Abstract
Development of the spine and thoracic cage consists of a complex series of events involving multiple metabolic processes, genes and signaling pathways. During growth, complex phenomena occur in rapid succession. This succession of events, this establishment of elements, is programmed according to a hierarchy. These events are well synchronized to maintain harmonious limb, spine and thoracic cage relationships, as growth in the various body segments does not occur simultaneously at the same magnitude or rate. In most severe cases of untreated progressive early-onset spinal deformities, respiratory insufficiency and pulmonary and cardiac hypertension (cor pulmonale), which characterize thoracic insufficiency syndrome (TIS), can develop, sometimes leading to death. TIS is the inability of the thorax to ensure normal breathing. This clinical condition can be linked to costo-vertebral malformations (e.g., fused ribs, hemivertebrae, congenital bars), neuromuscular diseases (e.g., expiratory congenital hypotonia), Jeune or Jarcho-Levin syndromes or to 50% to 75% fusion of the thoracic spine before seven years of age. Complex spinal deformities alter normal growth plate development, and vertebral bodies become progressively distorted, perpetuating the disorder. Therefore, many scoliotic deformities can become growth plate disorders over time. This review aims to provide a comprehensive review of how spinal deformities can affect normal spine and thoracic cage growth. Previous conceptualizations are integrated with more recent scientific data to provide a better understanding of both normal and abnormal spine and thoracic cage growth.
Keywords: Spine, Thorax, Thoracic cage, Growth, Early-onset spinal deformity, Children
Core tip: Development of the spine and thoracic cage is a complex series of events involving multiple metabolic processes, genes and signaling pathways. During growth, complex phenomena follow a rapid succession. This succession of events, this establishment of elements, is programmed according to a hierarchy. Complex spinal deformities alter normal growth plate development and vertebral bodies become progressively distorted, perpetuating the disorder. Therefore, many scoliotic deformities can become growth plate disorders over time.
INTRODUCTION
Growth is the basis of development. Normal spines are characterized by symmetric and harmonious growth, although spinal growth is the product of more than 130 growth plates working at different paces. In severe spinal deformities, growth becomes asymmetrical as a result of growth plate disorganization. Complex spinal deformities alter normal growth plate development, and vertebral bodies become progressively distorted, perpetuating the disorder. Therefore, many scoliotic deformities can become growth plate disorders over time[1-5].
Development of the spine and thoracic cage is a complex series of events involving multiple metabolic processes, genes and signaling pathways. During growth, complex phenomena follow each other in rapid succession. This succession of events, this establishment of elements, is programmed according to a hierarchy. These events are well synchronized to maintain harmonious limb, spine and thoracic cage relationships, as growth in the various body segments does not occur simultaneously at the same magnitude or rate[5-9].
The slightest error or modification can lead to a malformation or deformity that has negative effects on standing and sitting height; thoracic cage shape, volume and circumference; and lung development. These distortions alter the virtuous circle of growth[1,6,8,10-12].
Spinal deformities influence the various skeletal and organic components of the thoracic cage and cavity; however, these influences are not fully understood[1-3,5,8,10,11].
Only comprehensive knowledge of normal growth parameters allows a better understanding of both normal and abnormal spine and thoracic cage growth and of the pathologic changes in a growing spine and chest resulting from a spinal deformity[1-3].
As a spinal deformity progresses, not only is spinal growth affected, but the size and shape of the thoracic cage are also modified. In a “domino effect”, this distortion of the thorax eventually interferes with lung development and cardiac function, leading affected children to develop potentially lethal TIS and cor pulmonale[11]. Cardiac and respiratory problems can develop after precocious vertebral arthrodesis or as a consequence of pre-existing severe vertebral deformities and can vary in pattern and timing according to the existing degree of the deformity[1-3,5,7-10,12-15].
The aim of this work is to provide a comprehensive review of how spinal deformities can affect normal spine and thoracic cage growth.
RESEARCH
This review aims to provide a comprehensive review of how spinal deformities can affect normal spine and thoracic cage growth. The work is based on a search of English and French literature from 1970 to 2013 in the PubMed database. A manual search of the references cited in the retrieved articles was also performed. Some of the data presented in this article were gathered from studies performed in the 1980s and 1990s and their applicability to populations of different ethnicities, geographical regions and developmental stages has yet to be elucidated. The universal applicability of these results is therefore open to debate. Previous conceptualizations have been integrated with newer scientific data to provide a basis for a better understanding of both normal and abnormal spine and thoracic cage growth.
