endothelial growth factor) leads to leaky vessels that regress, whereas prolonged exposure promotes formation of more stable vessels129. To address this, novel polymers that degrade slowly and sustain release of growth factors have been employed130. However, it is unlikely that a single growth factor will be sufficient to promote neovascularization and limit adverse remodeling. Therefore, development of proangiogenic therapies will likely require combination therapy comprising multiple growth factors, such as FGF-2, HGF, MCP-1, GM-CSF, PDGF-BB, TGF-β131. In addition, careful selection of end points in trial design and appropriate methods for evaluating those end points may increase the likelihood of success of future proangiogenic therapies. Mode of delivery as well as timing of delivery may also be important.
As some of these growth factors tend to promote salvage of ischemic myocardium, early treatment may prove beneficial. Conversely, a study employing a mouse model of cardiac-specific induction and inactivation of a VEGF-sequestering soluble receptor reported that VEGF activity even at late stages of heart remodelling was sufficient to rescue function132. This study also suggested that a point-of-no-return may still exist, as augmenting neovascularization at late time points did not reverse fibrosis or myocyte hypertrophy.
Metabolic remodelling
Biology
Patients with diabetes and obesity are at increased risk of developing coronary artery disease, hypertension, and heart failure133. Under normal physiological conditions, the metabolic demands of the heart are met by metabolism of fatty acids and glucose, and to a lesser extent lactate and ketone bodies134. With the onset of insulin resistance and obesity-driven type II diabetes, uptake of metabolic substrates into cardiomyocytes becomes dysfunctional; fatty acid utilization is increased at the expense of glucose, which contributes to myopathy characterized by ventricular dilation, cardiomyocyte hypertrophy and death, interstitial fibrosis, and perturbations of diastolic relaxation. In animal models of obesity, triglycerides accumulate in the heart coupled with impaired mitochondrial function to oxidize the increased lipid load135. Several molecular and cellular mechanisms have been implicated in diabetic cardiomyopathy, including disordered activation of forkhead transcription factors, mTOR (mammalian target of rapamycin), microRNAs, mitochondrial dysfunction, the unfolded protein response, proteasome activation, and autophagy136, 137.
The term “obesity paradox” has been coined to describe the association between obesity and improvements in heart failure outcomes138; among patients with similar heart failure severity, obese patients manifest improved survival compared with normal-weight patients139, and higher BMIs are associated with lower mortality risk140. Whether this association relates to mechanism is unknown, but conceivably may be attributed to depression of the neurohumoral system or an increase in nutritional or metabolic reserve. For example, the adipokine, leptin, which regulates appetite and energy balance has direct cardioprotective effects against ischemia/reperfusion injury141.
The obesity paradox was tested in animal model, where insulin-insensitive rats were fed a high-fat diet and compared to insulin-insensitive lean rats, allowing for measurement of an effect of obesity in isolation. Obese rats manifested relative ischemia/reperfusion tolerance associated with activation of RISK (reperfusion injury salvage kinase) and nitric oxide synthase signalling pathways142.
The myocardium itself can have direct effects on metabolism within other organs. For example, natriuretic peptides such as ANF (atrial natriuretic factor) and BNP (B-type natriuretic peptide) are secreted from cardiomyocytes in response to stress143. These peptides, circulating levels of which are elevated in heart failure, have lipolytic effects on adipose tissue, which is specific to primates144. Recently, it was reported that cardiomyocyte-specific expression of MED13, a transcriptional regulator, or pharmacologic inhibition of miR-208a, antagonizes high-fat diet-induced obesity and improves insulin sensitivity and glucose tolerance145.
As a Therapeutic Target
Therapy that specifically targets cardiomyopathy due to obesity and diabetes does not currently exist. However, some strategies targeting weight loss manifest benefit to the heart. Weight loss from lifestyle changes or bariatric surgery is associated with decreases in LV dimensions, wall thickness, mass, and left atrial dimensions146. Removal of subcutaneous fat by liposuction does not elicit beneficial metabolic changes147. Also, whereas orlistat (a gastrointestinal lipase inhibitor) and sibutramine (a monoamine reuptake inhibitor) both to lead to weight loss and glycemic homeostasis, they have no significant effects on cardiac structure or dimensions148.
