Abstract
Bone is one of the most preferential metastatic target sites of breast cancer. Bone possesses unique biological microenvironments in which various growth factors are stored and continuously released through osteoclastic bone resorption, providing fertile soil for circulating breast cancer cells. Bone-disseminated breast cancer cells in turn produce osteotropic cytokines which modulate bone environments. Under the influences of breast cancer-produced cytokines, osteoblasts express elevated levels of Ligand for receptor activator of nuclear factor-κB (RANKL) and stimulate osteoclastogenesis via binding to the receptor receptor activator of nuclear factor-κB (RANK) and activating its downstream signaling pathways in hematopoietic osteoclast precursors, which causes further osteoclastic bone destruction. Establishment of crosstalk with bone microenvironments (so called vicious cycle) is an essential event for metastatic breast cancer cells to develop bone metastasis. RANKL and RANK play a central role in this crosstalk. Moreover, recent studies have demonstrated that RANKL and RANK are involved in tumorigenesis and distant metastasis independent of bone microenvironments. Pharmacological disruption of the RANKL/RANK interplay should be an effective therapeutic intervention for primary breast tumors and bone and non-bone metastasis. In this context, denosumab, which is neutralizing monoclonal antibody against RANKL, is a mechanism-based drug for the treatment of bone metastases and would be beneficial for breast cancer patients with bone metastases and potentially visceral organ metastases.
Keywords: Bone metastasis, Osteoclasts, Bone resorption, Osteoblasts, Stromal cells, Epithelial-mesenchymal transition, Bone pain
INTRODUCTION
Due to dramatic advancement of cumulative anti-cancer treatments, surgeons and medical oncologists are confidently able to control primary tumors these days. In contrast, however, metastasis to distant organs is still uncontrollable and has been one of the primary causes of increased mortality and morbidity in cancer patients. Hence, control of distant organ metastasis is very important and an ultimate goal in the treatment and management of cancer patients.
Breast cancer frequently spreads to bone as well as lung, liver and brain[1]. Notably, bone metastases are not readily detected, because they are asymptomatic until patients complain bone pain in most cases. Furthermore, it usually develops at later stages of illness and many cancer patients die before bone metastases are clinically detected and become problematic. However, over the last a decade bone metastasis has come under the spotlight since it causes many problems in the management of cancer patients who survive long enough to develop bone metastases due to improved anti-cancer therapies. Metastases to bone per se do not directly affect survival of cancer patients but virtually worsen quality of life in these patients by causing devastating bone pain and skeletal-related events (SREs) including pathological fractures, spinal compression and hypercalcemia, which indirectly lead to earlier death[1]. Furthermore, treatments of bone metastases significantly increase medical care cost[2]. Of note, there is increased frequency of bone metastases in breast, lung and prostate cancer of which incidence is sharply rising in developed countries. It is, therefore, evident that control of bone metastasis will be critical to properly manage cancer patients under good quality of life and also reduce healthcare costs.
In this article, mechanism of bone metastasis in breast cancer is overviewed at cellular and molecular levels with a special focus on the role of ligand for receptor activator of nuclear factor-κB (RANKL) and receptor activator of nuclear factor-κB (RANK). Furthermore, the pharmacological actions and clinical benefits of the anti-RANKL neutralizing monoclonal antibody denosumab in the management of bone metastasis in breast cancer are reviewed and discussed.
CANCER METASTASIS TO DISTANT ORGANS
There are multiple biological steps in distant metastasis of cancer[3]. However, it can be divided into two broad processes from the view point of organ-selective metastasis, namely before and after cancer cells arrest in target organs (Figure 1). Before cancer cells reach distant target organs, they grow and invade into the surrounding tissues at primary site, induce angiogenesis to support primary tumor development and enter the circulation (intravasation), escape from host immune cell attack by forming cell aggregates and migrate to their target organ. Cancer cells migrating in the circulation are called circulating tumor cells (CTCs), which are proposed to be a promising target to interrupt distant metastatic cascades[4]. This process is likely common for all metastatic cancer cells regardless of the target organ.
Figure 1.