GROWTH OF THE SPINE
Neurocentral synchondrosis
Neurocentral synchondrosis is a physis in the spine located at the junction of the pedicle and the vertebral body and is important in the growth of the vertebral body and the posterior arch. In a growing pig model, unilateral transpedicular screw fixation traversing the neurocentral synchondrosis can produce asymmetric growth of the synchondrosis and create scoliosis with the convexity on the side of the screw fixation. However, in humans, neurocentral synchondrosis fuses at approximately age nine, and by five years of age, the spinal canal has already grown to approximately 95% of its final size. Therefore, perivertebral arthrodesis performed after age five has no influence on the size of the spinal canal[1,2,4,16].
Sitting height
Sitting height correlates strictly with trunk height and is, on average, approximately 34 cm at birth and 88 and 92 cm at the end of growth for girls and boys, respectively. In children with severe spinal deformities, loss of sitting height is related to the severity of the deformity. For this reason, it is important to monitor changes in the sitting height rather than in the standing height in children with scoliosis. During the first three years of life or if a child has a neurologic disorder or a collapsing spine, it is recommended that the sitting height be measured in the supine position.
Growth is a succession of acceleration and deceleration phases comprising three periods (Figure 1). The first period is from birth to age five and is characterized by a gain in sitting height of 27 cm, with 12 cm of growth occurring during the first year of life. The second period is from age five to ten years and is a quiescent phase, as sitting height increases by 2.5 cm/year. The third period corresponds to puberty and is characterized by a gain in sitting height of approximately 12 cm[1-3]. At the beginning of puberty, the average remaining growth in the sitting height is approximately 12.5 cm for boys and approximately 11.5 cm for girls; 2/3 of this growth occurs during the “peak height velocity” or “acceleration phase”. The average remaining growth in sitting height during the “deceleration phase” is approximately 4 cm for boys and 3.5 cm for girls [1-4,10].
Figure 1.
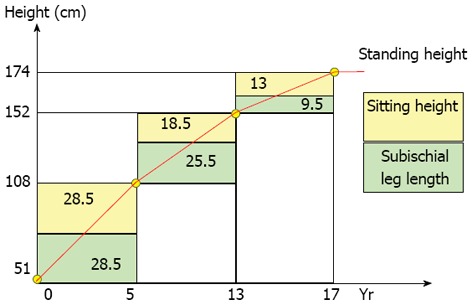
Sitting and standing height. From birth to age five, the development of the trunk is substantial. Between age 5 and puberty, the lower extremities grow more than the trunk and the spinal growth rate decreases from 2.2 to 1.1 cm/year. During puberty, the trunk grows more than the lower extremities. At the beginning of puberty, the remaining standing height is approximately 20 cm, of which 2/3 is at the level of the trunk and 1/3 is at the level of the lower extremities. At Risser I (menarche), the remaining growth of the trunk is approximately 3 to 4 cm.
T1-S1 spinal segment
Assessment of the T1-S1 spinal segment is important, as many spinal deformities originate in this segment. At birth, the T1-S1 segment measures approximately 20 cm and reaches 45 cm at skeletal maturity (Figure 2). The height of the spine accounts for 60% of total sitting height, and the head and pelvis account for the remaining 40%. The T1-S1 segment accounts for approximately 50% of the sitting height; two-thirds of this segment is thoracic spine, and one-third is lumbar spine. The T1-S1 segment grows approximately 10 cm during the first five years of life (2 cm/year), approximately 5 cm between ages five and 10 (1 cm/year), and approximately 10 cm between age 10 and skeletal maturity (1.8 cm/year) (Table 1). In patients who developed scoliosis before four years of age, early surgery does not modify the deformation produced by the scoliosis or preserve respiratory function, even when the anterior growth of the spine is arrested. Therefore, it is very important for a surgeon to consider the state of skeletal maturity and the amount of growth remaining in the spinal segment to be fused[1,2,5,6,11].
Figure 2.
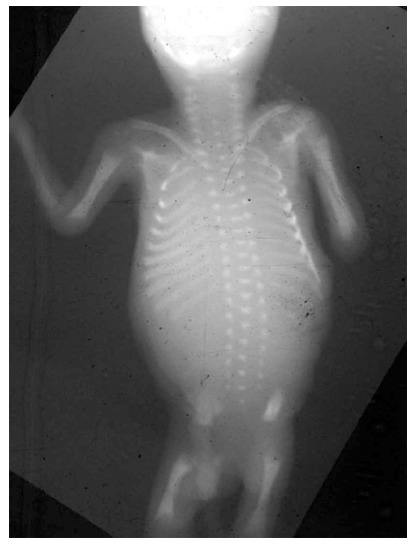
Spine at birth. At birth, only 30% of the spine is ossified. At birth, the T1-S1 segment measures approximately 20 cm and reaches 45 cm at skeletal maturity.
