Abstract
The present study examined the hypothesis that inhibitory visual selection mechanisms play a vital role in memory by limiting distractor interference during item encoding. In Experiment 1a we used a modified spatial cueing task in which 9-month-old infants encoded multiple category exemplars in the contexts of an attention orienting mechanism involving suppression (i.e., inhibition of return, IOR) versus one that does not (i.e., facilitation). At test, infants in the IOR condition showed both item specific learning as well as abstraction of broader category information. In contrast, infants in the facilitation condition did not discriminate across novel and familiar test items. Experiment 1b confirmed that the learning observed in the IOR condition was specific to spatial cueing of attention and was not due to timing differences across the IOR and facilitation conditions. In Experiment 2, we replicated the results of Experiment 1, using a within-subjects design to explicitly examine learning and memory encoding in the context of concurrent suppression. These data show that developing inhibitory selective attention enhances efficacy of memory encoding for subsequent retrieval. Furthermore, these results highlight the importance of considering interactions between developing attention and memory systems.
Keywords: selective attention, memory, encoding, spatial cueing
In everyday life, individuals understand that paying attention helps learning and memory. While this experience is deeply intuitive, an emerging body of research is beginning to elucidate the precise mechanisms supporting reciprocal interactions between attention and memory systems. On the one hand, previous learning generates expectations that can be used to direct attention to behaviorally relevant information (Stokes, Atherton, Patai, & Nobre, 2012; Yu & Smith, 2011). Conversely, effective allocation of attention is crucial for successful learning and subsequent memory retrieval (Ciaramelli, Grady, & Moscovitch, 2008; Fernandes & Moscovitch, 2000; Yi & Chun, 2005). Selective attention involves two core processes – enhanced processing of attended stimuli and concurrent suppression of irrelevant information (Desimone & Duncan, 1995). This concurrent enhancement and suppression ensures that attended information is selected while interference from distractors is minimized. Here we asked whether this reciprocal process, in diminishing noise from interference and focusing resources on attended information, promotes more effective encoding for subsequent memory retrieval. More broadly, we assert that attention and memory are deeply intertwined, such that development in one of these systems will likely have equally important implications for the other. Given that orienting of attention and eye movements is one of infants’ primary means of exploration, the development of visual selective attention skills during the first year of life may be especially relevant for the naïve learner immersed in cluttered environments (Gibson, 2003).
Numerous studies have demonstrated that selective attention processes have important implications for early learning (Amso & Johnson, 2006; S. P. Johnson, Slemmer, & Amso, 2004; Reid & Striano, 2005; Ross-Sheehy, Oakes, & Luck, 2011; Wu, Gopnik, Richardson, & Kirkham, 2011; Yu & Smith, 2011). One way that selective attention supports learning is by driving targeted sampling of meaningful information. For example, infants who demonstrated successful learning in perceptual completion and statistical learning tasks showed different patterns of eye movements and attention orienting relative to those who showed less robust learning (Amso & Johnson, 2006; S. P. Johnson, et al., 2004; Yu & Smith, 2011). Furthermore, infants showed greater learning of predictable events/statistical regularities when eye gaze cues directed their attention towards the relevant information (Wu, et al., 2011; Wu & Kirkham, 2010). Thus, selective attention can influence what is learned via efficient sampling of visual information.
Attention cueing also enhances performance on learning and memory tasks, both in adulthood (Hauer & MacLeod, 2005; Schmidt, Vogel, Woodman, & Luck, 2002) and during development (Astle, Nobre, & Scerif, 2010; Reid & Striano, 2005; Reid, Striano, Kaufman, & Johnson, 2004; Ross-Sheehy, et al., 2011). For example, the availability of spatial attention cues supported 6-month-old infants’ encoding of multi-element arrays into visual short-term memory (Ross-Sheehy, et al., 2011). Similarly, eye gaze cues elicited differential processing of object features among 4-month-old infants, as infants at this age responded to a non-cued object as a novel item relative to a gaze-cued object (Reid & Striano, 2005; Reid, et al., 2004). Together, these studies suggest that attention cues also impact how much infants learn; however, the mechanistic nature of this interaction remains unclear.
Critically, attention orienting is not uniform; instead, orienting can be driven by different underlying attention mechanisms. Some orienting mechanisms elicit the suppression component of selective attention while others do not (Posner & Cohen, 1984; Tipper, 1985). As such, the nature of the attention mechanisms underlying visual orienting, and particularly whether suppression is involved, may have important implications for subsequent processing of the attended information. Our working hypothesis is that relative to orienting powered by excitation alone, concurrent suppression of interfering information should lead to a less noisy signal for the attended information, and thus lead to more robust encoding.
We capitalized on the classic spatial cueing task (Posner, 1980) to examine the role of selection via suppression in infants’ encoding. By varying a single parameter, the spatial cueing task can index different orienting mechanisms that differentially elicit inhibitory selective attention processes. In this task, attention is engaged at a central location while a cue flashes in the periphery. After a delay, a target appears in the same cued location or in the opposite, non-cued location. When the cue-to-target delay is very short (< 250 ms), individuals respond faster to targets appearing in the cued location. This facilitation effect reflects a mechanism in which attention is reflexively captured by the peripheral cue and is already engaged at the cued location when the target appears (Posner, 1980; Posner & Cohen, 1984). However, when the cue is followed by a longer delay (> 250 ms), attention instead becomes suppressed at the cued location and individuals typically respond faster to targets in the non-cued location, an effect known as inhibition of return (IOR) (Klein, 2000; Posner, Rafal, Choate, & Vaughan, 1985). Importantly, while facilitation of orienting does not require the inhibitory component of selective attention, IOR effects on orienting are inherently inhibitory.
In the present study, we examined whether inhibitory selective attention mechanisms influence the quality of encoding among 9-month-old infants. Given that IOR cueing effects only begin to emerge between 4-6 months of age (Butcher, Kalverboer, & Geuze, 1999; M. H. Johnson & Tucker, 1996), we anticipated that this older age group would show reduced variability in spatial cueing effects, allowing us to test the strongest form of our hypothesis. Infants were familiarized with multiple category exemplars during the encoding phase. At test, they saw a completely novel item, a familiar item seen during encoding to index single item learning, and a novel item derived from the familiar category to index generalization of category learning. We reasoned that infants would look longer at items that they did not recognize from the encoding phase (i.e., a novelty response), reflecting learning of specific items. Within this framework, generalization of learning is reflected by similar looking times to the familiar and novel items from the encoded category, suggesting that infants respond to the novel item as familiar despite never seeing that specific item during familiarization (Quinn, 1987).
Based on our hypothesized mechanism, we predict that encoding during familiarization will be more effective in the context of inhibitory selective attention. To preview, the results of Experiment 1 confirm that orienting to a target location in the context of IOR resulted in enhanced learning of specific category exemplars presented in that location and abstraction of broader category information. In contrast, orienting in the context of facilitation resulted in neither item-specific learning nor generalization of learning. Experiment 1b confirms that the learning observed in the IOR condition is driven by spatial cueing of attention and cannot be attributed to timing differences across the facilitation and IOR conditions. Finally, Experiment 2 shows that the suppression associated with IOR supports enhanced learning and generalization of category exemplars appearing in the unsuppressed location, relative to those appearing in the suppressed location, in the same infants. Together, these results suggest that selective attention mechanisms involving suppression can support enhanced encoding efficacy in infancy.