Recently, a proteasome inhibitor, MG-132, was shown to manifest anti-oxidative and anti-inflammatory functions in an animal model of diabetic cardiomyopathy149. In addition, inhibition of phosphodiesterase-5 with tadalafil attenuated inflammation, improved fasting glucose and triglyceride levels, decreased body weight and reduced infarct size in an ischemia/reperfusion injury model in obese, diabetic mice150. Synthetic mimetics of natriuretic peptides have been approved for treatment of acute heart failure, although the largest study so far of nesiritide, a recombinant form of human B-type natriuretic peptide, failed to detect improvements in mortality or rehospitalization151.
Electrophysiological remodeling
Patients with left ventricular hypertrophy are at significantly increased risk of malignant arrhythmias, accounting for a substantial component of the mortality associated with cardiac hypertrophy. Indeed, arrhythmia, especially ventricular tachyarrhythmia, is a major cause of death in patients with left ventricular heart failure. Sustained ventricular tachycardia and/or ventricular fibrillation can occur immediately post-MI, during the remodelling process, or late following injury.
In recent years, “electrical remodeling”, a term which encompasses alterations in multiple electrogenic transport processes within the cardiac myocyte, has emerged as an important pathophysiological mechanism in many types of cardiac pathology152, 153. Yet, our understanding of mechanisms underlying the myriad facets of electrical remodeling is limited. As a result, means of treating hypertrophy-associated arrhythmias remain disappointingly ineffective. Also, there is substantial evidence that alterations in transmembrane Ca2+ fluxes – a central feature of electrical remodeling – contribute to the pathogenesis of hypertrophy and failure by abnormally activating Ca2+-responsive signaling pathways.
Mechanisms underlying ventricular arrhythmia are multifactorial, but they derive, at least in part, from disordered electrical currents arising from prolongation of ventricular action potentials153. The resulting delay in the recovery of excitability, a consistent feature of ventricular hypertrophy, predisposes to early and late after-depolarizations. Superimposition of myocardial fibrosis, with altered electrotonic coupling between cells, slowed conduction, and dispersion of refractoriness, exacerbates the pro-arrhythmic phenotype.
Lengthening of ventricular cardiomyocyte action potential duration is commonly observed in both cardiac hypertrophy and failure, a phenotype which contrasts with the action potential shortening in the stressed (fibrillating) atrium. In the setting of excessive afterload, action potential duration increases more in subepicardial myocytes than in subendocardial myocytes154. In a canine model of pacing-induced heart failure, action potential duration increased significantly more in mid-myocardial cells than in subepicardial cells155.
Action potential prolongation is caused by a wide range of changes in myocyte ion channels and electrogenic ion transporters (reviewed elsewhere153). Briefly, loss of voltage-gated Na+ channel inactivation leading to a late inward sodium current is increased in failing cardiomyocytes156. In addition, down-regulation of outward K+ currents157, up-regulation of inward Ca2+ currents, and changes in Ca2+ current inactivation all contribute158, 159. Indeed, in many models of heart failure, diminished outward, repolarizing current secondary to down-regulated K+ channel levels (particularly Ito) is observed152, 160.
In contrast with heart failure, up-regulated inward Ca2+ current contributes to action potential prolongation in ventricular hypertrophy, particularly in models of modest hypertrophy. In fact, the density of L-type Ca2+ current (ICa,L) may be inversely correlated with disease progression, being increased in mild-to-moderate hypertrophy and decreased in severe hypertrophy and failure. Importantly, entry of small amounts of Ca2+ from the extracellular space triggers release of much larger amounts of Ca2+ from intracellular stores, amplifying even modest changes in inward Ca2+ flux. Also, in many species, membrane impedance is relatively high during phase 2 of the action potential, so changes in ICa,L have significant effects on action potential morphology and duration.
The Na+-Ca2+ exchanger (NCX), which catalyzes the bidirectional exchange of three Na+ ions for a single Ca2+ ion, is a major mechanism of Ca2+ elimination during diastole. As one net positive charge moves per reaction cycle, NCX generates a transmembrane current that approaches one-half the magnitude of ICa,L. Alterations in NCX activation in heart disease can contribute to late after-depolarizations and triggered ventricular activity.