Table 1.
Evaluation of T1–T12 and L1–L5 spinal segments from birth to skeletal maturity
| Developmental stage |
Spinal segment |
|||
|
Boys |
Girls |
|||
| T1-T12 | L1-L5 | T1-T12 | L1-L5 | |
| Newborn | 11 | 7.5 | 11 | 7.5 |
| Child | 18 | 10.5 | 18 | 10.5 |
| Young | 22 | 12.5 | 22 | 12.5 |
| Adult | 28 | 16 | 26 | 15.5 |
Values are averages and expressed in cm[15].
T1-T12 spinal segment
T1-T12 is the posterior pillar of the thoracic cage and an important segment. It measures, on average, approximately 12 cm at birth, 18 cm at five years of age, and 27 cm at skeletal maturity. The thoracic spine constitutes 30% of the sitting height, and each single thoracic vertebra and its disc account for 2.5% of the sitting height. In normal children, the longitudinal growth of the thoracic spine is approximately 1.3 cm/year between birth and five years, 0.7 cm/year between the ages of five and 10 years, and 1.1 cm/year during puberty. A precocious arthrodesis of the T1-T12 segment affects thoracic growth and lung development. In young children with progressive deformities, there is a decrease in longitudinal growth and a loss of the normal proportionality of trunk growth[1-3,5,11]. Untreated progressive early-onset spinal deformities have been associated with short trunk, short stature and, often, respiratory insufficiency. In untreated patients, the loss of vital capacity in those with early-onset scoliosis has been shown to be 15% greater than in those with adolescent idiopathic scoliosis. Emans et al[15] showed that pelvic inlet width, measured by computerized tomograms or plain radiographs, is an age-independent predictor of the expected thoracic dimensions in unaffected children and adolescents. That study also established normal range standards for chest and spine dimensions to assist in the assessment of treatment outcomes[2,15].
The twelve thoracic vertebral bodies have costal facets that articulate with the costal heads of the ribs on each side and two transverse processes that articulate with the tubercles these ribs. Together, these synovial articulations constitute the costo-vertebral joint and play an essential role in elevating and depressing the ribs to increase the anterior-posterior and transverse diameters of the thoracic cavity during respiration.
Respiratory problems can develop after a precocious vertebral arthrodesis or as a consequence of pre-existing severe vertebral deformities and can vary in pattern and timing according to the existing degree of deformity. Variations in the extent of experimental arthrodesis also have different effects on both growth and thoraco-pulmonary function. Early spinal fusion for progressive scoliosis further limits spinal growth, leading to diminished thoracic height and a long-term loss of vital capacity. Therefore, early spinal fusion, especially if performed in the thoracic region, is a cause of respiratory insufficiency and adds loss of pulmonary function to the pre-existing spinal deformity. Karol et al[13] reported that a thoracic spine height of 18 cm or more is necessary to avoid severe respiratory insufficiency. In addition, they showed that children undergoing a precocious spinal fusion exhibit reductions in thoracic depth and shorter T1-T12 segments compared to normal subjects. The forced vital capacity may decrease by 50% of the predicted volume if more than 60% of the thoracic spine, eight thoracic vertebrae, is fused before eight years of age. In their clinical work, Karol et al[13] confirmed some of the previously published experimental findings published [5].
L1-L5 spinal segment
The lumbar vertebrae have well-developed vertebral bodies and spinous, transverse, and superior articular processes that provide attachment sites for ligaments and muscles (erector spinae and transversospinalis muscles). The L1-L5 length is, on average, approximately 7.5 cm at birth and 16 cm at skeletal maturity. The lumbar spine constitutes approximately 18% of the sitting height, and a single lumbar vertebra and its disc account for 3.5% of sitting height. At 10 years of age, the lumbar spine has reached approximately 90% of its final height but only 60% of its definitive volume. A perivertebral arthrodesis of the lumbar spine performed after 10 years of age results in a minimal loss of sitting height[1,2].
GROWTH OF THE CHEST
Thoracic cage volume, circumference and shape
At birth, the thoracic cage volume is approximately 6% of its final size and reaches 30% by age five and 50% by age 10. Between age 10 and skeletal maturity, the thoracic cage volume doubles before it ultimately stops growing. Not all types of thoracic growth progress at the same speed. At five years of age, the trunk has reached approximately 66% of its final height, whereas thoracic volume is only 30% of its definitive size.
The thoracic circumference corresponds to 95% of the sitting height and increases during the first five years of life and during puberty. On average, the thoracic perimeter is 32.3 cm in boys and 31.5 cm in girls at birth, and it attains a mean value of 89.2 cm and 85.4 cm, respectively.
