Abstract
Lymphatic filariasis is caused by three closely related nematode parasites: Wuchereria bancrofti, Brugia malayi and Brugia timori. These species have many ecological variants that differ in several aspects of their biology such as mosquito vector species, host range, periodicity, and morphology. Although the genome of B. malayi (the first genome sequenced from a parasitic nematode) has been available for more than five years, very little is known about genetic variability among the lymphatic dwelling filariae. The genetic diversity among these worms is not only interesting from a biological perspective, but it may have important practical implications for the Global Program to Eliminate Lymphatic Filariasis, as the parasites may respond differently to diagnostic tests and/or medical interventions. Therefore, better information on their genetic variability is urgently needed. With improved methods for nucleic acid extraction and recent advances in sequencing chemistry and instrumentation, this gap can be filled relatively inexpensively. Improved information on filarial genetic diversity may increase the chances of success for lymphatic filariasis elimination programs.
I. Introduction
Lymphatic filariasis (LF) is a neglected tropical disease caused by the filarial nematode parasites Wuchereria bancrofti, Brugia malayi, and Brugia timori. These worms are endemic in 72 countries in the tropics and sub-tropics where more than 1.4 billion people are at risk of infection (WHO, 2009). Estimates suggest that 120 million people are presently infected with one or more of the lymphatic filariae (WHO, 2009). Although many people with filarial infections are asymptomatic, some 40 million people have clinically evident disease (mostly hydroceles and lymphedema), making LF a leading cause of long-term disability (Gyapong et al., 2005).
Due to its significant medical, social, and economic impact, the World Health Organization (WHO) has targeted LF for elimination by the year 2020 (Ottesen, 2000). The Global Program to Eliminate Lymphatic Filariasis (GPELF) relies on mass administration of anthelmintic drugs to disrupt parasite transmission in endemic communities. By the end of 2005, mass drug administration (MDA) programs had reached nearly half of the global at-risk population (WHO, 2006). By halftime in 2010, MDA had successfully reduced disease rates in many areas (WHO, 2010, 2011); however, confounding factors impede the fight for global elimination. Among these factors are differences in the species and strains of the lymphatic filariae that affect various aspects of transmission, disease progression, diagnosis and treatment.
Filariasis research has not adequately explored variability among the lymphatic dwelling parasite species. Most molecular studies have focused on a lab-adapted zoophilic strain of B. malayi, the only lymphatic filarial parasite of humans that can be maintained in research laboratories. Though the behavior of this strain in a rodent model has been described in detail, little is known about its natural behavior in human or wild animal hosts. In contrast, the vast majority of field studies have focused on human infections with W. bancrofti, a parasite that has not been well characterized, mainly due to the lack of an experimental host and the inability to keep parasites in the laboratory. Despite recent advances that have made genome sequencing relatively cheap and easy, the inter- and intra-species variation among these parasites has not been thoroughly studied. Therefore, in this review, we discuss the diversity of lymphatic dwelling filarial nematodes and the potential impacts of this diversity on disease elimination efforts.
II. Parasite Species and Ecological Strains
a. Wuchereria bancrofti
Of the three lymphatic filarial species known to infect humans, W. bancrofti has the widest distribution. It is prevalent in Sub-Saharan Africa, south and southeast Asia, and it was introduced to countries in the Caribbean and Latin America with the slave trade (Michael and Bundy, 1997). W. bancrofti was once common in Japan, China, the Republic of Korea, Turkey, Egypt, and Oceania, but it is diminishing or gone from these areas due to disease elimination programs (WHO, 2010, 2011). Owing to its widespread distribution, W. bancrofti is responsible for some 90% of all cases of LF. Estimates suggested that before the launch of the GPELF approximately 115 million people were infected with W. bancrofti (Michael and Bundy, 1997). Since then, MDA efforts, human migrations, and population expansions have confounded these estimates, so updated information is urgently needed.