General Method
Stimuli and Apparatus
Task Design
The task consisted of a spatial cueing/encoding phase and a subsequent memory test phase. For the spatial cueing/encoding phase, stimuli included a central fixation, a cue, and a set of category exemplars. Category exemplars served as targets that appeared in the location of the expected attention bias (i.e., the cued location in the facilitation condition, the non-cued location in the IOR condition). Spatial cueing effects are typically assessed based on the difference in reaction times to items appearing in the cued and non-cued locations. Thus, it was necessary to include additional stimuli (foils) that appeared in the location opposite the expected attention bias. The fixation shape was presented in the center of the screen and loomed in and out (max size = 5.67 cm2) to sustain infants’ attention. The cue was a yellow ring (2.5 cm diameter). Targets and foils were 7.1 cm2. Cue, targets, and foils were presented 16° (19.41 cm) to the left or right of the central fixation.
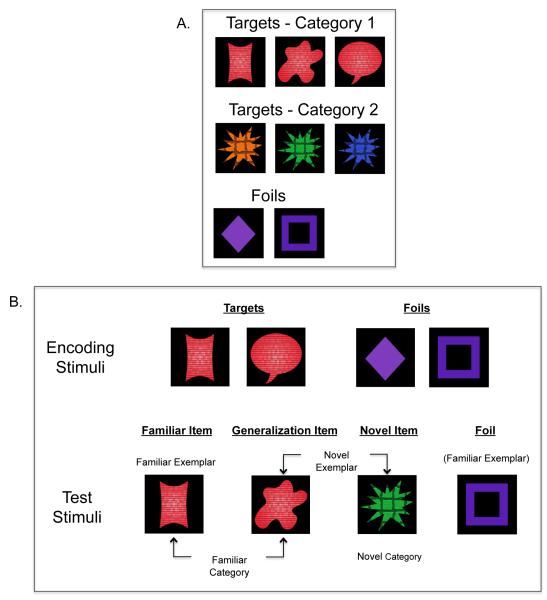
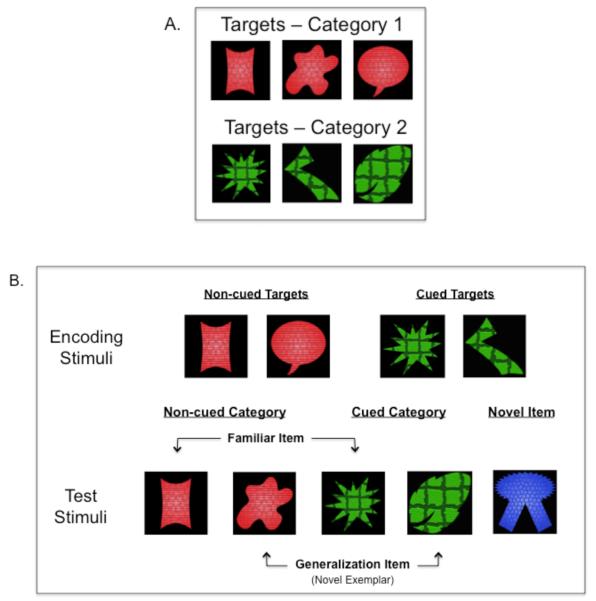
The complete set of targets and foils are depicted in Figure 1A. Targets were drawn from one of two categories consisting of three shapes. Category members always shared two features and varied on a third feature. Category 1 exemplars shared color and texture but varied on shape; Category 2 exemplars shared shape and texture but varied on color. These categories were loosely based on Younger and Cohen (1983), which showed that infants detect correlations across features, and Catherwood, Crassini, and Freiberg (1989), which showed that infants categorize across changing shapes when color is invariant. Two category exemplars served as targets during encoding and the third exemplar served as the generalization stimulus during the test phase (see below). Targets for encoding were counterbalanced across the two categories: half of the infants saw Category 1 exemplars during encoding and the remaining infants saw exemplars from Category 2. There were two foils, a purple diamond and a purple square. These were designed to be simple to calculate spatial cueing effects without requiring visual processing resources. Thus, all infants received repeated exposure to two targets (i.e., category exemplars) and two foils during the spatial cueing/encoding phase.
Figure 1.
A) Full set of target and foil stimuli used in Experiment 1. (B) Example of stimuli presented in the spatial cueing/encoding and test phases of the task.
There were four stimuli for the memory test phase (Figure 1B): 1) a familiar exemplar from the familiar category presented during encoding (Familiar Item), 2) a novel exemplar from the familiar category, to index abstraction of categories beyond basic item feature learning (Generalization Item), 3) a novel exemplar from the novel category (Novel Item), 4) one of the foils presented during encoding (Foil). The Generalization Item was the third category exemplar that had not been presented during encoding. The Novel Item was drawn from the category that had not been seen during encoding. Stimulus presentation order was counterbalanced.
Eye tracking apparatus
Infants sat on their parent’s lap about 70 cm from a 22″ monitor. Eye movements were recorded using a remote eye tracker (SensoMotoric Instruments RED system). A digital video camera with infrared night vision (Canon ZR960) was placed above the monitor to record infants’ head movements. The video camera provided live feed to the experimenter’s monitor, which was used for online coding during the test phase. The video output was additionally recorded as a digital file.
All stimuli were presented using the SMI Experiment Center software. Before the task, each infant’s point-of-gaze (POG) was calibrated by presenting a looming stimulus in the upper left and lower right corners of the screen. The same calibration stimulus was then presented in the four corners of the screen to validate the accuracy of the calibration. Average deviation was 2.3° (SD = 1.7°), suitable for assessing eye movements to the left and right periphery. The digital eye recording was used for offline coding of left/right eye movements if an accurate calibration or a stable POG was not obtained. Coded data were verified by similarly coding a subset of videos for infants who had successful eye movement recordings for comparison with SMI native software POG record output; reliability across the POG record and the coded data was high (r > .90, p < .05).
Experiment 1
Experiment 1 utilized a between-subjects design to examine infants’ learning in the contexts of two different orienting mechanisms, facilitation and IOR.
Experiment 1a
Method
Participants
The final sample included forty-eight 9-month-old infants (24 M, 24 F; M = 9 months, SD = 11 days). Thirteen infants were observed but excluded due to fussiness (9) or experimenter/technical error (4). Infants were recruited from the community via advertisements and birth records. Based on parental report, 80% were Caucasian, 7% were Hispanic, 2% were African-American, 3% were Asian, 7% were mixed-race, and 2% declined to provide information. Infants were excluded from the study if they had been born early (< 36 weeks), had low birth weight (< 5 lbs), or had any history of serious health problems. All families received compensation for participating.