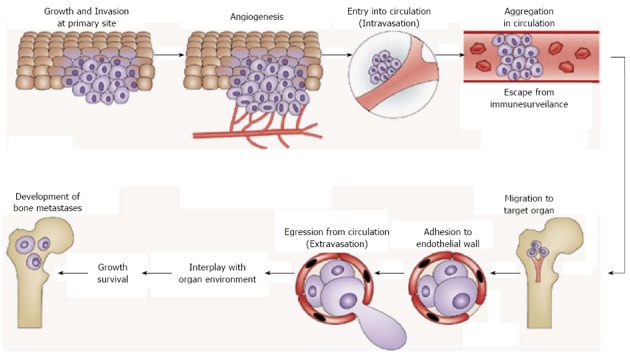
Steps of distant metastasis. Distant metastasis of cancer can be divided into two broad processes including before and after cancer cell arrest in target organs. Before the arrival at distant target organs, cancer cells go through growth, invasion, angiogenesis, intravasation, escape from host immune surveillance, and migration to their target organ. Cancer cells in the circulation are called circulating tumor cells (CTCs). This process is common for all metastatic cancer cells. CTCs reached their target organ subsequently show extravasation and finally arrest in the target organ. These cancer cells are called disseminated tumor cells (DTCs). DTCs change their phenotype via interactions with bone. Establishment of the interactions with bone environments is the most critical step for disseminated breast tumor cells to develop bone metastases. This process is unique depending on the target organs. Adapted from Ref. 14 and modified.
Next process is unique depending on the target organ in which CTCs migrate. After CTCs reach their target organ, here bone, they egress circulation (extravasation) and arrest in the target organ. These cancer cells are called disseminated tumor cells (DTCs). It is shown that there is a significant correlation between the detection of DTCs in bone marrow and higher risk of recurrence and disease-specific death in breast cancer[5]. DTCs change their phenotype under the influence of bone environments to adjust to and proliferate and survive in bone (Figure 1). Consistent with this notion, we found that the bone-seeking clone of the MDA-MB-231 human breast cancer shows altered biological phenotype from parental and brain-seeking clone that allow them to selectively home and colonize bone[6,7]. Establishment of the interactions with bone environments is the most critical step for disseminated breast tumor cells to develop bone metastases. Hence, dissection of bone environments at cellular and molecular levels in the context of cancer cell colonization is important to understand the mechanism of bone metastasis in breast cancer.
RANKL EXPRESSION AND VICIOUS CYCLE IN BONE ENVIRONMENTS
Bone is a storehouse of growth factors such as insulin-like growth factors (IGF), transforming growth factor-β (TGF-β), fibroblast growth factors, platelet-derived growth factors and bone morphogenetic proteins[8]. These growth factors are continually released into the bone marrow cavity via osteoclastic bone resorption during physiological bone remodeling (Figure 2). Thus bone is fertile soil for metastatic cancer cells to colonize. In this regard, bone represents the organ that exteriorized the concept of “Seed and Soil” theory proposed by Paget[9] more than 120 years ago. Bone-derived IGF is shown to promote proliferation and suppress apoptosis in breast cancer cells by activating Akt/NF-κB pathway[10]. On the other hand, bone-derived TGF-β stimulates the production of osteoclast-activating cytokines such as parathyroid hormone-related protein (PTH-rP)[11], prostaglandin E2 (PGE2)[12] and interleukin-11 (IL-11)[13] in breast cancer cells. These factors in turn further stimulate osteoclastic bone resorption, followed by enhanced release of bone-stored growth factors, thus establishing “vicious cycle” (Figure 2)[7,14-16].
Figure 2.