W. bancrofti can be divided into three major subtypes based on the periodicity of microfilaria (Mf) in the peripheral blood of infected patients: nocturnally periodic, nocturnally subperiodic and diurnally subperiodic (Sasa, 1976). These three subtypes have been further divided into ecological races based on their vector preference (Sasa, 1976). In many cases, the ecological races are exquisitely well adapted to a particular mosquito species. Various cross-transmission experiments have shown that vector competence is related to biting habits as well as the specific anatomical features and physiological properties of the insect (Bryan et al., 1990; Bryan and Southgate, 1988a, b; Buse and Kuhlow, 1979; Jayasekera et al., 1980; Pichon, 2002; Snow et al., 2006; Southgate and Bryan, 1992; Zielke and Kuhlow, 1977). Therefore, a given strain may be preferentially transmitted by a particular mosquito species even when other mosquito species are readily available.
Mf periodicity generally corresponds to the biting habits of the predominant mosquito vector in a given geographical area. The nocturnally periodic strains, whose Mf are only present in peripheral blood at night, are transmitted primarily by Culex quinquefasciatus in urban areas of Asia, East Africa and the Americas and by Anopheles mosquitoes in rural areas (particularly in sub-saharan Africa) (Bockarie et al., 2009; Hawking, 1957). However, a few reports implicate Mansonia species as vectors in West Africa (Toumanoff, 1958; Ughasi et al., 2012). Nocturnally subperiodic strains, whose Mf are present in peripheral blood at all times with peak densities around midnight, were once common in Thailand and in the Andaman and Nicobar Islands of India where Ochlerotatus (Aedes) niveus and related species served as vectors (Dhamodharan et al., 2008; Kalra, 1974; Pothikasikorn et al., 2008). Diurnally subperiodic W. bancrofti, transmitted by day-biting mosquitoes of the Aedes polynesiensis group, are prevalent in the Pacific region east of Wallace’s line (Moulia-Pelat et al., 1993).
Regardless of the strain or ecological type, all W. bancrofti are strictly anthropophilic. Despite various attempts to develop a laboratory life cycle, no viable, non-primate host has been identified (Ash and Schacher, 1971; Cross et al., 1979, 1981; Dissanaike and Niles, 1965). The only other recognized species of the genus Wuchereria, W. kalimantani, is an Anopheles-transmitted parasite of the silvered leaf monkey; this species is restricted to the island of Borneo and is not known to infect humans or non-primates (Atmosoedjono et al., 1993; Palmieri et al., 1980). Therefore, no member of the genus Wuchereria can be maintained in a host that is amenable to the laboratory setting.
b. Brugia malayi
B. malayi is found in tropical regions of South and Southeast Asia, occasionally overlapping with the range of W. bancrofti (Michael and Bundy, 1997). In areas where the two species are both present, they may co-infect the same host, but they do not utilize the same vector species. Two major forms of B. malayi have been recognized: anthropophilic and zoophilic (Partono and Purnomo, 1987).
Anthropophilic B. malayi are transmitted by Anopheles mosquitoes that breed in open swamps or rice patties, restricting this form of the parasite to rural areas (Fischer et al., 2000; Partono et al., 1977; Partono and Purnomo, 1987; Vythilingam et al., 1996). In accordance with the biting habits of the principal vector, anthropophilic strains exhibit nocturnal periodicity and exclusively infect humans. They may develop in laboratory models (e.g., cats and rodents) under experimental conditions, but the life cycle is difficult to maintain due to shortened periods of Mf production and decreased parasite survival rates (T. Supali, personal communication).
Zoophilic B. malayi are transmitted by Mansonia mosquitoes. These strains show varying patterns of periodicity, but are mainly nocturnally subperiodic. In Southeast Asia, they are readily passed between humans and wild and domestic animal hosts by their zoophilic vectors. Owing to their broad host range, parasites of this strain can be maintained in the laboratory in small animal models (e.g., gerbils, multimammate rats, etc.) using easily bred Aedes mosquitoes as vectors (e.g., Ae. egypti strain black-eyed Liverpool, Ae. togoi) (Ash and Riley, 1970). Mansonia transmitted B. malayi is assumed to have emigrated from South-east Asia to parts of the Indian subcontinent and to the southwestern coast of Sri Lanka in the Middle Ages. It has been hypothesized that the Malayan army introduced B. malayi infection to Sri Lanka during the 12th and 13th century (Schweinfurth, 1983). Unlike the ancestral strains of Southeast Asia, Mansonia transmitted B. malayi are nocturnally periodic in India and Sri Lanka and have not been found in animals (presumably due to the lack of an amenable non-human host).
