Abstract
Atrial fibrillation (AF) is a common and morbid cardiac arrhythmia that increases in prevalence with advancing age. The risk of ischemic stroke, a primary and disabling hazard of AF, also increases with advancing age. The aging of the population is anticipated to contribute to a rising burden of AF-related morbidity and economic costs, given the close association between the arrhythmia and aging. Recent biological, diagnostic, and therapeutic developments raise hope that AF-related stroke can be largely prevented. Yet despite advances in stroke prevention for patients with AF, numerous scientific and clinical knowledge gaps remain, particularly as these developments are applied to older adults. Given the public health importance of AF-related stroke in the elderly, a group of clinician-investigators convened on April 5, 2012 to identify promising areas for investigation that may ultimately reduce stroke-related morbidity. In this document, we summarize the meeting discussion and emphasize innovative topic areas that may ultimately facilitate the application of novel preventive, diagnostic, and therapeutic insights into the management of older adults with AF.
It is acknowledged that this report is limited by the opinions of those that participated in the meeting, and may not represent all of the issues that might be raised by other experts in this field.
Keywords: Atrial fibrillation, stroke, elderly, prevention
Introduction
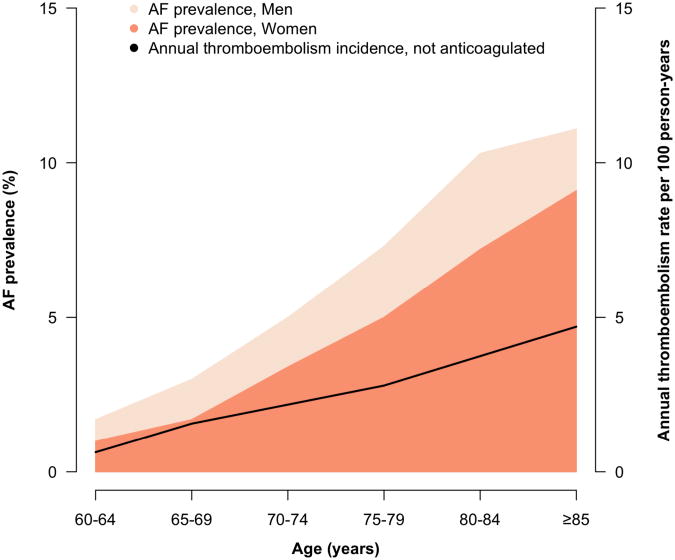
Atrial fibrillation (AF) is a common and morbid cardiac arrhythmia that increases in prevalence with advancing age.1 Approximately 800,000 strokes occur annually in the United States,2 with the proportion attributable to AF rising with mounting age (Figure 1). Among individuals age 80 years or greater, nearly one quarter of strokes are caused by AF.3 Strokes attributable to AF are disabling and carry increased risks of death and disability compared to strokes of other etiologies.4
Figure 1.
The sex-stratified prevalence of atrial fibrillation according to age is displayed.1 The annualized incidence of systemic thromboembolism among individuals not anticoagulated is shown.12
The substantial morbidity attributable to AF contributes to the estimated $26 billion annually in excess health care spending on individuals with the arrhythmia in the United States.5 Increased survival has led to more adults aging into their senior years, when they are more susceptible to developing AF.1 The morbidity, mortality, and costs associated with AF underscore the public health importance of the arrhythmia.