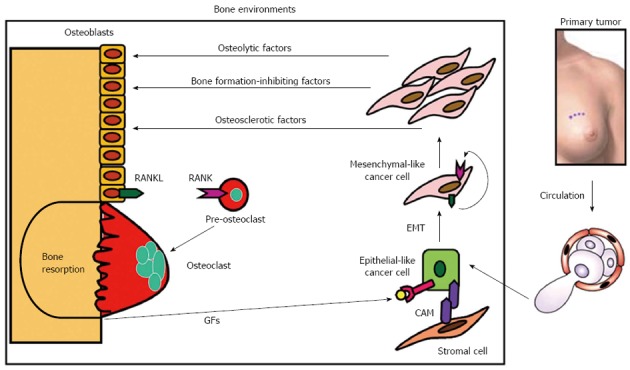
Vicious cycle between bone and breast cancer cells in bone metastasis. Bone-derived growth factors (GFs) such as insulin-like growth factors (IGF) and transforming growth factor-β (TGF-β) that are continually released via osteoclastic bone resorption promote proliferation and suppress apoptosis and stimulate the production of parathyroid hormone-related protein (PTH-rP), prostaglandin E2 (PGE2) and interleukin-11 (IL-11) in metastatic breast cancer cells migrating from primary site via circulation. Bone is fertile soil for metastatic cancer cells representing the concept of “Seed and Soil” theory proposed by Paget[9]. These osteolytic factors further stimulate osteoclastic bone resorption, followed by enhanced release of bone-stored growth factors, thus establishing “vicious cycle”. Prostate cancer may produce osteosclerotic factors and multiple myeloma is shown to produce bone formation-inhibiting factors[14-16]. These osteolytic factors up-regulate the production of ligand for receptor activator of nuclear factor-κB (RANKL) in osteoblasts/stromal cells, which then interacts with its receptor receptor activator of nuclear factor-κB (RANK) in the osteoclast precursors promoting osteoclastogenesis and bone resorption. RANKL and RANK play a central role in the establishment of the vicious cycle. Metastatic breast cancer cells themselves occasionally express RANKL and RANK to develop an autocrine stimulation of carcinogenesis or tumorigenesis. RANKL/RANK expression in breast cancer cells could be a predicting indicator for subsequent occurrence of bone metastasis. Some metastatic breast cancer cells reside in stromal cell niche via cell-cell contact that is mediated by cell adhesion molecules (CAMs) and stay dormant. Metastatic breast cancer cells undergo epithelial-mesenchymal transition (EMT) by changing cell shape from epithelial to mesenchymal and acquire more aggressiveness in the presence of bone-derived TGF-β.
Of note, these osteoclast-activating cytokines do not directly activate osteoclasts but they first bind and activate osteoblasts/stromal cells which express the receptors for these cytokines[17]. These cytokines up-regulate the production of RANKL that is a potent stimulator of osteoclast differentiation, activation and survival[18,19]. RANKL then interacts with its receptor RANK expressed in the hematopoietic osteoclast precursors and promotes osteoclastogenesis and bone resorption by mature osteoclasts (Figure 2). Thus the partnership between RANKL on osteoblasts and RANK on the hematopoietic osteoclast precursors plays a central role in the establishment and acceleration of the vicious cycle that is the driving force in the development and progression of bone metastases. Inhibition of bone metastasis by osteoprotegerin (OPG), a natural antagonist of RANKL, in preclinical models[20] verifies the importance of RANKL-RANK interplay in bone metastasis. Accordingly, design of pharmacological agents that interrupt the vicious cycle by targeting RANKL/RANK interplay would be a promising approach to effectively and selectively treat bone metastases in breast cancer patients.
RANKL/RANK AND MAMMARY GLAND MORPHOGENESIS
Mice deficient in RANKL or RANK exhibited disturbed mammary gland morphogenesis due to decreased differentiation and proliferation and increased apoptosis in mammary epithelial cells during lactation[21]. Later it was shown that RANKL[22] and RANK[23] were virtually involved in ductal side-branching, alveolar differentiation and lumen formation in mammary gland. Furthermore, RANKL was found to stimulate cell proliferation and suppressed apoptosis via inhibition of NF-κB kinase alpha (IKKα)-cyclin D1 signaling and Id2-p21 signaling in mammary epithelial cells[24,25]. Of note, prolactin- or progesterone-receptor null mice displayed identical mammary gland phenotype to RANKL- or RANK-deficient mice[26,27] and prolactin or progesterone stimulated RANKL expression at transcriptional levels in mammary epithelial cells. These results suggest that prolactin and progesterone promote mammary gland morphogenesis through transcriptionally up-regulating the expression of RANKL, which subsequently stimulates cell proliferation and inhibits apoptosis in mammary epithelial cells. Progesterone-RANKL-RANK axis plays an important role in lactating mammary gland morphogenesis in physiological condition.