Procedure
Spatial Cueing/Encoding
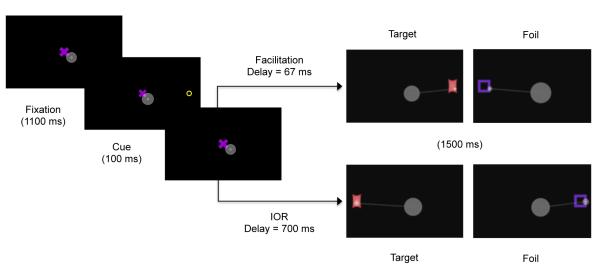
A schematic depiction of the task is presented in Figure 2. Infants were randomly assigned to the facilitation and IOR conditions. All parameters were identical across the facilitation and IOR conditions, except for the cue-target delay length. Trials began with presentation of the central fixation. After 1100 ms, the cue appeared on the left or right side of the fixation and remained for 100 ms. The cue was followed by a delay (facilitation = 67 ms; IOR = 700 ms), during which the central fixation remained visible. After this delay, the fixation disappeared and a target or foil appeared on the left or right side of the screen. Targets and foils remained visible for 1500 ms. An animated stimulus appeared between trials to refocus the infant’s attention to the center of the screen.
Figure 2.
Schematic of spatial cueing/encoding trials with sample superimposed scan path in grey. Infants remained fixated at the central location through the cue presentation and oriented to the left or right following target/foil presentation.
There were 56 spatial cueing/encoding trials, with an equal number of target and foil trials presented in random order. As noted, foils were included to allow for an accurate calculation of spatial cueing effects. Targets (i.e., category exemplars) appeared in the location that was consistent with the expected attention bias, whereas foils appeared in the location that was opposite from the expected attention bias. In the facilitation condition, the targets appeared in the cued location and foils appeared in the non-cued location. In the IOR condition, the targets appeared in the non-cued location while the foils appeared in the cued location. Two category exemplars were presented across the 28 target trials and the two foils were presented across the remaining 28 trials. Thus, the frequency of each individual stimulus was equivalent (14 trials for each stimulus).
Test
The test began after all encoding trials were complete. Test stimuli were presented individually in the center of the screen, with a total of four trials (Familiar Item, Generalization Item, Novel Item, Foil). The order of stimulus presentation was counterbalanced across infants. Attention-getters were presented between trials to reorient infants to the center of the screen. Trials could last up to 20 seconds. An experimenter (blind to the test displays) viewed the live video feed during the test phase and advanced to the next trial if the infant looked away for more than 2 seconds. Look durations at test were validated offline by two coders (r > .90, p < .001).
Data Processing
Spatial cueing/encoding
The two variables of interest for the spatial cueing/encoding phase of the task included saccade latencies and duration of looking at the targets and foils. Initial processing of the eye movement data was completed using the native SMI BeGaze analysis software. Three equivalent areas of interest (AOIs) were identified based on the central, left, and right stimulus locations. These AOIs were defined as a 14.2-cm2 region of space over each of these locations. Usable looks were defined as segments of the data in which the POG remained within 7.1 cm2 (5.8°) for at least 100 ms. This dispersion criterion was less than a third of the distance between the opposing target locations (24 cm/20°), allowing us to maximize usable data while clearly identifying left/right looks. Saccade latencies were computed based on the time at which a look lasting more than 100 ms first entered the relevant AOI. Duration of looking was computed by summing the duration of all looks that occurred within the AOI following target onset.
Individual trials were discarded if the infant looked away from the screen before looking at a target, if the infant looked at the cue/broke fixation prior to target onset, or if there was no eye tracking data available. Infants in the IOR condition looked at the cue at a higher rate (M = 13.8% of trials, SD = 11.5%) than infants in the facilitation condition (M = 7.6%, SD = 5.7%; t(46) = −2.36, p = .023). This increased rate of breaking fixation in the IOR condition was likely due to the longer cue-target delay period. Proportion of looks to the cue was standardized based on the group mean and utilized as a covariate for all analyses of test performance to control for this potentially confounding relationship. There were no differences across conditions in the percentage of trials excluded due to missing data or looks away. Trials were further filtered to exclude those with latencies that were less than 200 ms or greater than two SD above the infant’s mean latency. Latency values were standardized based on each infant’s individual mean to account for potential differences in baseline response times.
Test
Look durations for each test item were standardized based on each infant’s mean to account for individual differences in overall looking times at test. In addition, preferential looking scores were generated separately for the Generalization and Novel items by subtracting looking times to the Familiar item from looking times to each of the novel items. Positive scores indicate preferential looking to the novel item while negative scores indicate preferential looking to the Familiar item.
Results
Spatial Cueing/Encoding
Our first analysis examined whether infants showed the expected spatial cueing effects. Four infants were considered group outliers and were excluded from the spatial cueing analysis because the difference in their saccade latencies on cued versus non-cued trials was greater than 2 SD from the group mean (facilitation, n =3; IOR, n=1). Mean standardized saccade latencies were entered into a 2 (Trial type: cued, non-cued) × 2 (Encoding category: category 1, category 2) × 2 (Condition: facilitation, IOR) ANOVA. Results indicated a main effect of Trial type (F(1,40) = 4.33, p = .044, η2 = .10), with faster latencies to the cued location (M = 455.0 ms, SD = 117.32 ms) relative to the non-cued location (M = 463.62 ms, SD = 105.0 ms). However, there was also a significant Trial type × Condition interaction (F(1,40) = 29.76, p < .001, η2 = .48). Follow-up analyses revealed a significant effect of Trial type within the facilitation condition (F(1,20) = 51.71, p < .001, η2 = .61; Table 1), with expected faster latencies to the cued location (M = 419.70 ms, SD = 114.07 ms) compared to the non-cued location (M = 464.41 ms, SD = 104.61 ms). There was also a main effect of Trial type within the IOR condition (F(1,22) = 5.50, p = .028, η2 = .20; Table 1), with expected slower latencies to the cued location (M = 487.22 ms, SD = 113.14 ms) compared to the non-cued location (M = 462.90 ms, SD = 107.71 ms). Thus, infants in the facilitation condition showed the predicted facilitation of response times and infants in the IOR condition showed the predicted inhibition of response times to the cued location.
Table 1.
Mean reaction times to the cued and non-cued locations during the spatial cueing/encoding portion of the task.
Mean Reaction Times (ms)
| Cued Location Mean (SD) |
Non-cued Location Mean (SD) |
Difference Mean (SD) |
|
|---|---|---|---|
| Expt 1: Facilitation Condition |
419.70 (114.07) | 464.41 (104.61) | −44.70 (28.49) |
| Expt 1: IOR Condition |
487.22 (113.14) | 462.90 ms (107.71) | 24.32 (43.36) |
| Expt. 2 | 478.22 (101.21) | 458.61 (108.30) | 19.61 (52.27) |
Test
We were primarily interested in the impact of the facilitation and IOR mechanisms on infants’ looking patterns at test. However, there were two additional factors that could potentially impact responses to the test items. First, high rates of looking to the cue during encoding could interfere with the expected spatial cueing effects and lead to different looking patterns at test. As noted, these trials were excluded for the calculation of spatial cueing effects but still may have added variability to encoding during familiarization. Given this potentially confounding relationship, proportion of looks to the cue was included as a covariate during all analyses of looking times at test. Second, varying durations of looking to the targets during encoding across the facilitation and IOR conditions could drive different response patterns at test. Preliminary analyses indicated that infants in both conditions spent more cumulative time looking at the targets (i.e., category items; M = 16.12 s, SD = 6.69 s) than the foils (M = 13.26 s, SD = 5.88 s; F(1,46) = 31.43, p < .001, η2 = .41). As noted, the foils were intended to be simple and require less visual processing than targets. However, there was no difference across conditions in total looking times to the targets over all encoding trials (MFacilitation = 17.06 s, SD = 6.94 s, MIOR = 15.18 s, SD = 6.45 s; F(1, 46) = 0.95, p = .334).