The thoracic cage shape varies with age. At birth, the difference between the thoracic depth and width is minimal, and the thoracic depth/thoracic width ratio is very close to 1. Conversely, at skeletal maturity, the thoracic depth/thoracic width ratio is less than 1, as width increases more than depth. For this reason, the overall thoracic cage shape evolves from ovoid at birth to elliptical at skeletal maturity. At the end of growth, the thorax has an average depth of 21 cm in boys and 17.7 cm in girls and an average thoracic width of 28 cm and 24.7 cm in boys and girls, respectively. At skeletal maturity, the thoracic depth and width represent approximately 20% and 30% of sitting height, respectively. The thoracic cage is part of the rib-vertebral-sternal complex[1-4,6,8-11].
Emans et al[15] reviewed a total of 198 CT scans of healthy patients ranging from 0 to 21 years of age and showed that pelvic inlet width, measured by computerized tomograms or plain radiographs, is an age-independent predictor of the expected thoracic dimensions in unaffected children and adolescents.
Lung growth
Lung growth is a complex topic because different pulmonary structures and regions grow at different rates. At birth, a newborn has the same number of conducting airways as an adult. Tracheal caliber increases 2 to 3 fold between birth and skeletal maturity. In contrast, the peripheral regions of the lung that contain alveoli and pulmonary capillaries (acinar regions) undergo substantial postnatal growth and development. From infancy to adulthood, alveolar number increases by up to 6 fold. The alveolar-capillary surface area increases by more than 10 fold as a result of increased alveolar number, complexity and septation, as well as capillary development. Therefore, early-onset scoliosis adversely affects thoracic growth at a critical period of maximum respiratory growth, which induces irreversible changes in the thoraco-pulmonary structure[11,17-20].
Lung and thoracic cage growth volume increase in a non-linear fashion over the first twenty years of life, with rapid growth occurring before 4 years of age and during the pubertal growth spurt[11]. In the absence of spinal disease, the volumes of both the lungs and thorax are proportional to height, and norms for lung function in children, including lung volumes, are based primarily on standing height (Figure 3). Therefore, double extrinsic disturbances of the chest wall functions are a potential source of respirator failure, as thoracic cage deformities prevent hyperplasia of lung tissue and intrinsic alveolar hypoplasia. It is important to preserve both thoracic growth and lung volume during this critical period of life[7,11].
Figure 3.
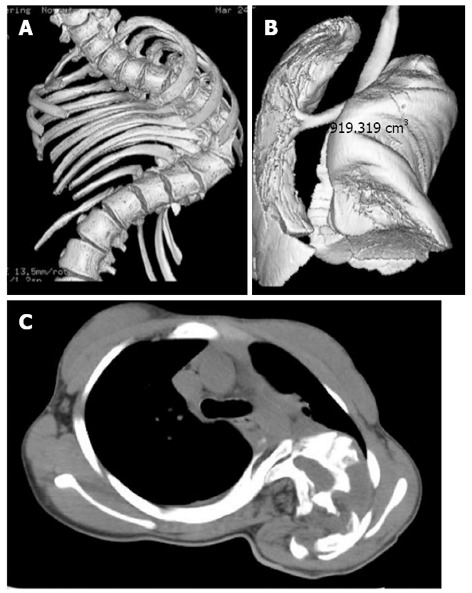
Thoracic cage distortion and lung compression. Lung (A) and thoracic cage (B) growth volumes increase in a non-linear fashion over the first twenty years of life. The volumes of both structures are proportional to height in the absence of spinal disease. Spinal deformities alter normal spine and thorax growth, prevent the lungs from expanding and lead to thoracic insufficiency syndrome (C).
In a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system, Gollogly et al[18] showed that lung parenchyma volume is a function of age (Figure 4).
Figure 4.
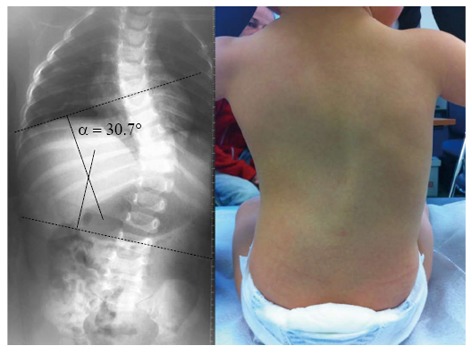
Early-onset spinal deformity. Thirteen-month-old female with progressive infantile scoliosis.