RANKL/RANK AND BREAST CANCER
Recent clinical studies demonstrated that progestin-containing hormone replacement therapy and contraceptives were significantly associated with increased incidence of breast cancer[28], suggesting the link between progesterone and breast cancer risk. Progesterone is shown to stimulate the proliferation of mammary stem cells[25,29], which possess the potential to transform preneoplastic cells. Of note, however, these mammary stem cells lack progesterone receptors, suggesting that the growth-stimulatory effects of progesterone are indirect and likely mediated by other neighboring molecules of which production is stimulated by progesterone. Administration of medroxyprogesterone acetate caused elevated induction of RANKL expression in mammary epithelial cells and RANKL promoted proliferation and inhibited apoptosis in mammary epithelial cells and mammary stem cells[26,27]. In addition, RANK is recently found to promote mammary tumorigenesis involving epithelial-mesenchymal transition[30]. Conversely, suppression of RANKL expression in mammary epithelial cells and mammary stem cells blocked progesterone-induced mammary morphogenesis and tumorigenesis, respectively. Together these results strongly suggest that progesterone-RANKL-RANK axis plays a critical role in the initiation of mammary tumors in an autocrine manner. Disruption of this axis could be a novel approach for the treatment and prevention of mammary gland tumor and carcinogenesis, respectively[25].
Of interest, Tan et al[31] have reported that CD4+CD25+FOXP3+ regulatory T lymphocytes infiltrating into mammary tumors produce elevated amounts of RANKL, which then stimulates mammary tumor cell proliferation and protects from apoptosis in a paracrine manner, leading to increased tumor burden and pulmonary metastases. Earlier studies have reported that the regulatory T cells, which negatively regulate tumor immunity, accumulate in the bone marrow cavity in patients with prostate cancer with bone metastases and multiple myeloma[32,33]. Thus, the paracrine regulation by regulatory T cell-derived RANKL contributes to the stimulation of tumor growth and metastasis in breast cancer. Targeting regulatory T cell RANKL may reverse anti-tumor immunity and could be a unique therapeutic intervention for breast cancer patients with bone metastasis.
Substantial cases of primary human breast tumors expressed RANK and RANKL. Of interest, RANKL expression was increased in breast cancers in young women. Furthermore, RANKL expression levels are also well-correlated with the number of mammary stem cells[34-37]. These results raise the possibility that breast cancers in premenopausal young women are more aggressive than those in postmenopausal old women. Further studies in clinical settings are required to prove this important notion. However, if this turns out to be the case, disruption of RANKL-RANK interplay would be more appropriate and effective in premenopausal breast cancer patients.
Inhibition of RANKL/RANK interplay using OPG-Fc combined with tamoxifen is shown to enhance anti-cancer action of tamoxifen, leading to a reduction in cancer colonization in bone and bone destruction in a preclinical animal model of estrogen receptor-positive breast cancer[38]. Combination of RANKL/RANK inhibitors with anti-cancer agents appears to be a practical therapeutic approach for breast cancer patients and its efficacy needs to be evaluated in clinical settings.
RANKL/RANK AND BREAST CANCER METASTASIS TO BONE
Circulating RANKL and OPG are proposed to be predicting biomarkers of bone metastases in breast cancer patients[39,40], although whether non-membrane-bound soluble RANKL is present at substantial levels in circulation in breast cancer patients is still controversial. Similarly, immunohistochemical examination of clinical samples showed that RANKL expression was elevated in breast, lung, prostate and thyroid cancers that metastasized to bone compared to those at primary site[41]. Moreover, there was a positive correlation between RANK expression levels in primary breast tumors and frequency of bone metastasis and poor survival[41]. On the other hand, survival of patients with breast cancers with increased expression of OPG, which disrupts RANKL-RANK interplay, is found to be prolonged[42]. Thus, RANKL/RANK/OPG expression ratio in tumors could be a diagnostic indicator for the subsequent occurrence of bone metastasis in the clinical course of breast cancer patients. The mechanism of increased frequency of skeletal metastasis in breast cancers that showed elevated RANKL or RANK expression is unknown. These breast cancers may be chemoattracted to bone by RANK or RANKL expressed in resident cells in bone marrow.