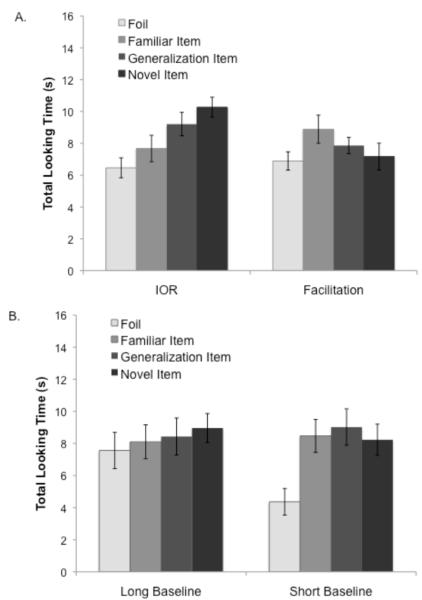
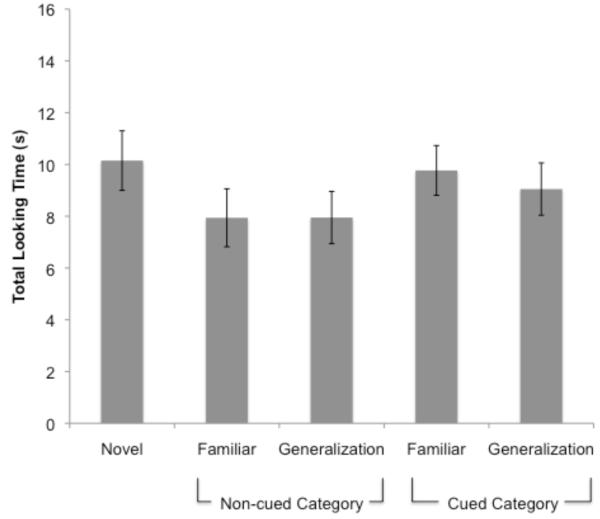
Looking times at test are presented in Figure 3A. Standardized looking times were compared using a 4 (Test Item: Familiar, Generalization Novel, Foil) × 2 (Encoding category: category 1, category 2) × 2 (Condition: facilitation, IOR) ANCOVA with standardized proportion of looks to the cue and standardized duration of looking to the targets and foils as covariates. Results indicated a significant Test Item × Condition interaction (F(3,123) = 4.04, p = .009, η2 = 09). Simple effects analyses indicated no significant effects within the facilitation condition; however, there was main effect of Test Item within the IOR condition (F(3,60) = 6.22, p = .001, η2 = .24).
Figure 3.
Total look durations to each test item during Experiment 1a (A) and Experiment 1b (B).
Follow-up analyses were conducted within the IOR condition to compare looking times to the Familiar versus Generalization items, as well as the Familiar, Foil, and Generalization items relative to the Novel test item. Infants spent more time looking at the Novel item (M = 10.27 s, SD = 4.87 s) relative to the Familiar item (M = 7.68 s, SD = 4.69 s; F(1,20) = 11.55, p = .003, η2 = .37), indicating effective learning of the Familiar item. Similarly, infants spent more time looking at the Novel item relative to the Foil (M = 6.46 s, SD = 4.51 s; F(1,20) = 17.02, p = .001, η2 = .46). In addition, infants spent more time looking at the Novel item compared to the Generalization item (M = 9.21 s, SD = 5.58; F(1,20) = 5.31, p = .032, η2 = .21), suggesting that they perceived the Generalization item to be relatively familiar. Finally, there was no difference in looking times across the Generalization and Familiar items (F(1,20) = 1.98, p = .175), indicating that infants perceived these items as similar. Thus, infants in the IOR condition showed increased interest in the Novel item relative to both of the familiar test items and the Generalization item.
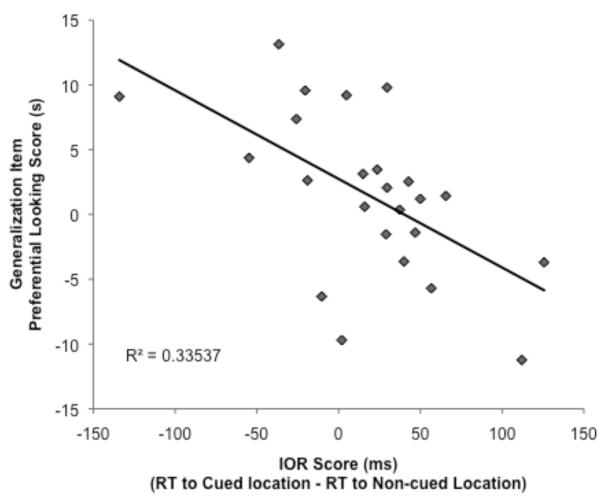
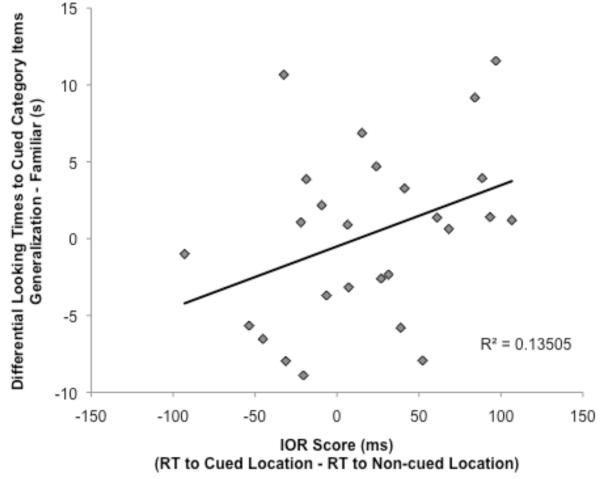
The final analysis related the extent of IOR to preferential looking scores for the Novel and Generalization items. Results showed a relationship between IOR scores and preferential looking scores for the Generalization item (r(24) = −.47, p = .02; Figure 4). Infants who showed the strongest IOR showed the least preferential looking to the Generalization item relative to the Familiar item, indicating better learning and abstraction of category information.
Figure 4.
Relation between IOR scores and dishabituation scores for the Generalization test item. Infants who showed the strongest IOR effects during encoding showed the least dishabituation to the Generalization item, indicating greater generalization of learning.