Effects of early-onset scoliosis on thoracic cage development
It is known that spinal deformations adversely affect thoracic development by changing the shape of the thorax and reducing its normal motility. The “rib-vertebral-sternal complex”, which encloses the three-dimensional thoracic cavity, tends to constitute an elastic structural model similar to a cube in shape[7]. However, in the presence of scoliosis, it becomes flat, rigid and elliptical and prevents the lungs from expanding. These deformities can be lethal in the most severe cases as a result of reciprocal interactions and influences among the various skeletal and organic components of the thoracic cage and cavity that are not well understood.
Several studies have focused on the anatomical influences of experimental arthrodesis on spinal growth, chest development, and thoraco-pulmonary function. These reports have demonstrated that early arthrodesis, as well as severe spinal deformities, can adversely affect the development of the spine and the thorax by changing their shape and reducing normal mobility[5-10,21].
Post-mortem studies showed that patients with early-onset deformities have fewer alveoli than expected and exhibit emphysematous changes in the existing alveoli[11,17]. These studies suggest that mechanical compression is not a factor in reducing the number of alveoli; this reduction is most likely due to a premature cessation of alveolar proliferation. Indeed, from the late fetal stage to four years of age, the number of alveoli increases by a factor of 10, and the development of the bronchial tree ends at approximately eight to nine years of age.
Canavese et al[5-9] evaluated the consequences of disturbed growth of vertebral bodies on the development of the ribs, sternum, and lungs. These consequences are much more evident when arthrodesis is performed at the critical T1-T6 segment of the thoracic spine. The development of the thoracic cage and lungs is a complex process that requires perfect synergy among the various components of the rib-vertebral-sternal complex. Alterations to any of these elements affect and change the development and growth of the others. Therefore, to preserve thoracic motility and permit a normal development of the respiratory tree, treatment should not only focus on the spine but also consider the rib-vertebral-sternal complex as a whole[5,6,8-11].
Maximal compensatory lung growth may well be limited to a specific time after birth and diminish after the period of alveolar multiplication is complete. Published estimates of this period of alveolar multiplication vary from 1 to 8 years of age. The optimal timing of surgical intervention to expand the thoracic cage, minimizing progressive postnatal pulmonary hypoplasia and maximizing compensatory lung growth, still needs to be determined. It is likely to be earlier rather than later in childhood[5-11,18-20], as the “golden” period for both thoracic spine and thoracic cage growth occurs between birth and 4 years of age and coincides with lung development[11,17-20].
DISCUSSION
Only comprehensive knowledge of normal growth parameters allows a better understanding of the pathologic changes in a growing organism resulting from an early-onset spinal deformity. As the spinal deformity progresses, in a “domino effect”, not only are spinal growth is affected, but size and shape of the thoracic cage are also modified. This distortion of the thorax interferes with lung development. Over time, the spinal disorder changes in nature from a mainly orthopedic issue to a severe pediatric systematic disease, with thoracic insufficiency syndrome[1-11,22,23] and cor pulmonale[14].
In normal children, the longitudinal growth of the thoracic spine is approximately 1.3 cm/year between birth and 5 years of age, 0.7 cm/year between the ages of 5 and 10 years and 1.1 cm/year during puberty.
The thoracic spine from T1 to T12 is the posterior pillar of the thoracic cage and is an important segment. A precocious arthrodesis of this segment can have repercussions on thoracic growth and lung development. In young children with progressive deformities, arthrodesis can result in decreased of longitudinal growth and a loss of normal proportionality of trunk growth. Karol et al[13] showed that a thoracic spine height of 18-22 cm or more is necessary to avoid severe respiratory insufficiency. They showed that children undergoing a precocious spinal fusion have a reduced thoracic depth and a shorter T1-T12 segment compared to normal subjects[13]. The forced vital capacity may decrease by 50% of the predicted volume if more than 60% of the thoracic spine, or 8 thoracic vertebrae, is fused before 8 years of age[11,17-19,21-24].
Untreated progressive early-onset spinal deformity has been associated with short trunk, short stature and often respiratory insufficiency. In untreated patients, the loss of vital capacity in those with early-onset scoliosis is 15% greater than in those with adolescent idiopathic scoliosis[1-5,11,12,24]. Moreover, spinal fusion is a cause of respiratory insufficiency and adds a loss of pulmonary function[13] to the pre-existing spinal deformity.
The time between birth and 8 years of age is the golden period for thoracic spine and thoracic cage growth and coincides with lung development. From birth to four years, the number of alveoli increases by a factor of 10, and the development of the bronchial tree ends at 8 to 9 years of age. Gollogly et al[18] showed in a CT scan study that lung parenchyma volume is a function of age. The lung parenchyma volume is approximately 400 cc at birth, 900 cc at 5 years of age, 1500 cc at 10 years of age, and is approximately 4500 cc for boys and 3500 for girls at skeletal maturity. Therefore, early-onset scoliosis adversely affects thoracic growth at the critical period of maximum “respiratory growth”, inducing irreversible changes in the thoraco-pulmonary structure[1-11,18-20,24].