Normal electrical conduction depends on cell-cell connections through gap junctions, such as connexin 43, and these connections can be disorganized in the failing heart disrupting normal impulse conduction161. Further, in the failing heart, phosphorylation of the ryanodine receptor by Ca2+/calmodulin-dependent protein kinase II (CaMKII) results in calcium leakage from sarcoplasmic reticulum (SR)162 with concurrent down-regulated expression of the sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) and reduced Ca2+ uptake into the SR163. The resulting depletion of SR Ca2+ stores coupled with elevations in cytoplasmic Ca2+ potentiates development of ventricular arrhythmia152.
Aging
Processes involved in maladaptive ventricular remodeling are age dependent, and most patients with heart disease are older. Indeed, one caveat to translating pre-clinical results into the human context is that most animal studies are performed using young animals. Mortality due to myocardial infarction increases with age, a fact not explained by larger infarcts164. Aging in mice is associated with an attenuated inflammatory response and decreased macrophage-mediated phagocytosis of dead cells165. In addition, aged mice have decreased numbers of myofibroblasts and perturbed extracellular matrix deposition resulting in malformed scar165. Cell death is also affected by age-related accumulation of mitochondrial damage and DNA mutations166. Cumulative organelle damage with age may also increase the need for clearance by autophagy, a process that declines with age51. Cardiomyocyte hypertrophy is more pronounced in aged heart, contributing to cardiac dysfunction167, and the intrinsic capacity of the heart to regenerate diminishes with aging46, 168.
Environmental Exposures
Cumulative environmental exposures alter disease risk, therapeutic responsiveness, biomarker expression, and cellular phenotypes in the heart. This can begin as early as during fetal development. Epidemiological data suggest that an adverse intrauterine environment increases the risk of cardiovascular disease in adulthood169. For example, prenatal hypoxia leads to altered expression of proteins such as protein kinase C epsilon, heat shock protein 70, and endothelial nitric oxide synthase169. However, these environmental exposures are not always mimicked reliably in preclinical studies using laboratory animals. Even when non-genetically modified laboratory rodents are fed a high fat diet to induce obesity and cardiac dysfunction, they do not develop atherosclerotic plaques as seen in humans. In chimpanzees, heart disease is primarily mediated by aberrant myocardial fibrosis and not vascular atherosclerotic plaque despite high levels of cholesterol and LDL170.
Recently, sialic acid N-glycolylneuraminic acid (Neu5Gc), a molecule not synthesized in humans but found in red meat and milk products, was identified in the endothelium of human atherosclerotic plaque171. This sugar, foreign to humans but not other mammals, promotes generation of antibodies and inflammation and is associated with carcinoma progression in humans172. In a recent epidemiological study of major dietary sources, high red meat intake was associated with coronary heart disease173. These data raise the possibility that this sugar could be used as a biomarker for patient stratification and therapeutic effectiveness, as well as being a therapeutic target itself (e.g. generation of neutralizing antibodies). Recent evidence implicates intestinal microbiota in the link between red meat consumption and cardiovascular risk174, as bacterial metabolism of red meat-derived l-carnitine can promote atherogenesis175.
Sex Differences
Coronary artery disease, the leading cause of HFrEF, occurs more commonly in men than women6, 176. By contrast, HFpEF affects women more commonly than men by a proportion of 2:1. Underlying mechanisms remain unclear, although sex differences have been described in cardiac structure, left ventricular diastolic function, ventricular-arterial stiffness, and aging177. Males, both human and animal models, tend to develop eccentric LV remodeling in response to stress, whereas females develop concentric remodeling177. In addition, women display enhanced regression of LV hypertrophy after aortic valve replacement compared to men, suggesting enhanced susceptibility to afterload stress in women178. Cardiac structural differences have also been demonstrated in regards to concentric LV remodeling and systolic hypertension which are enhanced in aging women179, 180. This may be exacerbated by the co-morbidities of aging, such as obesity, diabetes, and physical inactivity which may occur more frequently in women than men181, 182.