Discussion
Experiment 1a suggests that the facilitation and IOR attention mechanisms support differential encoding of category exemplars. Infants in the IOR condition showed both learning of the familiar category exemplar and generalization of learning to a novel exemplar whereas infants in the facilitation condition showed no evidence of learning. This differential learning was evident despite the fact that all presentation parameters were equated across the two conditions, except for the cue-target delay length, and infants in the two conditions spent the same amount of time looking at the category exemplars during encoding. These data suggest that orienting mediated by the suppression mechanism associated with IOR promotes more effective encoding compared to orienting driven by the excitation mechanism associated with facilitation.
However, Experiment 1a does not eliminate the possibility that similar effects would be observed in the absence of spatial cueing. For example, it remains possible that the short versus long cue-target delay may have contributed to differential learning across the two conditions. Though these delay lengths are critical for eliciting the facilitation and IOR attention mechanisms, they result in different total trial durations. Over the course of 56 encoding trials, this difference may have allowed for more extensive encoding in the IOR condition by providing more time for consolidation of items that were encoded during the previous trial.
To address this possibility, we conducted a control experiment consisting of two baseline no-cue conditions with timing that mirrored the facilitation and IOR conditions. Doing so allowed us to both examine learning in the absence of attention cues and examine potential trial duration effects. If the differential learning observed in Experiment 1a was due to timing differences alone, similar effects should be evident with the same timing despite the absence of cueing.
Experiment 1b
Experiment 1b examined infants’ category learning in the absence of spatial cueing of attention using a short baseline condition with timing that mirrored the previous facilitation condition and a long baseline condition with timing that mirrored the previous IOR condition.
Method
Participants
The final sample included forty-eight 9-month-old infants (26 M, 22 F; M = 9 months, SD = 10 days). Seven infants were tested but excluded due to fussiness (3) or technical errors (4). Based on parental report, 81% were Caucasian, 13% were Hispanic, 4% were African-American, and 2% were Asian. Exclusion criteria were the same as those reported above. All families received compensation for participating.
Procedure
Encoding
Trials began with presentation of the central fixation, which remained visible for 1267 ms in the short baseline condition and 1900 ms in the long baseline condition. The fixation was immediately followed by presentation of the target or foil on the left or right side of the screen. Targets and foils remained visible for 1500 ms. There were 56 trials, with an equal number of targets and foils and 14 trials for each individual stimulus.
Test
The test phase was identical to that of Experiment 1a.
Data Processing
Encoding
Saccade latencies and looking times to the targets were computed using the same process described in Experiment 1a. Individual trials were discarded based on the criteria described previously. Infants in the long baseline condition looked away more frequently (M = 35.0% of trials, SD = 14.0%) than infants in the short baseline condition (M = 27.2%, SD = 11.9%; t(46) = 2.06, p = .045). Trials were filtered to exclude those with latencies that were less than 200 ms or greater than two SD above the infant’s mean latency. Saccade latencies were standardized as described in the previous experiment.
Test
Data processing for the test phase was identical to that of Experiment 1a.
Results
Encoding
We first examined whether there were differences in saccade latencies to the targets and foils. Two infants in the long baseline condition were excluded from this analysis because the difference in their latencies to targets versus foils was greater than 2 SD from the group mean. Results of a 2 (Trial type: target, foil) × 2 (Encoding category: category 1, category 2) × 2 (Condition: short, long) ANOVA indicated a main effect of Trial type (F(1,42) = 10.27, p = .003, η2 = .20), with faster latencies to targets (M = 465.11 ms, SD = 119.95 ms) than foils (M = 481.27 ms, SD = 114.62 ms). Follow-up analyses indicated that this effect was driven by infants who saw category 2 exemplars (variable colors) during encoding (MTarget = 462.78 ms, SD = 121.02 ms, MFoil = 487.58 ms, SD = 120.89; F(1, 20) = 8.08, p = .01, η2 = .29). These faster responses to category 2 targets is likely attributable to greater salience of changing colors across trials in the absence of a preceding cue. There was no difference in saccade latencies among infants who saw category 1 exemplars during encoding.
Test
Preliminary analyses indicated that infants spent more time looking at the targets (M = 13.25 s, SD = 6.37 s) than the foils (M = 10.73 s, SD = 5.29 s; F(46) = 26.51, p < .001, η2 = .37) during encoding. However, there was no difference in total looking times to the targets across the two conditions (MShort = 12.27 s, SD = 7.04 s; MLong = 14.23 s, SD = 5.6 s; F(1,46) = 1.14, p = .291).
Looking times at test were compared using a 4 (Test Item: Familiar, Generalization, Novel, Foil) × 2 (Encoding category: category 1, category 2) × 2 (Condition: short, long) ANCOVA with standardized looking times to the target and foil during encoding treated as covariates. Results indicated a main effect of Test Item (F(3,126) = 5.38, p = .002, η2 = .11). Follow-up comparisons indicated that the Foil was the only item with shorter looking times (M = 6.09 s, SD = 4.86 s) relative to the remaining test items (MFamiliar = 8.29 s, SD = 5.05 s; F(1,45) 7.74, p = .004, η2 = .17; MGen = 8.73 s, SD = 5.55 s; F(1,45) 7.74, p = .008, η2 = .15; MNovel = 8.60 s, SD = 4.56 s; F(1,45) = 15.56, p < .001, η2 = .26). There were no significant differences in looking times across the remaining test items and the Test Item × Condition interaction was not significant (F(3,126) = 1.14, p = .34, η2 = .03).
We again examined whether looking patterns during encoding predicted looking patterns at test. Correlation analyses indicated that saccade latencies were not related to infants’ preferential looking scores at test.
Discussion
Experiment 1 revealed that infants showed greater learning and abstraction of category exemplars when encoding occurred in the context of inhibitory selective attention, relative to basic facilitation of orienting. Experiment 1b confirmed that this differential learning was dependent on spatial cueing and was not due to timing differences alone. Together, these results provide support for the proposed mechanism in which suppression of interfering information leads to a more robust signal and enhanced learning of items appearing in the attended location.
A more stringent test of this interpretation of the data would involve a within-subjects design that tests learning of items in the cued/suppressed location versus those in the opposing, non-cued location. If our proposed mechanism were correct, we would expect infants to show enhanced learning of items appearing in the non-cued location relative to those in the cued/suppressed location. In Experiment 1 we were unable to directly examine the impact of suppression on learning across target locations; the cued items (the foils) were intended to be simple to minimize processing demands, precluding a clear comparison of infants’ learning of the cued and non-cued items. As such, we conducted a final experiment in which we examined learning of category exemplars appearing in the non-cued location compared to those appearing in the cued/suppressed location.
Experiment 2
Experiment 2 utilized a within-subjects design with IOR cueing to examine the impact of inhibitory selective attention on learning of items appearing in the cued/suppressed location versus those in the non-cued/unsuppressed location.