Campbell et al[22,23] described thoracic insufficiency syndrome, the inability of the thorax to ensure normal breathing. They showed that an opening wedge thoracostomy can increase the thoracic volume (“parasol effect”)[11]. It is important to perform this procedure before the end of bronchial tree development at 8 years of age[1-4,6,8-11,17-20,22,23,25]. However, this procedure has drawbacks, including an increase in the stiffness of the thoracic cage resulting in an increase in the amount of energy needed to breathe.
The crankshaft phenomenon is the progression of a spinal deformity when the anterior portion of the spine continues to grow while the posterior portion is blocked by arthrodesis[26,27]. Golberg et al[12] showed that in patients who develop scoliosis before the age of 4, early surgery does not modify the deformation produced by the scoliosis and does not preserve respiratory function, even when the anterior growth of the spine is arrested. Therefore, it is very important for a surgeon to consider the state of skeletal maturity and the amount of growth remaining in the spinal segment to be fused. Similarly, Dubousset et al[21] showed that severe spinal deformities lead to penetration of the apical portion of the deformity inside the thoracic cage (“endo-thoracic hump”) and described the “spinal penetration index”[21].
It is now known that spinal deformations adversely affect the development of the thorax by changing its shape and reducing its normal motility. Metha et al[24] demonstrated that a unilateral deformity of the spine or the thorax induces scoliosis and thoracic cage deformities with asymmetrical lung volume[24]. Moreover, Canavese et al[6] reported that the “rib-vertebral-sternal complex”, which encloses the three-dimensional thoracic cavity, tends to constitute an elastic structural model similar to a cube in shape. In the presence of scoliosis, it becomes flat, rigid and elliptical and prevents the lungs from expanding. These deformations can be lethal in the most severe cases and result from mutual interactions and influences among the various skeletal and organic components of the thoracic cage. Alterations in some elements affect the development and growth of the others[6-11]. These influences are much more evident when an arthrodesis is carried out in the “critical portion” (T1-T6 segment) of the thoracic spine[1-3,5,7-11]. Early spinal fusion, especially if performed in the thoracic region, results in respiratory insufficiency and adds loss of pulmonary function to the pre-existing spinal deformity[11,17-19,21-24]. Therefore, arthrodesis carried out in the thoracic spine at an early age does not address the impact of the deformity on lung parenchyma development or the preservation of pulmonary function. Its ability to prevent deformity progression has been questioned. In children with spinal deformities with strong progression potential, expansible materials can be used to support the expansion of the thoracic cage and lung growth[1-3,5,11,20,22-25].
Modern techniques and instrumentation control only one plane of the deformity, as distraction forces are applied to the spine or to the thoracic cage. Over the past few years, several studies demonstrated that near-normal growth can be attained with the vertical expandable prosthetic titanium rib, growing rods or a Shilla-type procedure. All of these techniques aim to restore normal spinal growth by controlling the progression of the deformity[26-30].
However, there is currently no instrumentation that is able to control the three-dimensional nature of early-onset spinal deformities. To preserve thoracic motility and permit the normal development of the respiratory tree, treatments should not focus only on the spine but should consider the ”rib-vertebral-sternal complex” as a whole[2-6]. In very young children, surgery should be limited as much as possible and extensive arthrodesis of the spine should be avoided (Figure 5).
Figure 5.
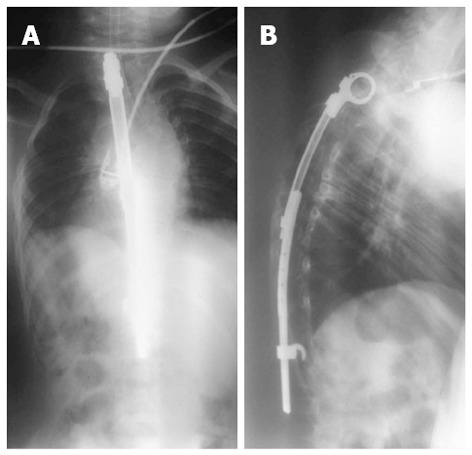
Controlled growth. Eight-year-old male with congenital scoliosis treated with “spine to rib” vertical expandable prosthetic titanium rib. Treatment of the growing spine is a unique challenge and involves preservation of the thoracic spine, thoracic cage, and lung growth without reducing spinal motion.
CONCLUSION
A deformed spine does not grow normally!