c. Brugia timori and other Brugia spp
B. timori has the most restricted geographic range of the lymphatic dwelling filarial species. It is only found in Indonesia and Timor-Leste, where it replaces B. malayi in areas east of the Wallace line. B. timori is biologically similar to nocturnally periodic B. malayi in its Mf periodicity, use of an Anopheline vector (in this case, A. barbirostris), and in its restriction to human definitive hosts. Like B. malayi, this species may be co-endemic with W. bancrofti and may co-infect the same human host, but the two parasites are transmitted by different vector species. For example, on Alor island W. bancrofti is transmitted by A. subpictus in coastal areas while B. timori is transmitted by A. barbirostris near rice patties (Supali et al 2002).
Several other Brugia species occur in parts of Asia, Africa and the Americas, including the U.S.A. These species infect various mammals and occasionally cause zoonotic infections in humans, but these cases are rare and almost never lead to the production of Mf (Dissanaike et al., 2000; Orihel and Beaver, 1989; Orihel and Eberhard, 1998; Tan et al., 2011). Some Brugia species hybridize under experimental conditions, and fertile crosses between B. malayi, B. pahangi, and B. patei were reported (Suswillo et al., 1978; Trpis et al., 1981). If hybridization occurs in nature, it may have contributed to intra-specific variation.
III. Disease Pathology
Symptoms of LF range from sub-clinical lymphangiectasia to severe edema and elephantiasis for both brugian and bancroftian filariasis. Hydrocele, scrotal elephantiasis, and chyluria are only seen with bancroftian filariasis. One report detailed a population of Javanese transmigrant farmers that rapidly developed high rates of elephantiasis after settling in a B. timori endemic area of West Flores, Indonesia; this could be taken as an indication that B. timori is a particularly virulent species, but it is very likely that this phenomenon was due (at least in part) to the immunological naïveté of the host population, as farmers immigrating from other endemic areas did not develop the same rates of disease (Partono et al., 1978; Partono and Purnomo, 1978).
The clinical manifestations of LF vary not only between species but also between strains of the same species and between different geographical locations. In 2002, Supali et al. reported W. bancrofti Mf prevalence rates of nearly 20% in parts of Indonesia, and up to 29% of adult males in surveyed communities presented with hydrocele (Supali et al., 2002). On Lihir Island, Papua New Guinea, W. bancrofti Mf prevalence was comparable (approximately 24%), but fewer than 2% of men presented with hydrocele (Hii et al., 2000). Lymphedema of the leg was uncommon in both areas. However, in an area in Haiti where Mf prevalence was 25%, approximately 5% of women suffered from elephantiasis of the leg (Eberhard et al., 1996). Based on an integrated analysis of clinical and parasitological data, Dreyer et al. proposed that the diverse clinical manifestations might result from variations in the mechanisms of pathogenesis (Dreyer et al., 2000). These variations are not well understood, but they could be influenced by differences in the parasite strains or populations present in a given location.
IV. Diagnostics
Differences in parasite species and strains can be distinguished by the tests that are commonly used to diagnose filarial infections in endemic areas. Some diagnostic assays are able to detect a wide array of filarial species and strains, while others are specific to a given type. Discrepancies in the utility and sensitivity of the various diagnostic methods presently in use are reflective of differences in the morphological and genetic structure of the worms. The tests to be discussed include: morphological examination, host antibody detection, parasite antigen detection, and parasite DNA detection.
a. Morphology
Adult stage filarial worms of different species are easily differentiated by size and physical appearance. Unfortunatly, the worms that cause LF are hidden in deep lymphatic vessels and are rarely recovered. Aside from laboratory strains of B. malayi, only a few examples of each species have been collected and examined. Therefore, little is known about morphological variation among naturally occuring lymphatic filariae at the adult stage.