Method
Participants
The final sample included twenty-six 9-month-old infants (9 M, 17 F; M = 9 months, 10 days, SD = 12 days). Four infants were observed but excluded due to fussiness (3) or experimenter/technical error (1). Based on parental report, 73.1% of participants were Caucasian, 11.5% were Hispanic, 11.5% were African-American, and 3.8% were Asian. Exclusion criteria were the same as reported above. All families received compensation for participating.
Stimuli
The stimuli used for Experiment 2 are depicted in Figure 5. Targets were drawn from two categories consisting of three shapes (Figure 5A). For both categories, exemplars shared color and texture but varied on shape. Two exemplars from each category, counterbalanced across infants, served as targets during the encoding phase and the third exemplar served as the generalization stimulus at test. The fixation and cue stimuli were identical to those in Experiment 1.
Figure 5.
A) Full set of target stimuli used in Experiment 2. (B) Example of stimuli presented in the spatial cueing/encoding and test phases of the task.
During the encoding phase “cued” targets appeared in the cued location and “non-cued” targets appeared in the non-cued location. Because this experiment used IOR timing, we expected the cued targets to be suppressed while infants’ attention would be biased towards the non-cued targets. Targets were counterbalanced so that half of the infants saw cued targets from Category 1 and non-cued targets from Category 2 while the remaining infants saw cued targets from Category 2 and non-cued targets from Category 1. Thus, all infants received repeated exposure to four targets (two cued, two non-cued).
The memory test phase included five stimuli (Figure 5B): 1) a familiar target from the cued category (Familiar Cued Item), 2) a novel exemplar from the cued category (Cued Generalization Item), 3) a familiar target from the non-cued category (Familiar Non-cued Item); 4) a novel exemplar from the non-cued category (Non-cued Generalization Item), and 5) a completely novel item (Novel Item).
Procedure
Spatial Cueing/Encoding
The procedure for the spatial cueing/encoding trials was identical to the IOR condition of Experiment 1a. There were a total of 56 spatial cueing/encoding trials, with an equal number of cued and non-cued targets presented in random order. Two different exemplars were presented across the 28 cued target trials and two new exemplars were presented across the remaining 28 non-cued target trials. Thus, the frequency of each individual stimulus was equivalent (14 trials for each stimulus).
Test
The test began after all encoding trials were complete. Test stimuli were presented individually in the center of the screen, with a total of five trials (Familiar Cued Item, Cued Generalization Item, Familiar Non-cued Item, Non-Cued Generalization Item, Novel Item). The order of stimulus presentation was pseudo-randomized to ensure that each of the five test items was presented first with equal frequency. The timing of the trials was identical to Experiment 1.
Data Processing
Spatial cueing/encoding
As before, our primary variables of interest from the spatial cueing/encoding phase were saccade latencies and duration of looking at the targets. These variables were computed using the same procedure as Experiment 1. Individual trials were discarded for saccade latency analysis based on the criteria described previously. The proportion of trials that were excluded was similar to that observed in Experiment 1a (F(2, 72) = 1.5, p = .231). Trials were further filtered to exclude those with latencies less than 200 ms or greater than two SD above the infant’s mean latency. Saccade latencies were standardized in the same manner as Experiment 1. IOR scores were computed by subtracting each infant’s mean latency to the cued location from their mean latency to the non-cued location. Positive scores indicate stronger IOR effects (i.e., greater suppression).
Test
Data processing for the test phase was identical to Experiment 1.
Results
Encoding
We first verified the expected differences in saccade latencies to the cued and non-cued targets. Results of a 2 (Trial type: cued, non-cued) × 2 (Encoding category: category 1, category 2) ANOVA indicated a main effect of Trial type (F(1,24) = 4.63, p = .042, η2 = .16; Table 1), with slower latencies to the cued targets (M = 478.22 ms, SD = 101.21 ms) relative to the non-cued targets (M = 458.61 ms, SD = 108.30 ms). Thus, as a group infants demonstrated the predicted IOR effect.
Test
Preliminary analyses confirmed that infants showed similar cumulative looking times to the cued (M = 11.69 s, SD = 4.83 s) and non-cued targets (M = 11.86 s, SD = 6.13s; F(1,24) = 0.09, p = .764) during the spatial cueing/encoding phase.
Looking times at test are presented in Figure 6. Our primary question concerned whether infants learned the non-cued items to a greater extent than the cued items. To examine this question we compared infants’ responses to the Familiar items from these categories to their responses to the Novel test item. Looking times to these items were entered into a repeated-measures ANCOVA with looking times to targets during encoding as covariates. Results indicated a main effect of test item (F(2,46) = 3.25, p = .048, η2 = .12). Follow-up comparisons indicated that infants spent more time looking at the Novel item (M = 10.15 s, SD = 5.86 s) relative to the Familiar item from the non-cued category (M = 7.94 s, SD = 4.63 s, F(1,23) = 6.77, p = .02). In contrast, there was no difference in looking times across the Novel item and the Familiar item from the cued category (M = 8.91 s, SD = 4.15 s, F(1,23) = 2.15, p = .156). These results suggest that infants effectively learned the non-cued targets but did not discriminate between the cued targets and the completely novel item.
Figure 6.
Looking times to the Novel test item and test items drawn from the cued and non-cued categories.
We next examined whether the extent of generalization of learning varied across the cued and non-cued categories. We entered standardized looking times at test into a 2 (Trial type: cued, non-cued) × 2 (Test item: Familiar, Generalization) × 2 (Encoding category: Category 1, Category 2) ANCOVA with standardized proportion of looks to the cue and standardized IOR scores as covariates. As noted in Experiment 1, high rates of looking to the cue during encoding could interfere with IOR and lead to different looking patterns at test. Proportion of looks to the cue was standardized based on the group mean and included as a covariate to account for this possibility. In addition, given the wide variability in IOR scores in our sample, we included these scores as a covariate to examine whether the extent of generalization varied based on the strength of infants’ IOR effects.
Results indicated only a significant Trial type × Test item × IOR score interaction (F(1,22) = 4.65, p = .042, η2 = .175). Follow-up analyses indicated that there were no significant effects of Test item or IOR score for the non-cued category. Thus, infants showed similar looking times to the Familiar and Generalization items from the non-cued category. It is important to recall that infants spent less time looking at the non-cued targets relative to the Novel item, indicating successful learning of these items (Figure 6). Here, the same infants did not differentiate between the familiar and novel items from the non-cued category, suggesting generalized learning of the non-cued category.
In contrast, for the cued category there was a significant Test item × IOR score interaction (F(1,22) = 6.86, p = .016, η2 = .24). Infants who showed the strongest IOR (i.e., the strongest suppression) showed a greater novelty response to the Generalization item relative to the Familiar item (r = .48, p = .016; Figure 7). This preferential looking to the Generalization item suggests that infants who demonstrated the strongest suppression at the cued location showed little generalized learning of the cued category.
Figure 7.
Relationship between IOR scores and differential looking times to the Familiar and Generalization items from the cued category. Infants who showed the strongest IOR (i.e. greatest suppression) at encoding showed the least generalized learning of the cued category.