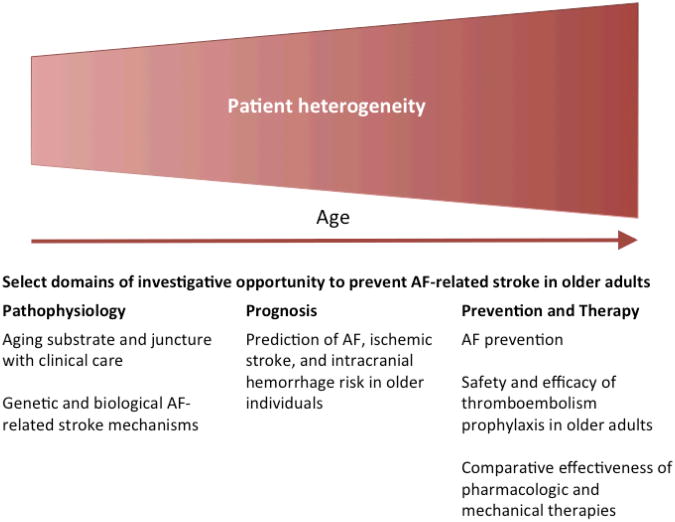
On April 5, 2012, the American Federation for Aging Research convened a seminar comprised of clinician investigators to examine developments in disciplines related to AF and stroke in older adults. The aims of the meeting were to discuss gaps in knowledge related to the prevention of stroke in older patients with AF, and to identify promising areas for investigation that may ultimately reduce stroke-related morbidity. Investigators presented lectures summarizing clinical and scientific evidence related to geriatric medicine, AF susceptibility, and stroke prevention in AF. The knowledge gaps were proposed by individual investigators during the seminar, and were agreed upon by each of the investigators after debate and discussion. Funding for the seminar was unrestricted. In this document, we summarize the meeting discussion and emphasize innovative topic areas (Figure 2).
Figure 2.
The risks of atrial fibrillation and ischemic stroke both increase with age, contributing to increased patient heterogeneity that may also accompany aging. Investigation focused on several promising pathophysiological, prognostic, preventive and therapeutic domains may help define and improve standards of care for elderly patients with atrial fibrillation. Novel insights in these domains may yield improvements in clinical outcomes and prevent atrial fibrillation-related strokes.
Aging physiology and the juncture of geriatrics and cardiology
The impact of age on AF is not only causal, but also affects the presentation of patients with the arrhythmia and management decisions.6 Age-related physiological changes that may increase vulnerability to AF include vascular stiffening, decreased endothelial performance and increased ventricular diastolic filling delay.7 Declines in cognition, functional status, and vitality also are associated with the aging process, and can complicate AF management. Furthermore, with respect to the interplay between AF and stroke, the prevalence of risk factors for stroke in patients with AF increases with age. Indeed, age is independently associated with stroke risk among patients with AF.8
Additional challenges include medication compliance and greater susceptibility to side effects that diminish quality of life. Furthermore, older AF patients may face declining social and financial resources that compound limitations in quality of life and compliance, and further exacerbate management complexity.
Defining safe and effective care for the spectrum of older adults poses challenges for clinicians trying to manage patients with AF. The presentation of older patients ranges from those who are robust and active, to those who are cognitively challenged, sedentary, and frail. The seemingly straightforward principle of patient-centered AF management becomes especially complex for an aging population. Implications of rate control, cardioversion, and anticoagulation may take on entirely different dimensions in each older patient, especially in the context of the multi-morbidity and polypharmacology that are more likely to occur with advancing age. Some older patients may derive a greater benefit from cardiovascular therapies, whereas others may only experience disproportionate treatment burden and risks.6
Aims/knowledge gaps
Examine relationships among markers of aging physiology that indicate increased risk of AF and treatment-related adverse effects.
Determine whether specific patient features (physiologic as well as clinical) can be used to systematically guide treatment strategies in older adults.
Increase the proportion of older adults with complex medical, economic, and psychosocial factors in clinical trials of AF treatments including rate and rhythm control strategies, and novel stroke prevention therapies.
Determine the physiologic implications of aging on AF and stroke mechanisms.
Stroke prevention of AF in the current era and opportunity for innovation
The presumed mechanisms of stroke in patients with AF involve impaired atrial mechanical function, thrombus formation in the left atrial appendage, and thromboembolism, resulting in cerebral ischemia and infarction. The population attributable risk of stroke for AF increases from 1.5% in individuals age 50-59 years to 23.5% in those age 80-89 years.3 Stroke prevention in patients with AF is directed at inhibiting left atrial appendage thrombus formation.