Developing larvae (late L1 through L3) are readily obtained through collection and dissection of insect vectors. Since W. bancrofti, B. malayi and B. timori do not employ the same vectors, these three species can often be differentiated based on the species of the infected mosquito. However, difficulties may arise in distingushing the human filarial parasites from parasites of wild and domestic animals, since larval stages have relatively few defining characters and since animal parasites are poorly described (Bain and Chabaud, 1986). The inability to clearly differentiate animal parasites complicates studies of intra-specific variation in the lymphatic filaria since larvae with minute physical differences may belong to different species rather than to different strains of the same species.
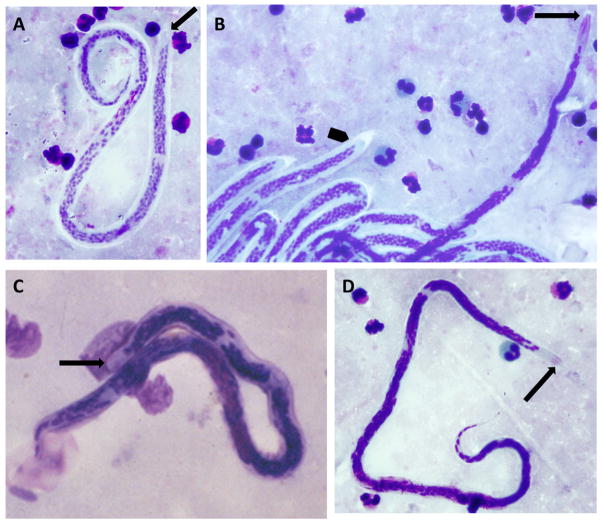
LF has historically been diagnosed by detection of circulating Mf in peripheral blood. The sheathed Mf of W. bancrofti, B. malayi and B. timori are shown in Figure 1. Assuming an identical preservation and staining protocol, B. malayi Mf are generally larger than W. bancrofti Mf and they are readily distinguished by densely packed nuclei, the presences of two isolated nuclei at the tip of the tail, and the absence of nuclei in the cephalic space (Figure 1 A, B, C). Microfilariae of B. timori, which also have very densley packed nuclei, are longer than those of B. malayi with a longer nucleus-free cephalic space (Figure 1B, D). There are a few reports detailing intra-specific variation among W. bancrofti Mf, including an Indian strain of W. bancrofti with particularly large Mf, but little conclusive data are available (Jitpakdi et al., 1999; Kaushal et al., 2012; Paily et al., 2009). However, given the potential for variation and given the dramatic plumping and/or dehydration that may result from preservation and staining, absolute size is not the best feature for morphological differentiation.
Figure 1.
Mf of lymphatic filariae stained by Giemsa: (A) W. bancrofti, (B) mixed infection, (C) B. malayi, and (D) B. timori. Panel (B) depicts the anterior end of a single B. timori Mf (arrow) next to W. bancrofti Mf (arrowhead) in a patient from central Flores. Note the long nucleus free cephalic space and the densely packed nuclei in B. timori. Arrows point to the cephalic space.
b. Antibody Detection
A number of crude and recombinant filarial antigens have been used to detect circulating anti-filarial antibodies as a marker for exposure and present or past infection. In general, there is strong serological cross-reaction between antigens of the three lymphatic filarial species, as well as to other filarial species. For example, the commercial BrugiaRapid test that employs a recombinant B. malayi antigen to detect anti-filarial IgG4 antibodies is equally sensitive to antibodies against B. timori (Supali et al., 2004). The same test may also detect antibodies against W. bancrofti infection, but with decreased sensitivity (Rahmah et al., 2003). A second commercially available ELISA format test relies on another recombinant B. malayi antigen, but it cannot be used to differentiate infections with B. malayi, B. timori and W. bancrofti (Weil et al., 2011). Thus, existing antibody assays are not helpful for studying variability of lymphatic filariae despite their utility for diagnosis.