RANKL/RANK AND EPITHELIAL-MESENCHYMAL TRANSITION IN BREAST CANCER
Cancer cells are genetically unstable[43]. They readily change their biological phenotype and acquire new malignant capacities according to the environments to which they are exposed[44]. Epithelial-mesenchymal transition (EMT) is a process in which the epithelial-like cancer cells change their cell shape to mesenchymal fibroid morphology, accompanying with increased aggressiveness including mobility, invasiveness, distant metastasis and resistance to chemotherapy[45]. Since bone is a preferential target site of metastasis for breast cancer, it is plausible to reason that breast cancer cells show EMT when they arrest in and colonize bone. We found that mRNA expression of Snail, a well-recognized mesenchymal marker[46], was markedly increased in breast cancer in bone compared with that at orthotopic mammary fat pad in mice, while E-cadherin, a representative epithelial marker, was profoundly decreased in these cells in bone. Snail is a transcription repressor and known to inhibit E-cadherin expression. These results suggest that bone environments cause EMT in breast cancer cells metastasized in bone. Subsequent in vitro experiments to examine which constituent of bone environments promotes EMT in breast cancer cells showed that TGFβ, which is stored in bone and continually released in active forms from bone by bone resorption[10], promoted EMT. TGFβ is a powerful stimulator of EMT[47]. Of note, we found that OPG inhibited TGFβ-stimulated EMT, suggesting an involvement of RANKL and RANK interplay. Consistent with this result, RANKL also stimulated EMT via up-regulating Snail expression in breast cancer cells. These results suggest that RANKL produced in breast cancer cells mediates TGFβ-promoted EMT in an autocrine manner. They also suggest that intrinsic expression of RANK in breast cancer cells may exacerbate their malignant behaviors once these breast cancer cells arrest in bone in which RANKL is abundantly available in autocrine and paracrine fashion. It is therefore expected that suppression of RANKL actions in bone not only inhibits bone metastases but also consequent acquisition of aggressive autonomous behaviors of breast cancer. Consistent with our results, Palafox et al[30] recently have reported that RANK induces EMT in human mammary epithelial cells and promotes tumorigenesis and metastasis. Study on the role of RANKL/RANK in EMT in breast cancer in bone may allow us to design novel therapeutic approaches.
RANKL/RANK AND BONE CANCER PAIN
Bone pain is one of the major complications that seriously affect quality of life in cancer patients with bone metastases[1]. More than 70% of cancer patients with bone metastases suffer from devastating bone pain. Although the precise mechanism of bone pain is still unclear, the long-standing clinical observations that specific inhibitors of osteoclastic bone resorption bisphosphonates (BPs) reduce bone pain[48] suggest a potential role of osteoclasts, which play a central role in osteolytic bone metastases. Osteoclasts dissolve bone minerals by releasing protons through the vacuolar type proton pump (V-H+-ATPase)[49], thereby locally creating acidic microenvironments in bone. Acid is a well-known cause of pain[50]. Consistent with this notion, we reported that acidosis created by bone-resorbing osteoclasts is partially responsible for inflammation-induced bone pain[51-53]. The important role of osteoclasts in causing bone cancer pain is further supported by the finding that OPG reduced bone cancer pain in animal models[54-56]. Furthermore, a recent clinical report describes that denosumab reduces bone pain due to cancer metastasis to bone[57]. These results support the notion that RANKL causes bone pain by activating osteoclastic bone resorption that creates acidic microenvironments by releasing protons. Blockade of RANKL/RANK interplay thus is a promising novel therapeutic intervention for bone pain in cancer patients with bone metastases.
DENOSUMAB IN BREAST CANCER PATIENTS WITH BONE METASTASIS
Denosumab is a fully human IgG2 monoclonal neutralizing antibody to RANKL[19]. Denosumab is administered via subcutaneous injection and because of its large molecular weight, denosumab is not excreted from kidney, and it is not metabolized in liver[58]. Different from bisphosphonates that inhibit bone resorption by inducing apoptosis in mature bone-resorbing osteoclasts, denosumab inhibits not only bone resorption by mature osteoclasts but also osteoclast formation from hematopoietic precursors and survival[59].
Phase II clinical study showed significant effectiveness and safety of denosumab in the treatment of breast cancer patients with bone metastases[60]. Body et al[61] described that denosumab was effective at blocking bone resorption and preventing SREs in breast and prostate cancer patients who had not responded adequately to earlier bisphosphonate therapy. These results provide with useful information for the question as to whether denosumab should be a first line treatment to prevent SREs in cancer patients and if cancer patients who previously were refractory to bisphosphonate treatment can be treated with denosumab. Stopeck et al[62] reported that denosumab was significantly more effective than zoledronic acid at delaying the first onset of SRE in breast cancer patients with bone metastasis. Concise description about the results of these clinical studies on breast, prostate and lung cancers and multiple myeloma is reported elsewhere[63].
Regarding adverse effects, denosumab as well as bisphosphonates is associated with osteonecrosis of the jaw (ONJ). There was no difference in the incidence rate of ONJ between bisphosphonate- and denosumab-treated cancer patients with skeletal metastases in the randomized double-blind controlled trial[64]. However, the incidence of hypocalcemia was significantly higher in cancer patients receiving denosumab than bisphosphonates[65]. Cautious periodical monitoring of blood ionized calcium levels and supplementation of calcium and vitamin D are strongly recommended for cancer patients receiving denosumab.