Prophylactic systemic anticoagulation is advocated for many individuals with AF, since stroke risk is greatly increased, especially in older adults.9 Pooled data indicate an estimated 68% reduction in ischemic stroke risk among those treated with vitamin K antagonists relative to placebo,10 and a 52% reduction relative to those treated with aspirin.11 Clinical outcomes correlate with the time spent in therapeutic range on warfarin, which poses an ongoing challenge. Risk stratification schemes comprised of clinical factors can help clinicians identify patients likely to receive the greatest benefit from systemic anticoagulation. However, current methods have limited ability to discriminate individuals who will and will not develop a stroke. Revised clinical prediction algorithms and inclusion of biomarkers may enhance risk discrimination.
The net clinical benefit of warfarin appears greatest in older patients,12 yet it is estimated that about 60% or more of older adults with AF eligible for warfarin, and without contraindications, are not prescribed systemic anticoagulation.13 Although experience with novel anticoagulants remains limited, incorporation of dabigatran into clinical practice does not appear to have increased the proportion of individuals treated with anticoagulants.14 A multitude of physician, patient, and health-care-system-related factors may explain the discrepancy between seeming eligibility and lack of prescription of anticoagulants in older patients.13 Such aspects include increased risk of bleeding in the elderly as well as diminished cognition and treatment burden that can confound management of some older patients. Other aspects relate to intrinsic limitations of vitamin K antagonists, such as a narrow therapeutic window that is bounded by substantial risks of ischemic stroke and hemorrhage in patients with sub- or supra-therapeutic anticoagulation, respectively. Vitamin K antagonists also impose dietary constraints, have a delayed time to therapeutic onset and offset, and frequently interact with medications, which is particularly problematic in the elderly given the prevalence of polypharmacy. Limitations of vitamin K antagonists have created an opportunity for novel therapeutic methods for stroke prevention in patients with AF.
Aims/knowledge gaps
Determine the impact of stroke prevention on morbidity and long-term survival in the extreme elderly.
Determine methods to improve time in therapeutic range among older patients taking vitamin K antagonists.
Define and predict risks of stroke and bleeding in the extreme elderly.
Develop and refine net-clinical benefit prediction tools for anticoagulant prescription in older patients.
Seek to better understand modifiable barriers to anticoagulant under-prescription in the elderly.
Novel anticoagulants for stroke prevention in AF
Several novel anticoagulants were recently found to be at least noninferior to warfarin with respect to thromboembolism prophylaxis in patients with AF.15-17 In each of these trials, the average participant age was at least 70 years, lending some generalizability of the findings to older adults. Principal differences between novel anticoagulants and vitamin K antagonists have been summarized.18 Novel agents approved by the Food and Drug Administration (FDA) include the thrombin inhibitor dabigatran (Pradaxa) and the Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis). Another factor Xa inhibitor, edoxaban, is currently under study. Generally, these novel anticoagulants have rapid onset, fixed dosing, few drug interactions, and no dietary constraints. Coagulation monitoring is not required with novel agents. The trials of dabigatran, rivaroxaban, and apixaban demonstrated that these novel anticoagulants led to fewer intracranial hemorrhages than warfarin, a particularly important benefit for older patients who are at higher intrinsic risk of such devastating bleeding events. Counterbalancing these advantages are the challenges with monitoring patient drug compliance, higher “out-of-pocket” expense, lack of drug reversal in the event of hemorrhage, and lack of long-term experience with the drugs. Considerations specific to each novel anticoagulant have been reviewed elsewhere.18 Reports of life-threatening hemorrhages in patients treated with the first-licensed novel anticoagulant, dabigatran, have prompted advisories from the FDA and other drug agencies and recommendations for more vigilant monitoring of renal function.19 This is particularly important among individuals older than 75 years. At this point, there is no clear evidence that bleeding risk in real-world use of dabigatran exceeds what would be expected from the randomized trial results. However, it is clear we need better information on the real-world experience with these novel agents to better guide the anticoagulant decision in older patients with AF.