One possible mechanism underlying sexual dimorphism in heart disease involves mutations in mitochondrial DNA. Mitochondrial DNA encodes proteins associated with oxidative phosphorylation and is inherited from the mother's egg. Therefore, mutations in mitochondrial DNA would be passed on only by women, which can lead to family cohorts in which the offspring of female members are at risk for disease, whereas the offspring of male members are not. Some mitochondrial DNA mutations or seemingly neutral mitochondrial DNA polymorphisms may not be pathogenic in offspring immediately, but rather lead to inability to adapt to aging or environmental exposures, triggering emergence of pathology later in life. In fact, mitochondria harboring mutant DNA may selectively proliferate in response to a defect in the respiratory electron transport chain, rendering these mutant mitochondria more prevalent in post-mitotic cells such as cardiac myocytes. Sex-specific hormones also affect mitochondria. Ubiquinol-cytochrome-c reductase, a component of complex III within the respiratory electron transport chain, is reduced in the absence of ovarian hormones183.
Unlike the Y chromosome, the X chromosome is enriched in genes essential for development and viability. X-chromosome silencing occurs to inactivate one of the two X chromosomes in female cells. Originally, it was thought that silencing is maintained throughout the individual's lifespan; however, it has been shown more recently that loss of X-chromosome silencing can occur with aging184. Furthermore, approximately 15% of X-linked genes escape inactivation in a manner which differs across regions of the X chromosome185. Genomic imprinting of complex traits can also depend on sex186.
Conclusion
Heart failure is exploding in incidence and prevalence around the world. Defined by clinical criteria, this syndrome derives from a wide range of underlying disease etiologies and is marked by a diverse spectrum of structural, functional, electrophysiological, cellular, and molecular events. At one level, it comes as little surprise that only a small number of therapeutic strategies have emerged with efficacy, given these complexities. The effects of genetic, neurohumoral, environmental, and age-related influences -- and more -- combine to dictate pathogenesis and clinical outcome. Ultimately, these complexities must be elucidated in the context of the individual patient to optimize therapeutic success. That the myocardium comprises a host of cell types, each manifesting unique transcriptional, signaling, remodeling, proliferative, and death responses, underscores the seemingly insurmountable complexity of the challenge we face. However, unequivocal successes achieved already, and the expanding scope of the problem, will continue to drive progress in this fascinating field.
Acknowledgments
We thank members of the Hill lab for helpful discussions and critique.
Funding Sources: This work was supported by grants from the NIH (HL-080144, HL-0980842, HL-100401), Cancer Prevention and Research Institute of Texas (CPRIT, RP110486P3), the American Heart Association-DeHaan Foundation (0970518N), and the Fondation Leducq (11CVD04).
Footnotes
Conflicts of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 3.Hill JA, Olson EN. Muscle: Fundamental biology and mechanisms of disease. Academic Press; 2012. [Google Scholar]
- 4.Goldberg LR. Heart failure. Ann Intern Med. 2010;152:ITC61–15. doi: 10.7326/0003-4819-152-11-201006010-01006. quiz ITC616. [DOI] [PubMed] [Google Scholar]
- 5.Xie M, Burchfield JS, Hill JA. Pathological ventricular remodeling: Therapies. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.001879. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soufer R, Wohlgelernter D, Vita NA, Amuchestegui M, Sostman HD, Berger HJ, Zaret BL. Intact systolic left ventricular function in clinical congestive heart failure. Am J Cardiol. 1985;55:1032–1036. doi: 10.1016/0002-9149(85)90741-6. [DOI] [PubMed] [Google Scholar]
- 8.Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail. 2011;4:538–540. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 9.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 10.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 11.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Nakayama M, Talbot M, Nevo E, Fetics B, Gerstenblith G, Becker LC, Kass DA. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol. 1999;33:1602–1609. doi: 10.1016/s0735-1097(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 16.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123:19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linke WA. Sense and stretchability: The role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Shah S. Review: Heart failure with preserved ejection fraction in african americans. Ethn Dis. 2012;22:432–438. [PubMed] [Google Scholar]