CONCLUSION
In conclusion, Because large bodies of scientific information on bone microenvironments have been accumulated at cellular and molecular levels, steps associated with the development and advancement of bone metastasis are relatively well characterized. In particular, the importance of the interactions between metastatic cancer cells and bone cellular constituents including osteoclasts and osteoblasts is unique and specific for bone metastasis. RANKL and RANK are expressed in a variety of bone marrow-resident cells including osteoblasts/stromal cells, hematopoietic cells and immune cells at substantial levels and play primary and essential roles in mediating these interactions. Thus RANKL/RANK is a logic target in design of bone-modifying agents that inhibit bone metastasis by interrupting the vicious cycle.
In addition to the critical role in bone metastasis, early results obtained in RANKL- or RANK-deficient mice clearly demonstrated that RANKL and RANK were important in normal mammary gland morphogenesis during lactation. However, these findings did not attract intense interests until recently. Over the last several years, however, the importance of RANKL and RANK not only in normal mammary morphogenesis but also in the acquisition of aggressive neoplastic phenotype including promoted cell proliferation, resistance to apoptosis, carcinogenesis, tumorigenesis, suppressed tumor immunity and EMT have been revisited. Furthermore, involvement of RANKL/RANK in the development and advancement of tumors other than breast cancer is also revealed[19,20]. These results raise the possibility that RANKL/RANK can be categorized as oncogene. Based on this concept, the bone-modifying agents including bisphosphonates and denosumab are able to inhibit cancer cell colonization in bone and secondary metastasis to distant organs from bone as well as bone metastasis. This direction of research may lead us to gain novel insights into cancer biology and ideas for the development of effective therapeutic approaches.
Footnotes
P- Reviewers Tarantino U, Korovessis P S- Editor Huang XZ L- Editor A E- Editor Zhang DN
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–2342. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- 3.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer--clinical applications. Nat Rev Clin Oncol. 2010;7:693–701. doi: 10.1038/nrclinonc.2010.171. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Slodkowska EA. Circulating and disseminated tumor cells in the management of breast cancer. Am J Clin Pathol. 2009;132:237–245. doi: 10.1309/AJCPJI7DEOLKCS6F. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 7.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 8.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986;261:12665–12674. [PubMed] [Google Scholar]
- 9.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 10.Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y, Yoshikawa H, Rosen CJ, Mundy GR, Yoneda T. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res. 2012;72:4238–4249. doi: 10.1158/0008-5472.CAN-11-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massagué J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraga T, Myoui A, Choi ME, Yoshikawa H, Yoneda T. Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor-beta enhances bone metastases in breast cancer. Cancer Res. 2006;66:2067–2073. doi: 10.1158/0008-5472.CAN-05-2012. [DOI] [PubMed] [Google Scholar]
- 13.Morinaga Y, Fujita N, Ohishi K, Tsuruo T. Stimulation of interleukin-11 production from osteoblast-like cells by transforming growth factor-beta and tumor cell factors. Int J Cancer. 1997;71:422–428. doi: 10.1002/(sici)1097-0215(19970502)71:3<422::aid-ijc20>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 15.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 16.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 18.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 20.Dougall WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2012;18:326–335. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 21.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. 2009;328:127–139. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol. 2007;27:1442–1454. doi: 10.1128/MCB.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 25.Schramek D, Sigl V, Penninger JM. RANKL and RANK in sex hormone-induced breast cancer and breast cancer metastasis. Trends Endocrinol Metab. 2011;22:188–194. doi: 10.1016/j.tem.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro S, Farmer RD, Stevenson JC, Burger HG, Mueck AO. Does hormone replacement therapy cause breast cancer? An application of causal principles to three studies. Part 4: the Million Women Study. J Fam Plann Reprod Health Care. 2012;38:102–109. doi: 10.1136/jfprhc-2011-100229. [DOI] [PubMed] [Google Scholar]
- 29.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 30.Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Muñoz P, Viñals F, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–2888. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]