Aims/knowledge gaps
Determine real-world safety and efficacy of novel anticoagulants in older adults.
Directly compare the net clinical benefit of different anticoagulants in clinically relevant subgroups of older patients with AF.
Develop clinical tools to guide selection of anticoagulants in different clinical scenarios.
Determine if novel anticoagulants lead to a larger percentage of older AF patients receiving high quality, guideline-recommended stroke prophylaxis.
Can AF be prevented in older adults?
The lifetime risk of developing AF is about 25% after the age of 40 years.20 The risk of AF increases steadily with advancing age, with an estimated prevalence of greater than 9% among individuals aged 80 years or greater.1 Advancing age also is a risk factor for stroke in patients with AF. Therefore, preventing AF will also presumably prevent AF-related stroke and other morbidity.
Numerous clinical risk factors for AF have been identified, some of which are modifiable.9 Recent risk prediction algorithms can identify individuals at greatest risk of developing AF.21,22 Such algorithms may facilitate efforts to test measures for utility in preventing AF.
Currently available data suggest that certain interventions might prevent incident AF. A recent randomized controlled trial comparing aggressive to usual blood pressure control demonstrated a reduced risk of incident AF in patients randomized to the tight control arm.23 Meta-analyses indicate that the administration of angiotensin converting enzyme inhibitors or angiotensin receptor blockers may be associated with reduced risks of AF, particularly in patients with heart failure.24 Nevertheless, efforts to prevent the arrhythmia have not been extensively tested. As such, current guidelines emphasize treatment of existing AF and prevention of adverse AF consequences. Areas of potential investigation within the realm of AF prevention include assessing the applicability of predictors of AF generated in largely homogeneous cohorts to other cohorts, races, and countries; identification of individuals for prospective studies and randomized controlled trials; and the development and utilization of valid and predictive biomarkers as a benchmark for treatment.
Aims/knowledge gaps
Determine the applicability of AF prediction algorithms to older adults of different races and ethnic groups.
Replicate and improve AF prediction algorithms.
Develop clinical trials to test primary prevention of AF.
Can insights from AF genetics inform stroke risk and prevention?
Recent findings illustrate a heritable component underlying AF. Evidence suggests there is about a 40% increased risk of AF among those with an affected first-degree relative, and that AF heritability persists into older ages.25 Genetic variants associated with AF implicate cardiac ion channels, transcription factors involved in cardiopulmonary development, and signaling molecules in the pathogenesis of AF.26 Elucidating the mechanisms linking most common variants to AF presents an opportunity for biological investigation.
AF susceptibility variants on chromosomes 4q25 and 16q22 have been tested and associated with ischemic stroke in samples of European descent.27 The risk between these variants and stroke appears to be most prominent for cardioembolic stroke, implicating AF as the causal mechanism in stroke pathogenesis. Associations between other discovered AF-associated genetic variants and AF, and the potential clinical utility of assessing AF genetic risk for minimizing stroke risk, requires formal testing.
Pharmacogenetics represents a potentially promising area for the application of genetic discoveries. In one analysis, a clinical dosing algorithm comprised of clinical factors and genotypes in the warfarin dose-related genes VKORC1 and CYP2C9 accounted for a greater proportion of variance in warfarin dose requirements than a clinical algorithm alone (about 45% vs. 27%, respectively).28 Future studies are necessary to determine whether genotype-guided dosing improves clinical outcomes in patients taking warfarin as well as novel anticoagulants.
Aims/knowledge gaps
Identify genetic markers for AF and determine how these variants interact with aging and stroke risk.
Test whether AF-associated genetic variants improve stroke risk prediction in older adults.
Test the clinical utility of genotype-guided thromboembolism prophylaxis in older adults.
Determine the cost-effectiveness of genotype-guided diagnosis and management strategies.