A critical analysis of all growth parameters over time will allow for an improved understanding the full magnitude of the deficits induced by early-onset spinal deformities. Spinal and thoracic growth obey strict rules and can be controlled only by adhering to their requirements.
Four different scenarios can be identified: (1) The clinical picture worsens, and the abnormal growth leads to a deficit that sustains the deformity and continues to worsen (“snowball effect”). Reduced body mass index due to weight loss weakens the respiratory muscles and makes breathing more difficult; (2) The clinical picture remains stable; (3) The clinical picture improves slightly with the improvement of clinical parameters, including weight, vital capacity, and sitting height; and (4) The clinical picture returns to normal. In this ideal scenario, all clinical parameters return to normal and the deficit induced by the deformity is eliminated. Unfortunately, this is unlikely to happen, as most children with severe spinal deformities present with a short trunk, significant loss of vital capacity, and disproportionate body habitus at skeletal maturity. Surgical strategies must consider the complete life span of the patient and should provide answers to two basic questions: (1) What is the functional benefit? and (2) What is the morbidity risk?
It must be emphasized that the thoracic cage is part of the deformity (rib-vertebral-sternal complex). There is a normal interaction between the spine, thoracic cage, and lungs. Both early-onset spinal deformities and precocious spinal arthrodesis alter spinal growth and affect the development the thorax by changing its shape and reducing its normal mobility. Treatment of the growing spine is a unique challenge and involves preservation of the thoracic spine, thoracic cage, and lung growth without reducing spinal motion. It is no longer generally accepted that a short, straight spine produced by an early fusion is better that a long, curved spine.
Footnotes
P- Reviewers Hyun SJ, Kasai Y, Wang JC S- Editor Wen LL L- Editor A E- Editor Wang CH
References
- 1.Dimeglio A, Bonnel F. Le rachis en croissance. Paris, France: Springer Verlag; 1990. [Google Scholar]
- 2.Dimeglio A, Canavese F. The growing spine: how spinal deformities influence normal spine and thoracic cage growth. Eur Spine J. 2012;21:64–70. doi: 10.1007/s00586-011-1983-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimeglio A, Canavese F, Charles YP. Growth and adolescent idiopathic scoliosis: when and how much? J Pediatr Orthop. 2012;31:S28–S36. doi: 10.1097/BPO.0b013e318202c25d. [DOI] [PubMed] [Google Scholar]
- 4.Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1:102–107. [Google Scholar]
- 5.Canavese F, Dimeglio A, Volpatti D, Stebel M, Daures JP, Canavese B, Cavalli F. Dorsal arthrodesis of thoracic spine and effects on thorax growth in prepubertal New Zealand white rabbits. Spine (Phila Pa 1976) 2007;32:E443–E450. doi: 10.1097/BRS.0b013e3180bc2340. [DOI] [PubMed] [Google Scholar]
- 6.Canavese F, Dimeglio A, D’Amato C, Volpatti D, Granier M, Stebel M, Cavalli F, Canavese B. Dorsal arthrodesis in prepubertal New Zealand white rabbits followed to skeletal maturity: Effect on thoracic dimensions, spine growth and neural elements. Indian J Orthop. 2010;44:14–22. doi: 10.4103/0019-5413.57280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles YP, Diméglio A, Marcoul M, Bourgin JF, Marcoul A, Bozonnat MC. Influence of idiopathic scoliosis on three-dimensional thoracic growth. Spine (Phila Pa 1976) 2008;33:1209–1218. doi: 10.1097/BRS.0b013e3181715272. [DOI] [PubMed] [Google Scholar]
- 8.Canavese F, Dimeglio A, Granier M, Beraldo P, Bonnel F, Stebel M, Daures JP, Canavese B, Cavalli F. Selective dorsal T1-T6 fusion of the thoracic spine and effects on thorax growth: experimental study in prepuberal New Zealand White rabbits. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:490–497. doi: 10.1016/j.rco.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Canavese F, Dimeglio A, Stebel M, Galeotti M, Canavese B, Cavalli F. Thoracic cage plasticity in prepubertal New Zealand white rabbits submitted to T1-T12 dorsal arthrodesis: computed tomography evaluation, echocardiographic assessment and cardio-pulmonary measurements. Eur Spine J. 2013;22:1101–1112. doi: 10.1007/s00586-012-2644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimeglio A, Canavese F. Progression or not progression? How to deal with adolescent idiopathic scoliosis during puberty. J Child Orthop. 2013;7:43–49. doi: 10.1007/s11832-012-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbarnia BA, Campbell RM, Dimeglio A, Flynn JM, Redding GJ, Sponseller PD, Vitale MG, Yazici M. Fusionless procedures for the management of early-onset spine deformities in 2011: what do we know? J Child Orthop. 2011;5:159–172. doi: 10.1007/s11832-011-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg CJ, Gillic I, Connaughton O, Moore DP, Fogarty EE, Canny GJ, Dowling FE. Respiratory function and cosmesis at maturity in infantile-onset scoliosis. Spine (Phila Pa 1976) 2003;28:2397–2406. doi: 10.1097/01.BRS.0000085367.24266.CA. [DOI] [PubMed] [Google Scholar]