- 31.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atanackovic D, Cao Y, Luetkens T, Panse J, Faltz C, Arfsten J, Bartels K, Wolschke C, Eiermann T, Zander AR, et al. CD4+CD25+FOXP3+ T regulatory cells reconstitute and accumulate in the bone marrow of patients with multiple myeloma following allogeneic stem cell transplantation. Haematologica. 2008;93:423–430. doi: 10.3324/haematol.11897. [DOI] [PubMed] [Google Scholar]
- 33.Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–161. doi: 10.4161/onci.1.2.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, Shamseddine AI. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Veronesi P, Torrisi R, Montagna E, Luini A, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (& lt; 35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–1981. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 37.Azim HA, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, Haibe-Kains B, Piccart MJ, Sotiriou C, Loi S. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 38.Canon J, Bryant R, Roudier M, Branstetter DG, Dougall WC. RANKL inhibition combined with tamoxifen treatment increases anti-tumor efficacy and prevents tumor-induced bone destruction in an estrogen receptor-positive breast cancer bone metastasis model. Breast Cancer Res Treat. 2012;135:771–780. doi: 10.1007/s10549-012-2222-2. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim T, Sacanna E, Gaudio M, Mercatali L, Scarpi E, Zoli W, Serra P, Ricci R, Serra L, Kang Y, et al. Role of RANK, RANKL, OPG, and CXCR4 tissue markers in predicting bone metastases in breast cancer patients. Clin Breast Cancer. 2011;11:369–375. doi: 10.1016/j.clbc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Mercatali L, Ibrahim T, Sacanna E, Flamini E, Scarpi E, Calistri D, Ricci M, Serra P, Ricci R, Zoli W, et al. Bone metastases detection by circulating biomarkers: OPG and RANK-L. Int J Oncol. 2011;39:255–261. doi: 10.3892/ijo.2011.1001. [DOI] [PubMed] [Google Scholar]
- 41.Huang L, Cheng YY, Chow LT, Zheng MH, Kumta SM. Tumour cells produce receptor activator of NF-kappaB ligand (RANKL) in skeletal metastases. J Clin Pathol. 2002;55:877–878. doi: 10.1136/jcp.55.11.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A, Ortega C, Porta C, Galluzzo S, Armento G, et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One. 2011;6:e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dave B, Mittal V, Tan NM, Chang JC. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012;14:202. doi: 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Herreros AG, Peiró S, Nassour M, Savagner P. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J Mammary Gland Biol Neoplasia. 2010;15:135–147. doi: 10.1007/s10911-010-9179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 48.Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y, Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 49.Brisson L, Reshkin SJ, Goré J, Roger S. pH regulators in invadosomal functioning: proton delivery for matrix tasting. Eur J Cell Biol. 2012;91:847–860. doi: 10.1016/j.ejcb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ion channels. Am J Physiol Cell Physiol. 2012;303:C699–C710. doi: 10.1152/ajpcell.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 52.Yoneda T, Hata K, Nakanishi M, Nagae M, Nagayama T, Wakabayashi H, Nishisho T, Sakurai T, Hiraga T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone. 2011;48:100–105. doi: 10.1016/j.bone.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Yoneda T, Hata K, Nakanishi M, Nagae M, Nagayama T, Wakabayashi H, Nishisho T, Sakurai T, Hiraga T. Molecular events of acid-induced bone pain. IBMS BoneKey. 2011;8:195–204. [Google Scholar]
- 54.Clohisy DR, Mantyh PW. Bone cancer pain and the role of RANKL/OPG. J Musculoskelet Neuronal Interact. 2004;4:293–300. [PubMed] [Google Scholar]
- 55.Roudier MP, Bain SD, Dougall WC. Effects of the RANKL inhibitor, osteoprotegerin, on the pain and histopathology of bone cancer in rats. Clin Exp Metastasis. 2006;23:167–175. doi: 10.1007/s10585-006-9026-x. [DOI] [PubMed] [Google Scholar]
- 56.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25:99–104. doi: 10.1007/s00774-006-0734-8. [DOI] [PubMed] [Google Scholar]
- 57.Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, Patrick DL, Clemons M, Tonkin K, Masuda N, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119:832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 58.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24:23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 59.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 61.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, Fizazi K. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 62.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 63.Coleman RE. Bone cancer in 2011: Prevention and treatment of bone metastases. Nat Rev Clin Oncol. 2012;9:76–78. doi: 10.1038/nrclinonc.2011.198. [DOI] [PubMed] [Google Scholar]
- 64.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, Diel IJ, Takahashi S, Shore N, Henry DH, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 65.Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, Richardson GE, Siena S, Maroto P, Clemens M, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]