Preventing intracerebral hemorrhage
Intracerebral hemorrhage (ICH) is among the most feared complications of anticoagulant treatment.29 Over 50% are dead within 30 days of the event and only 20% of ICH victims are independent at six months. Although warfarin substantially reduces the risk of disability following AF-related strokes, warfarin use increases the risk of ICH two- to five-fold and anticoagulant-related ICH has an even poorer prognosis. There currently is no effective treatment in the acute phase of ICH, so prevention is an attractive strategy.
The risk of ICH appears to be disproportionately high in patients with cerebral amyloid angiopathy (CAA),29 a common age-related process characterized by accumulation of amyloid β (Aβ) peptide in cerebral blood vessels. A diagnosis of CAA in a patient with a prior lobar ICH precludes further warfarin use in nonvalvular AF.29 Beyond secondary prevention, however, risk stratification for ICH in patients with AF is limited.
Over the past decade, lobar cerebral microbleeds on MRI have been established as a specific marker of CAA pathology.30 Furthermore, a number of non-specific MRI markers have been associated with the presence and severity of CAA, including white matter T2-hyperintensities, altered diffusivity and anisotropy, and silent infarcts.
Noninvasive imaging of cerebral Aß is a new approach with potential for noninvasive diagnosis of cerebrovascular amyloid and prediction of incident hemorrhages. Important advances were made using the Aß ligand Pittsburgh Compound B (PiB), but clinical application (and regulatory approval) of this agent has been limited by its short half-life requiring onsite synthesis. More recent radiopharmaceuticals labeled with fluorine 18 (18F), such as fluorine 18–labeled florbetapir (18F-AV-45), have longer half-lives and have made brain amyloid imaging commercially viable. Cerebrospinal fluid amyloid beta and total tau levels appear to discriminate individuals with CAA from healthy controls.31 The importance of isolated microbleeds without prior intracerebral hemorrhage needs to be explored as such small lesions are increasingly being recognized with brain MRIs.
Although novel oral anticoagulants may confer a reduced ICH risk as compared to warfarin,15-17 the potential benefit may be offset by an increased number of individuals at risk if they are more widely utilized than warfarin. Overall, more research is needed in identifying genetic, radiologic, and serologic markers of hemorrhagic risk both in the general population and in high risk patients in order to further individualize management approaches in patients with AF.
Aims/knowledge gaps
Assess the value of non-invasive methods such as amyloid imaging, MRI, and genotyping for predicting hemorrhage risk in patients with early or mild CAA pathology.
Determine whether existing neuroimaging modalities can inform intracranial bleeding risk in older patients with AF.
Develop novel methods for stratifying intracranial hemorrhagic risk.
Formally test strategies to prevent intracranial hemorrhage in anticoagulated patients with AF.
Preventing stroke through mechanical approaches
Potential limitations of pharmacologic therapy in older individuals may create a role for mechanical approaches to stroke prevention in this population. Existing guidelines do not endorse restoration of sinus rhythm for the prevention of thromboembolism. Nevertheless, observational data suggest that stroke risk may be reduced in patients who have undergone successful restoration of sinus rhythm through catheter ablation.32 With catheter ablation for AF being extended as a therapeutic option to older individuals,33 the impact of this procedure on stroke risk needs to be better understood.
Local mechanical approaches to stroke prevention may be advantageous in patients with AF at risk of stroke, particularly among subsets with contraindications to systemic anticoagulation. Mechanical alternatives to pharmacologic therapy broadly include surgical and catheter-based left atrial appendage endocardial (e.g., Watchman) or epicardial exclusion (e.g., Lariat) (Table).
Table.
Nonpharmacologic options for stroke prevention in atrial fibrillation.
| Method | Availability | Long-term efficacy |
|---|---|---|
| Surgical left atrial appendage ligation or amputation | Available | Unclear |
| Percutaneous left atrial appendage ligation (e.g., Lariat) | Available | Unclear |
| Percutaneous left atrial appendage occlusion (e.g., Watchman) | Under FDA review | Unclear |
FDA = Food and Drug Administration
The PROTECT-AF trial was a randomized trial comparing warfarin to a percutaneous endocardial left atrial appendage occlusion device (Watchman) for a composite of stroke, systemic embolism and cardiovascular death prevention in 707 patients with AF and increased stroke risk.34 The average age of participants in the trial was about 72 years. The left atrial appendage occlusion strategy was noninferior to the continued warfarin therapy strategy, though primary safety events were more frequent in the intervention group than in the control group. The FDA has not approved the Watchman device for use in the United States and instead recommended further safety and efficacy data, which is being reviewed in the context of the PREVAIL trial (clinicaltrials.gov identifier NCT01182441).