In sum, the results of Experiment 2 suggest that infants effectively learned items that appeared in the non-cued location. Furthermore, within the non-cued category, infants did not differentiate between the Familiar and Generalization items, indicating generalization of the non-cued category information to a novel exemplar. However, the same infants did not discriminate the cued category items from the Novel item, indicating poor learning. Yet even as both the Familiar and Generalization items within the cued category were not different from a completely Novel test item, infants’ responses to these items varied based on the extent of suppression that occurred during encoding.
General Discussion
The present study examined the role of attentional processes at encoding in modulating the efficacy of learning and memory. We utilized a spatial cueing task to compare 9-month-old infants’ encoding of category exemplars in the context of an orienting mechanism involving location suppression (IOR) versus a selection mechanism that does not require suppression (facilitation). Infants demonstrated the predicted spatial cueing effects, as they oriented faster to cued items in the facilitation condition but instead oriented faster to non-cued items in the IOR condition. Furthermore, this small difference in orienting dynamics resulted in differential learning. In Experiment 1, infants in the IOR condition showed learning of specific category exemplars presented during encoding as well as abstraction of broader category information. In contrast, infants in the facilitation condition showed no evidence of learning. Experiment 2 replicated this effect using a within-subjects design, as infants showed robust learning and abstraction of non-cued category exemplars but also showed ineffective learning of items appearing in the cued/suppressed location. To our knowledge, this is the first developmental investigation demonstrating that the suppression component of attention mechanisms driving orienting has a critical impact on the efficacy of learning and memory.
Evidence for generalized category learning is typically based on two patterns of responses at test (Quinn, 1987): First, infants show reduced looking to a familiar stimulus relative to a completely novel item, indicating effective learning of the stimulus presented during encoding. Second, infants fail to recover interest to a novel exemplar from the familiar category, suggesting that they respond to the novel item as familiar, despite never seeing that specific item during encoding. In Experiment 1, infants in the IOR condition showed both responses, suggesting that they learned the specific features of the exemplars presented during encoding and abstracted the broader category information. Furthermore, infants who showed the strongest IOR also showed the most effective generalization of category learning. In contrast, infants in the facilitation condition showed no discrimination across test items, suggesting that they did not learn the specific category items or the broader category information.
Although the results of Experiment 1 provide the first indication that inhibitory selective attention drives differential learning, Experiment 2 offers a more stringent test of the impact of suppression on encoding efficacy. The results of Experiment 2 indicate that suppression at the cued location supports robust encoding at the opposing location, as learning and generalization was specific to items appearing in the non-cued location. For these items, infants showed both of the requisite response patterns, including reduced looking times relative to a completely novel item and generalization of looking time from the familiar category exemplar to a novel exemplar (i.e., the Generalization item). This is consistent with the proposed mechanism in which suppression of interfering information supports a more robust signal and enhanced encoding at the attended location.
However, as a group, the same infants in Experiment 2 showed ineffective learning of the cued items, despite equivalent look durations to the cued and non-cued targets during encoding. Furthermore, within this context of generally poor learning, the variability in infants’ learning of the cued category was best predicted by the extent of suppression that occurred in that location, as indexed by IOR scores; infants who showed the greatest suppression (i.e., highest IOR scores) at the cued location showed the least abstraction of general category information for exemplars appearing in that location. This finding provides important and novel insight into how precise spatiotemporal dynamics of attentional mechanisms can influence learning and memory.
Notably, infants only demonstrated successful learning and generalization when targets were encoded in the context of IOR. In Experiment 1, effective learning was observed only in the IOR condition; in Experiment 2 learning was evident only for items that appeared in the non-cued location. We observed no evidence of learning in either baseline condition or the facilitation condition and looking times to the cued test items in Experiment 2 were similar to those observed in the long baseline condition of Experiment 1 (Table 2). Together, these patterns suggest that the differential learning associated with IOR was due to enhanced encoding efficacy.
Table 2.
Comparison of looking times at test across the long baseline condition in Experiment 1 and the cued category items in Experiment 2.
Total Looking Time (s)
| Familiar Item Mean (SD) |
Generalization Item Mean (SD) |
Novel Item Mean (SD) |
|
|---|---|---|---|
| Expt 1: Long Baseline |
8.11 (1.06) | 8.44 (1.15) | 8.97 (0.90) |
| Expt 2: Cued Location |
8.77 (0.96) | 9.05 (1.01) | 10.15 (1.15) |
It should be underscored that this differential learning cannot be attributed to differences in looking to the targets during encoding. In Experiment 1, infants in the two conditions accumulated similar amounts of looking time to the targets during encoding, yet only infants in the IOR condition showed effective learning. In Experiment 2, infants similarly showed no differences in looking to the cued and non-cued targets during encoding, yet showed differential recognition of these items at test. Furthermore, the results of Experiment 1b verify that the observed differential encoding efficacy cannot be attributed to simple differences in trial duration. This dissociation between look durations during encoding and encoding efficacy is consistent with previous work indicating that overt looking does not always directly correspond to attentional processing (Oakes, Madole, & Cohen, 1991; Richards, 1997); instead, the nature of the underlying attentional mechanisms can have critical implications for learning.
The results of the no-cue baseline conditions also confirm that attention cueing is a crucial factor driving differential learning across the facilitation and IOR conditions. However, the results of Experiment 1 also demonstrate that spatial cueing alone does not drive differential learning; rather, encoding is only enhanced in the context of spatial cueing that elicits an inhibitory selective attention mechanism. In the Experiment 1, infants in the facilitation condition showed a larger spatial cueing effect on orienting latency than those in the IOR condition. Enhanced learning in the IOR condition despite this weaker cueing effect underscores the specificity of the link between encoding efficacy and inhibitory selective attention.
Previous work has shown that different forms of attention cueing can differentially impact learning. Wu and Kirkham (2010) showed that 8-month-old infants successfully learned predictable audiovisual events when a face directed orienting to the target location, but not when orienting was driven by a non-social attention cue. In the present study, the nature of the underlying attention mechanisms mediating orienting, rather than the features of the cue itself, modulated the efficacy of encoding. Though it has long been understood that the suppression component of selective attention mechanisms limits distractor interference, the present data add that, in doing so, this reciprocal process also supports increasingly sophisticated learning and memory outcomes. These data thus highlight a potential mechanism for the beneficial effects of attention cueing on learning and memory that have been observed in previous work.
Although we have used the terms learning and encoding interchangeably, our proposed mechanism is that attentional suppression influences the quality of the visual signal downstream of learning and memory systems. Because this signal theoretically serves as input to memory systems, this suppression is most likely to influence depth of encoding. Although the present results show differential recognition at test, in Experiment 1 all parameters were equated across conditions except for the facilitation/IOR attention manipulation at encoding. Similarly, in the within-subjects design of Experiment 2, all parameters were equated yet learning efficacy varied based on the placement of the category items relative to the location of the cue during encoding. As such, we are confident that inhibitory selection specifically modulated encoding in the present experiments. Nonetheless, because encoding is one stage in the learning and memory continuum, this effect of suppression on encoding will undoubtedly have implications for the efficacy of related consolidation and retrieval processes.