- 20.Bhuiyan T, Maurer MS. Heart failure with preserved ejection fraction: Persistent diagnosis, therapeutic enigma. Curr Cardiovasc Risk Rep. 2011;5:440–449. doi: 10.1007/s12170-011-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (pep-chf) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 23.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (seniors) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 24.Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, Sanz G. Clinical implications of increased plasma angiotensin ii despite ace inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 25.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev. 2005;10:23–29. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 26.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 28.Liggett SB. Pharmacogenomics of beta1-adrenergic receptor polymorphisms in heart failure. Heart Fail Clin. 2010;6:27–33. doi: 10.1016/j.hfc.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamal FA, Smrcka AV, Blaxall BC. Taking the heart failure battle inside the cell: Small molecule targeting of gbetagamma subunits. J Mol Cell Cardiol. 2011;51:462–467. doi: 10.1016/j.yjmcc.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 33.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 34.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 35.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? AJP - Heart and Circulatory Physiology. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 36.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: A review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91:645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- 38.de Groot PC, van Dijk A, Dijk E, Hopman MT. Preserved cardiac function after chronic spinal cord injury. Arch Phys Med Rehabil. 2006;87:1195–1200. doi: 10.1016/j.apmr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita M, Takano H, Takaichi S, Taenaka Y, Nakatani T. Influence of prolonged ventricular assistance on myocardial histopathology in intact heart. Ann Thorac Surg. 1996;61:640–645. doi: 10.1016/0003-4975(95)01087-4. [DOI] [PubMed] [Google Scholar]
- 40.Cao DJ, Jiang N, Blagg A, Johnstone JL, Gondalia R, Oh M, Luo X, Yang KC, Shelton JM, Rothermel BA, Gillette TG, Dorn GW, Hill JA. Mechanical unloading activates foxo3 to trigger bnip3-dependent cardiomyocyte atrophy. J Am Heart Assoc. 2013;2:e000016. doi: 10.1161/JAHA.113.000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florea VG, Henein MY, Rauchhaus M, Koloczek V, Sharma R, Doehner W, Poole-Wilson PA, Coats AJ, Anker SD. The cardiac component of cardiac cachexia. Am Heart J. 2002;144:45–50. doi: 10.1067/mhj.2002.123314. [DOI] [PubMed] [Google Scholar]
- 42.Oriyanhan W, Tsuneyoshi H, Nishina T, Matsuoka S, Ikeda T, Komeda M. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J Heart Lung Transplant. 2007;26:16–23. doi: 10.1016/j.healun.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Dorn GW., 2nd Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6:283–291. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- 44.Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res. 2012;94:293–303. doi: 10.1093/cvr/cvs109. [DOI] [PubMed] [Google Scholar]
- 45.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 50.Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z, Klionsky DJ. Eaten alive: A history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen S, Kepp O, Kroemer G. The end of autophagic cell death? Autophagy. 2012;8 doi: 10.4161/auto.8.1.16618. [DOI] [PubMed] [Google Scholar]
- 53.Tsujimoto Y, Shimizu S. Another way to die: Autophagic programmed cell death. Cell Death Differ. 2005;12(2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 54.Miyata S, Takemura G, Kawase Y, Li YW, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li LH, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 56.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of amp-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 57.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerczuk PZ, Kloner RA. An update on cardioprotection: A review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 61.Hayakawa K, Takemura G, Kanoh M, Li YW, Koda M, Kawase Y, Maruyama R, Okada H, Minatoguchi S, Fujiwara T, Fujiwara H. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation. 2003;108:104–109. doi: 10.1161/01.CIR.0000074225.62168.68. [DOI] [PubMed] [Google Scholar]
- 62.Chandrashekhar Y, Sen S, Anway R, Shuros A, Arland I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-i cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol. 2004;43:295–301. doi: 10.1016/j.jacc.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Wencker D, Chandra M, Nguyen K, Miao WF, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin ii induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 65.Sabbah HN, Sharov VG, Gupta RC, Todor A, Singh V, Goldstein S. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J Am Coll Cardiol. 2000;36:1698–1705. doi: 10.1016/s0735-1097(00)00913-x. [DOI] [PubMed] [Google Scholar]
- 66.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–712. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D'Alessandro DA, Michler RE, Del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Bass GT, Ryall KA, Katikapalli A, Taylor BE, Dang ST, Acton ST, Saucerman JJ. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2012;52:923–930. doi: 10.1016/j.yjmcc.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: Moving beneath the cell surface. Nat Rev Drug Disc. 2007;6:617–635. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 70.Sheehy SP, Huang S, Parker KK. Time-warped comparison of gene expression in adaptive and maladaptive cardiac hypertrophy. Circ CardiovascGenet. 2009;2:116–124. doi: 10.1161/CIRCGENETICS.108.806935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. P53-induced inhibition of hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 73.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 74.