c. Antigen Detection
Two antigen detection kits are commercially available for diagnosing W. bancrofti infection: the Og4C3 ELISA test (TropBio, Townsville, Australia) and the rapid format Binax Now Filariasis ICT Test (Alere, Portland, ME, USA) (Weil and Ramzy, 2007). These tests rely on monoclonal antibodies (mAbs) against filarial antigens from animal parasites: the cattle parasite Onchocerca gibsoni for the Og4C3 ELISA and the dog heartworm Dirofilaria immitis for the ICT test (More and Copeman, 1990; Weil and Liftis, 1987). The mAbs used in these tests detect their target epitopes in antigen preparations of various filarial nematode species; however, W. bancrofti is the only species that can be detected in human sera. This suggests that any antigens with these epitopes that are released by other filarial species are rapidly cleared from the circulation in humans. A few reports describe new tests that are able to detect circulating antigens from other filarial species in human blood (for examples, see Abdullah et al., 1993; Lalitha et al., 2002; Pandey et al., 2011; Wongkamchai et al., 2003), but these have not been independently verified and none are commercially available.
d. DNA Detection
Assays developed to detect filarial nematode DNA, whether by Southern blot, PCR-ELISA, or conventional PCR, have historically targeted repeated sequences in parasite genomes. For the most part, these sequences are species-specific. The first sequence employed for this purpose was the HhaI repeat present in B. malayi and B. pahangi. Probes designed to target this sequence successfully detected DNA from Brugia species but failed to hybridize with W. bancrofti DNA (McReynolds et al., 1986). Later, genus-specific conventional and real-time PCR assays were designed and implemented based on the HhaI repeat (Rao et al., 2006b; Triteeraprapab et al., 2001). Conversely, the long dispersed repeat (LDR1), which contains the SspI repeat, is a useful target that is specific for W. bancrofti (Fischer et al., 1999; McCarthy et al., 1996; Rao et al., 2006a; Williams et al., 1996; Zhong et al., 1996).
As more sequence data became available, assays were designed to amplify conserved DNA sequences and distinguish species based on the restriction digest patterns of the amplified fragments. Such loci include the glutathione peroxidase, cytochrome oxidase I, and the first internal transcribed spacer (Fischer et al., 2002; Nuchprayoon et al., 2005; Thanomsub et al., 2000). Recent sequencing studies have also noted species and strain specific polymorphism in the first internal transcribed spacer and cofactor-independent phosphoglycerate mutase isoform-1 (Dhamodharan et al., 2012; Fong et al., 2012). In 2010, Sakthidevi et al. devised a single-step PCR assay targeting portions of the abundant larval transcript-2 gene (Sakthidevi et al., 2010). PCR products from B. malayi are at least 200bp larger than those of W. bancrofti due to increased numbers of tandem repeats in the second and third introns, and this difference is easily detected by agarose gel electrophoresis. It is likely that more loci like alt-2 will be discovered as additional genome sequences are generated from W. bancrofti and other filarial species.
V. FR3 Strain of B. malayi and the State of Filarial Genomics
The FR3 strain of B. malayi has arisen as the choice “model” for laboratory studies of lymphatic dwelling and other filarial nematodes, and is provided free of charge for research purposes by the Filariasis Research Reagent Resource Center in the US (http://www.filariasiscenter.org) (Michalski et al., 2011). Originally isolated from a human patient, this zoophilic strain was sent from Malaysia to the UK in an experimentally infected cat by C. P. Ramachandran in the 1950’s. The characterization of experimental vectors (Ae. aegypti black-eyed Liverpool strain and Ae. togoi) and rodent hosts made it possible to establish and maintain a life cycle in the laboratory (Ash and Riley, 1970; Ramachandran, 1966; Ramachandran et al., 1960; Ramachandran et al., 1961; Ramachandran and Zaini, 1967, 1968a, b). Parasites of this strain have been passaged in laboratories throughout the world for more than 60 years.