The present evidence for differential encoding in the context of suppression is consistent with the neural mechanisms mediating selective attention. Numerous studies have demonstrated that frontoparietal selective attention networks modulate activity in visual cortex, with enhanced signal associated with information appearing in attended locations (Gandhi, Heeger, & Boynton, 1999; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999) and concurrent suppression of the signal associated with information appearing in unattended locations (Slotnick, Schwarzbach, & Yantis, 2003; Smith, Singh, & Greenlee, 2000). We raise the possibility that the net result may be a focus of resources on the attended location and hence a more robust, less noisy, signal for subsequent learning and memory. Indeed, the extent to which visual cortex signal is modulated by selective attention networks predicts adults’ behavioral performance on working memory tasks (Rutman, Clapp, Chadick, & Gazzaley, 2010; Zanto, Rubens, Thangavel, & Gazzaley, 2011), further suggesting that the role of selective attention in modulating visual cortex activity can have meaningful consequences for encoding efficacy.
Though the precise neural mechanisms underlying behavioral IOR effects in infancy are less clear, the existing adult/animal literature suggests that the role of suppression in enhancing encoding efficacy may be driven by frontoparietal modulation of visual cortex signal. As noted in the introduction, selection via suppression develops in the first several postnatal months (Hunnius, 2007; Richards, 2000; Rothbart & Posner, 2001). Therefore, the efficacy of learning and memory may be intertwined with these attentional processes and their level of sophistication. Having established this result, we can now pursue this important developmental question. Future work will also consider this interaction in more cluttered and naturalistic environments.
Naïve learners face the daily challenge of encoding and retrieving information about numerous objects/events appearing in noisy environments. The development of selective attention aids in this process by efficiently allocating processing resources to relevant information. In the present study we show that inhibitory selective attention mechanisms involving concurrent enhancement of attended information and suppression of unattended information can further enhance the efficacy of memory encoding for subsequent retrieval. Though attentional orienting mechanisms often appear uniform at the behavioral level, the present results highlight that the nature of the underlying attention mechanisms driving information gathering can critically impact the efficacy of learning and memory processes.
Footnotes
The authors gratefully acknowledge the National Institutes of Health (MH 07793 to DA) and the James S. McDonnell Foundation (Scholar Award in Understanding Human Cognition to DA) for their generous support of this work.
References
- Amso D, Johnson SP. Learning by selection: Visual search and object perception in young infants. Developmental Psychology. 2006;42(6):1236–1245. doi: 10.1037/0012-1649.42.6.1236. [DOI] [PubMed] [Google Scholar]
- Astle DE, Nobre AC, Scerif G. Attentional control constrains visual short-term memory: Insights from developmental and individual differences. The Quarterly Journal of Experimental Psychology. 2010;65(2):277–294. doi: 10.1080/17470218.2010.492622. doi: 10.1080/17470218.2010.492622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher PR, Kalverboer AF, Geuze RH. Inhibition of return in very young infants: A longitudinal study. Infant Behavior and Development. 1999;22(3):303–319. [Google Scholar]
- Catherwood D, Crassini B, Freiberg K. Infant response to stimuli of similar hue and dissimilar shape: Tracing the origins of the categorization of objects by hue. Child Development. 1989;60(3):752–762. [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M. Divided attention and memory: Evidence of substantial interference effects at retrieval and encoding. Journal of Experimental Psychology: General. 2000;129(2):155–176. doi: 10.1037//0096-3445.129.2.155. doi: 10.1D37//0096-3445.129.2.155. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences. 1999;96(6):3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ. The world is so full of a number of things: On specification and perceptual learning. Ecological Psychology. 2003;15(4):283–287. [Google Scholar]
- Hauer BJA, MacLeod CM. Endogenous versus exogenous attentional cuing effects on memory. Acta Psychologica. 2005;122:305–320. doi: 10.1016/j.actpsy.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Hunnius S. The early development of visual attention and its implications for social and cognitive development. Progress in Brain Research. 2007;164:187–209. doi: 10.1016/S0079-6123(07)64010-2. doi: 10.1016/S0079-6123(07)64010-2. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Slemmer JA, Amso D. Where infants look determines how they see: Eye movements and object perception performance in 3-month-olds. Infancy. 2004;6(2):185–201. doi: 10.1207/s15327078in0602_3. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. TRENDS in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Madole KL, Cohen LB. Infants’ object examining: Habituation and categorization. Cognitive Development. 1991;6(4):377–392. [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. Vol. X. Erlbaum Lawrence Associates; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2(3):211–228. [Google Scholar]
- Quinn PC. The categorical representation of visual pattern information by young infants. Cognition. 1987;17:145–179. doi: 10.1016/0010-0277(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Reid V, Striano T. Adult gaze influences infant attention and object processing: implications for cognitive neuroscience. European Journal of Neuroscience. 2005;21:1763–1766. doi: 10.1111/j.1460-9568.2005.03986.x. [DOI] [PubMed] [Google Scholar]
- Reid V, Striano T, Kaufman J, Johnson MH. Eye gaze cueing facilitates neural processing of objects in 4-month-old infants. NeuroReport. 2004;15:2553–2555. doi: 10.1097/00001756-200411150-00025. [DOI] [PubMed] [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33(1):22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36(1):91–108. [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes LM, Luck SJ. Exogenous attention influences visual short-term memory in infants. Developmental Science. 2011;14(3):490–501. doi: 10.1111/j.1467-7687.2010.00992.x. doi: 10.1111/j.1467-7687.2010.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Mechanism and variation in the development of attentional networks. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 353–363. [Google Scholar]
- Rutman A, Clapp W, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2010;22(6):1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Perception and Psychophysics. 2002;64(5):754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19(4):1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11(2):271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Atherton K, Patai EZ, Nobre AC. Long-term memory prepares neural activity for perception. Proceedings of the Nataional Academy of Sciences. 2012;109(6):E360–E367. doi: 10.1073/pnas.1108555108. doi: 10.1073/pnas.110855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: Inhibitory priming by ignored objects. Quarterly Journal of Experimental Psychology. 1985;37A:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Wu R, Gopnik A, Richardson DC, Kirkham NZ. Infants learn about objects from statistics and people. Developmental Psychology. 2011;47(5):1220–1229. doi: 10.1037/a0024023. doi: 10.1037/a0024023. [DOI] [PubMed] [Google Scholar]
- Wu R, Kirkham NZ. No two cues are alike: Depth of learning during infancy is dependent on what orients attention. Journal of Experimental Child Psychology. 2010;107(2):118–136. doi: 10.1016/j.jecp.2010.04.014. doi: 10.1016/j.jecp.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Yi D-J, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. The Journal of Neuroscience. 2005;25(14):3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger BA, Cohen LB. Infant perception of correlations among attributes. Child Development. 1983;54(4):858–867. [PubMed] [Google Scholar]
- Yu C, Smith LB. What you learn is what you see: using eye movements to study infant cross-situational word learning. Developmental Science. 2011;14(2):165–180. doi: 10.1111/j.1467-7687.2010.00958.x. doi: 10.1111/j.1467-7687.2010.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens M, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14(5):656–661. doi: 10.1038/nn.2773. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]