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen YH, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JXJ, Evans S, Chien KR. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unno K, Jain M, Liao R. Cardiac side population cells: Moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–1363. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 78.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013 doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta 4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 81.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 82.Zhou B, Pu WT. Epicardial epithelial-to-mesenchymal transition in injured heart. J Cell Mol Med. 2011;15:2781–2783. doi: 10.1111/j.1582-4934.2011.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russell JL, Goetsch SC, Aguilar HR, Frantz DE, Schneider JW. Targeting native adult heart progenitors with cardiogenic small molecules. ACS Chem Biol. 2012;7:1067–1076. doi: 10.1021/cb200525q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 85.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 86.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 87.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 91.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 92.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 93.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 94.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 95.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res. 2011;109:407–417. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massare J, Berry JM, Luo X, Rob F, Johnstone JL, Shelton JM, Bassel-Duby R, Hill JA, Naseem RH. Diminished cardiac fibrosis in heart failure is associated with altered ventricular arrhythmia phenotype. J Cardiovasc Electrophysiol. 2010;21:1031–1037. doi: 10.1111/j.1540-8167.2010.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dimas V, Ayers C, Daniels J, Joglar JA, Hill JA, Naseem RH. Spironolactone therapy is associated with reduced ventricular tachycardia rate in patients with cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:309–314. doi: 10.1111/j.1540-8159.2010.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 99.van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JF, Daemen MJ, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52:2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 100.van den Borne SW, Isobe S, Zandbergen HR, Li P, Petrov A, Wong ND, Fujimoto S, Fujimoto A, Lovhaug D, Smits JF, Daemen MJ, Blankesteijn WM, Reutelingsperger C, Zannad F, Narula N, Vannan MA, Pitt B, Hofstra L, Narula J. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC Cardiovasc Imag. 2009;2:187–198. doi: 10.1016/j.jcmg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin ii is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 102.Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin ii induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension. 1995;25:1252–1259. doi: 10.1161/01.hyp.25.6.1252. [DOI] [PubMed] [Google Scholar]
- 103.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin ii stimulates cardiac myocyte hypertrophy via paracrine release of tgf-beta(1) and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 104.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 105.Lopez B, Querejeta R, Varo N, Gonzalez A, Larman M, Ubago JLM, Diez J. Usefulness of serum carboxy-terminal propeptide of procollagen type i in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104:286–291. doi: 10.1161/01.cir.104.3.286. [DOI] [PubMed] [Google Scholar]
- 106.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–985. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 107.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–324. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA. Suppression of class i and ii histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aukrust P, Gullestad L, Lappegard KT, Ueland T, Aass H, Wikeby L, Simonsen S, Froland SS, Mollnes TE. Complement activation in patients with congestive heart failure: Effect of high-dose intravenous immunoglobulin treatment. Circulation. 2001;104:1494–1500. doi: 10.1161/hc3801.096353. [DOI] [PubMed] [Google Scholar]
- 110.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: A translational approach. Curr Mol Med. 2003;3:161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 111.Caforio AL, Mahon NG, Baig MK, Tona F, Murphy RT, Elliott PM, McKenna WJ. Prospective familial assessment in dilated cardiomyopathy: Cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 112.Mann DL. Targeted anticytokine therapy and the failing heart. Am J Cardiol. 2005;95:9C–16C. doi: 10.1016/j.amjcard.2005.03.007. discussion 38C-40C. [DOI] [PubMed] [Google Scholar]
- 113.Flores-Arredondo JH, Garcia-Rivas G, Torre-Amione G. Immune modulation in heart failure: Past challenges and future hopes. Curr Heart Fail Rep. 2011;8:28–37. doi: 10.1007/s11897-010-0044-2. [DOI] [PubMed] [Google Scholar]
- 114.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: An observational cohort study. Lancet. 2002;359:118–123. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- 115.Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, Walker BR. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90:859–865. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaya Z, Leib C, Katus HA. Autoantibodies in heart failure and cardiac dysfunction. Circ Res. 2012;110:145–158. doi: 10.1161/CIRCRESAHA.111.243360. [DOI] [PubMed] [Google Scholar]