- 13.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 14.Swank SM, Winter RB, Moe JH. Scoliosis and cor pulmonale. Spine (Phila Pa 1976) 1982;7:343–354. doi: 10.1097/00007632-198207000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Emans JB, Ciarlo M, Callahan M, Zurakowski D. Prediction of thoracic dimensions and spine length based on individual pelvic dimensions in children and adolescents: an age-independent, individualized standard for evaluation of outcome in early onset spinal deformity. Spine (Phila Pa 1976) 2005;30:2824–2829. doi: 10.1097/01.brs.0000190865.47673.6a. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Sucato DJ. Unilateral pedicle screw epiphysiodesis of the neurocentral synchondrosis. Production of idiopathic-like scoliosis in an immature animal model. J Bone Joint Surg Am. 2008;90:2460–2469. doi: 10.2106/JBJS.G.01493. [DOI] [PubMed] [Google Scholar]
- 17.Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976) 1992;17:1091–1096. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Gollogly S, Smith JT, White SK, Firth S, White K. The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system. Spine (Phila Pa 1976) 2004;29:2061–2066. doi: 10.1097/01.brs.0000140779.22741.33. [DOI] [PubMed] [Google Scholar]
- 19.Berend N, Rynell AC, Ward HE. Structure of a human pulmonary acinus. Thorax. 1991;46:117–121. doi: 10.1136/thx.46.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGroodt EG, van Pelt W, Borsboom GJ, Quanjer PH, van Zomeren BC. Growth of lung and thorax dimensions during the pubertal growth spurt. Eur Respir J. 1988;1:102–108. [PubMed] [Google Scholar]
- 21.Dubousset J, Wicart P, Pomero V, Barois A, Estournet B. Thoracic scoliosis: exothoracic and endothoracic deformations and the spinal penetration index. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:9–18. [PubMed] [Google Scholar]
- 22.Campbell RM, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85-A:399–408. doi: 10.2106/00004623-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Campbell RM, Hell-Vocke AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85-A:409–420. doi: 10.2106/00004623-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Mehta HP, Snyder BD, Callender NN, Bellardine CL, Jackson AC. The reciprocal relationship between thoracic and spinal deformity and its effect on pulmonary function in a rabbit model: a pilot study. Spine (Phila Pa 1976) 2006;31:2654–2664. doi: 10.1097/01.brs.0000244613.66055.b6. [DOI] [PubMed] [Google Scholar]
- 25.Rizzi PE, Winter RB, Lonstein JE, Denis F, Perra JH. Adult spinal deformity and respiratory failure. Surgical results in 35 patients. Spine (Phila Pa 1976) 1997;22:2517–2530; discussion 2531. doi: 10.1097/00007632-199711010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Dubousset J. Recidive d’une scoliose lombaire et d’un basin oblique après fusion precoce: le phénomène du Villebrequin. Paris, France: Proceeding Group Etude de la Scoliose; 1973. [Google Scholar]
- 27.Hefti FL, McMaster MJ. The effect of the adolescent growth spurt on early posterior spinal fusion in infantile and juvenile idiopathic scoliosis. J Bone Joint Surg Br. 1983;65:247–254. doi: 10.1302/0301-620X.65B3.6841390. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy RE, Sucato D, Turner JL, Zhang H, Henson MA, McCarthy K. Shilla growing rods in a caprine animal model: a pilot study. Clin Orthop Relat Res. 2010;468:705–710. doi: 10.1007/s11999-009-1028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroerlucke SR, Akbarnia BA, Pawelek JB, Salari P, Mundis GM, Yazici M, Emans JB, Sponseller PD. How does thoracic kyphosis affect patient outcomes in growing rod surgery? Spine (Phila Pa 1976) 2012;37:1303–1309. doi: 10.1097/BRS.0b013e318246d8a0. [DOI] [PubMed] [Google Scholar]
- 30.Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, Boachie-Adjei O, Karlin LI, Canale S, Poe-Kochert C, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533–2543. doi: 10.2106/JBJS.I.01471. [DOI] [PubMed] [Google Scholar]