The results of PROTECT-AF and other studies of left atrial appendage exclusion support the putative mechanism implicating the left atrial appendage in the development of stroke in patients with AF. As such, future mechanical left atrial appendage occlusion options hold potential promise.
Aims/knowledge gaps
Examine the impact of AF ablation on stroke risk in older individuals.
Compare the relative safety and efficacy of different left atrial appendage exclusion approaches to anticoagulation in older individuals.
Assess the cost-effectiveness of prolonged drug therapy versus mechanical left atrial appendage exclusion.
Determine the net clinical benefit of various thromboembolism prophylaxis strategies stratified by age.
Conclusion
The aging of the population is anticipated to contribute to an increasing burden of AF-related morbidity and economic costs, given the close association between AF risk and advancing age. Novel biological, diagnostic, and therapeutic developments raise hope that AF-related stroke largely can be prevented. Given the potential harm from current stroke preventive interventions, highly individualized approaches to stroke prevention are necessary in patients with AF. An improved appreciation for heterogeneity of older adults and formal testing of novel strategies in this population will be essential to minimize morbidity from this very common arrhythmia.
Acknowledgments
Funding: Dr. Lubitz is supported by an American Heart Association award 12FTF11350014. Dr. Benjamin receives NIH support from 1R01HL092577 and 1R01HL102214. Dr. Gurol receives NIH support from T32NS048005-08, 5RO1AG026484-08, 5P50NS051343_08. Dr. Besdine is supported by a John A. Hartford Foundation Center of Excellence in Geriatric Medicine Award. Dr. Singer received support from 1RC2HL101589 and from the Eliot B. and Edith C. Shoolman Fund of the Massachusetts General Hospital.
Sponsor's Role: American Federation of Aging Research and unrestricted educational grants from Bristol Meyers Squibb and Pfizer
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Authors' Contributions: All authors participated in the conception and design, drafting or revision of the manuscript, and final approval. All authors who contributed significantly have been included.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: Data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32:392–398. doi: 10.1161/01.str.32.2.392. [DOI] [PubMed] [Google Scholar]
- 5.Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 6.Forman DE, Rich MW, Alexander KP, et al. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 8.Stroke Risk in Atrial Fibrillation Working G. Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 10.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 11.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. JAMA. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 12.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Annals of internal medicine. 2009 Sep 1;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000 Jan 10;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 14.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 17.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 18.Katsnelson M, Sacco RL, Moscucci M. Progress for stroke prevention with atrial fibrillation: Emergence of alternative oral anticoagulants. Circulation. 2012;125:1577–1583. doi: 10.1161/CIR.0b013e31825498e8. [DOI] [PubMed] [Google Scholar]
- 19.Administration USFAD. FDA Drug Safety Communication: Safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate) http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm282820.htm.
- 20.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 21.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF Consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009 Aug 15;374:525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 24.Khatib R, Joseph P, Briel M, et al. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: A systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. doi: 10.1016/j.ijcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Lubitz SA, Yin X, Fontes JD, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellenguez C, Bevan S, Gschwendtner A, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurol ME, Greenberg SM. Management of intracerebral hemorrhage. Curr Atheroscler Rep. 2008;10:324–331. doi: 10.1007/s11883-008-0050-y. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeek MM, Kremer BP, Rikkert MO, et al. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–249. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: Results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 33.Santangeli P, BIASE LD, Mohanty P, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol. 2012;23:687–693. doi: 10.1111/j.1540-8167.2012.02293.x. [DOI] [PubMed] [Google Scholar]
- 34.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]