- 117.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, Lien E, Froland SS, Aukrust P. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–225. doi: 10.1161/01.cir.103.2.220. [DOI] [PubMed] [Google Scholar]
- 118.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, Gass A, Janosko K, Tokarczyk T, Kessler P, Mann DL, Feldman AM. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 119.Staudt A, Staudt Y, Dorr M, Bohm M, Knebel F, Hummel A, Wunderle L, Tiburcy M, Wernecke KD, Baumann G, Felix SB. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829–836. doi: 10.1016/j.jacc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 120.Staudt A, Hummel A, Ruppert J, Dorr M, Trimpert C, Birkenmeier K, Krieg T, Staudt Y, Felix SB. Immunoadsorption in dilated cardiomyopathy: 6-month results from a randomized study. Am Heart J. 2006;152:712, e711–716. doi: 10.1016/j.ahj.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 121.Torre-Amione G, Orrego CM, Khalil N, Kottner-Assad C, Leveque C, Celis R, Youker KA, Estep JD. Therapeutic plasma exchange a potential strategy for patients with advanced heart failure. J Clin Apher. 2010;25:323–330. doi: 10.1002/jca.20264. [DOI] [PubMed] [Google Scholar]
- 122.Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, Devereux RB. Vascular biomarkers in the prediction of clinical cardiovascular disease: The strong heart study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmauss D, Weis M. Cardiac allograft vasculopathy: Recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 124.Jugdutt BI, Menon V, Kumar D, Idikio H. Vascular remodeling during healing after myocardial infarction in the dog model: Effects of reperfusion, amlodipine and enalapril. J Am Coll Cardiol. 2002;39:1538–1545. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 125.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur Heart J. 2012;33:614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 126.Steg PG, Kerner A, Mancini GB, Reynolds HR, Carvalho AC, Fridrich V, White HD, Forman SA, Lamas GA, Hochman JS, Buller CE. Impact of collateral flow to the occluded infarct-related artery on clinical outcomes in patients with recent myocardial infarction: A report from the randomized occluded artery trial. Circulation. 2010;121:2724–2730. doi: 10.1161/CIRCULATIONAHA.109.933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–2010. doi: 10.1016/j.athoracsur.2003.11.003. discussion 2011. [DOI] [PubMed] [Google Scholar]
- 128.Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: Protein therapy. Cardiovasc Res. 2005;65:649–655. doi: 10.1016/j.cardiores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Dor Y, Djonov V, Keshet E. Induction of vascular networks in adult organs: Implications to proangiogenic therapy. Ann N Y Acad Sci. 2003;995:208–216. doi: 10.1111/j.1749-6632.2003.tb03224.x. [DOI] [PubMed] [Google Scholar]
- 130.Yao C, Prevel P, Koch S, Schenck P, Noah EM, Pallua N, Steffens G. Modification of collagen matrices for enhancing angiogenesis. Cells Tissues Organs. 2004;178:189–196. doi: 10.1159/000083730. [DOI] [PubMed] [Google Scholar]
- 131.Molin D, Post MJ. Therapeutic angiogenesis in the heart: Protect and serve. Curr Opin Pharmacol. 2007;7:158–163. doi: 10.1016/j.coph.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 132.Gordon O, Gilon D, He Z, May D, Lazarus A, Oppenheim A, Keshet E. Vascular endothelial growth factor-induced neovascularization rescues cardiac function but not adverse remodeling at advanced ischemic heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1642–1651. doi: 10.1161/ATVBAHA.112.248674. [DOI] [PubMed] [Google Scholar]
- 133.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 134.Hue L, Taegtmeyer H. The randle cycle revisited: A new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: Translational implications. Circ Res. 2012;111:1565–1577. doi: 10.1161/CIRCRESAHA.111.262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Battiprolu PK, Lopez-Crisosto C, Wang ZV, Nemchenko A, Lavandero S, Hill JA. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life sciences. 2013;92:609–615. doi: 10.1016/j.lfs.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality--fat or fiction? Nat Rev Cardiol. 2011;8:233–237. doi: 10.1038/nrcardio.2010.209. [DOI] [PubMed] [Google Scholar]
- 139.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: The obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 140.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: Body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 141.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donner D, Headrick JP, Peart JN, du Toit EF. Obesity improves myocardial ischaemic tolerance and risk signalling in insulin-insensitive rats. Dis Model Mech. 2013;6:457–466. doi: 10.1242/dmm.010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-a: Role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557–573. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M, Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R257–265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 145.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microrna governs systemic energy homeostasis by regulation of med13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alpert MA, Terry BE, Mulekar M, Cohen MV, Massey CV, Fan TM, Panayiotou H, Mukerji V. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80:736–740. doi: 10.1016/s0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]
- 147.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 148.Padwal RS, Majumdar SR. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Sun W, Du B, Miao X, Bai Y, Xin Y, Tan Y, Cui W, Liu B, Cui T, Epstein PN, Fu Y, Cai L. Therapeutic effect of mg-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: Roles of nrf2 and nf-kappab. Am J Physiol Heart Circ Physiol. 2013;304:H567–578. doi: 10.1152/ajpheart.00650.2012. [DOI] [PubMed] [Google Scholar]
- 150.Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC. Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PloS One. 2012;7:e45243. doi: 10.1371/journal.pone.0045243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Hill JA. Electrophysiological remodeling in heart failure. J Mol Cell Cardiol. 2010;48:619–632. doi: 10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Tandan S, Hill JA. Calcineurin-dependent ion channel regulation in heart. 2012 doi: 10.1016/j.tcm.2013.05.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: A mechanism of abnormal impulse conduction in heart failure. Circulation. 2006;113:1849–1856. doi: 10.1161/CIRCULATIONAHA.106.615682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 156.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: Role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaab S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 158.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 159.Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, Sugianto J, Johnstone JL, Sun Y, Hill JA. Ca2+/calmodulin-dependent protein kinase ii-dependent remodeling of ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283:25524–25532. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hill JA. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc Med. 2003;13:316–322. doi: 10.1016/j.tcm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 161.Mueller EE, Momen A, Masse S, Zhou YQ, Liu J, Backx PH, Henkelman RM, Nanthakumar K, Stewart DJ, Husain M. Electrical remodelling precedes heart failure in an endothelin-1-induced model of cardiomyopathy. Cardiovasc Res. 2011;89:623–633. doi: 10.1093/cvr/cvq351. [DOI] [PubMed] [Google Scholar]
- 162.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 163.Movsesian MA, Karimi M, Green K, Jones LR. Ca(2+)-transporting atpase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation. 1994;90:653–657. doi: 10.1161/01.cir.90.2.653. [DOI] [PubMed] [Google Scholar]
- 164.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The investigators of the gruppo italiano per lo studio della sopravvivenza nell'infarto miocardico (gissi-2) N Engl J Med. 1993;329:1442–1448. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- 165.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Susic D, Frohlich ED. The aging hypertensive heart: A brief update. Nat Clin Pract Cardiovasc Med. 2008;5:104–110. doi: 10.1038/ncpcardio1091. [DOI] [PubMed] [Google Scholar]
- 168.Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3(1):S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 169.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–666. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Finch CE. Evolution in health and medicine sackler colloquium: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107(1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, Chen X, Witztum JL, Varki NM, Varki A. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hedlund M, Ng E, Varki A, Varki NM. Alpha 2-6-linked sialic acids on n-glycans modulate carcinoma differentiation in vivo. Cancer Res. 2008;68:388–394. doi: 10.1158/0008-5472.CAN-07-1340. [DOI] [PubMed] [Google Scholar]
- 173.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, Didonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: Impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 178.Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, Dworatzek E, Mahmoodzadeh S, Schubert C, Becher E, Hampl H, Hetzer R. Regression of myocardial hypertrophy after aortic valve replacement: Faster in women? Circulation. 2010;122:S23–28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- 179.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 180.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 181.De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, Lee ET, Howard BV. Sex differences in obesity-related changes in left ventricular morphology: The strong heart study. J Hypertens. 2011;29:1431–1438. doi: 10.1097/HJH.0b013e328347a093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: A community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huynh H, Servant N, Chalifour LE. Ubiquinol-cytochrome-c reductase 7.2 kda protein of mitochondrial complex iii is steroid-responsive and increases in cardiac hypertrophy and hypertension. Can J Physiol Pharmacol. 2007;85:986–996. doi: 10.1139/y07-086. [DOI] [PubMed] [Google Scholar]
- 184.Bennett-Baker PE, Wilkowski J, Burke DT. Age-associated activation of epigenetically repressed genes in the mouse. Genetics. 2003;165:2055–2062. doi: 10.1093/genetics/165.4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in x-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 186.Hager R, Johnstone RA. The genetic basis of family conflict resolution in mice. Nature. 2003;421:533–535. doi: 10.1038/nature01239. [DOI] [PubMed] [Google Scholar]