Little is known about the ecology of the FR3 strain in nature, and human B. malayi infection has nearly been eliminated from mainland Malaysia. However, the life cycle of these parasites in experimental animals has been studied in great detail. Mf take 12–14 days to develop to the infective stage in Ae. aegypti (Ash and Riley, 1970). After introduction into the jird host, the larvae molt twice (once at 7–9 days and again at 28–31 days) to reach the adult stage (Ash and Riley, 1970). The full duration of the pre-patency period (i.e., time from introduction of infective larva to appearance of Mf in the blood, which includes development, finding a partner, mating, and production of Mf) is 12 weeks (Ash and Riley, 1970). The pre-patency period of this strain in humans has not been determined, but a small number of experimental studies indicate a great deal of variability, with periods ranging from 3 to 5 months (Nutman, 1991).
Genomic studies of B. malayi started with the generation of expressed sequence tags (ESTs). In 1996, Blaxter et al reported the first 364 genes expressed from infective L3 by generating spliced leader and non-spliced leader cDNA libraries (Blaxter et al., 1996). Studies comparing profiles of genes expressed in infective, cultured versus irradiated (Li et al., 2006) and infective versus post-infective (Gregory et al., 1997) L3 followed as the techniques for extracting biological material, isolating RNA, and sequencing improved. The sequences generated by these studies enabled the construction of the first cDNA oligonucleotide microarray and the subsequent comparison of genes expressed in male and female B. malayi (Li et al., 2006) and also helped to annotate the first 10MB of the B. malayi genome (Whitton et al., 2004).
In 2007, the ~90 Mb genome of the FR3 strain of B. malayi was sequenced with 9x coverage (Ghedin et al., 2007). Annotation of the genome resulted in ~11,500 protein coding genes. While this dataset only represented 65% to 80% of the inferred 14,500 to 17,800 genes, the analysis of this first draft genome produced from any helminth provided a wealth of information about filarial adaptation to its human and vector hosts and its relationship with the Wolbachia endosymbiont. Due to the complex life cycle of B. malayi compared to the free-living model nematode C. elegans, the B. malayi genome reveals a unique evolutionary history leading to the conservation of long-range gene linkage with rearrangements in local gene order (Scott and Ghedin, 2009). The genome sequence launched the field of filarial biology in to the genomic age, resulting in the first publically available LF microarray chip (http://www.filariasiscenter.org).
It should be noted that the B. malayi genome is still incomplete, and information has been added since 2007. The present version includes 18,348 genes (with 21,332 predicted proteins including unique isoforms, see: WormBase release WS230) with completeness estimated at 93% based on the conserved eukaryotic gene mapping protocol (Parra et al., 2007). Recent papers describing transcription profiles across the life cycle (Choi et al., 2011; Li et al., 2012) and proteomics studies (Bennuru et al., 2011; Bennuru et al., 2009; Hewitson et al., 2008; Moreno and Geary, 2008) have taken advantage of the more complete genome and provided novel insights into the biology of B. malayi at the molecular level.
While new sequencing projects are in progress (Brindley et al., 2009) and sequence reads from the W. bancrofti genome are available from the NCBI sequence read archive (project number SRP000772), B. malayi is still the only filarial parasite with a nearly complete, fully annotated, and published genome. Since the FR3 strain has been adapted to the rodent model and inbred in laboratories for so long, it may differ in significant ways from the original outbred parasite. Clearly there could be important differences between the TRS strain and outbred periodic B. malayi and B. timori. Further studies are urgently needed to document the natural variation of lymphatic filarial parasites in relation to laboratory strains, and we believe that sequencing of clinical isolates of lymphatic dwelling filarial parasites is a high priority.
VI. Natural Variation in Filarial Genomes
a. Inter-Species Variation Among the Lymphatic-Dwelling Filariae
As mentioned above, only the FR3 strain of B. malayi has been sequenced, annotated and published, and very little is known about genetic variation among lymphatic filarial parasites. For W. bancrofti and B. timori, only common phylogenetic markers (e.g., 5s and 18s ribosomal RNA genes) and the mitochondrial genomes have been reported (Fong et al., 2008; McNulty et al., 2012; Ramesh et al., 2012; Xie et al., 1994). Thus far, it seems that the lymphatic-dwelling species share a high degree of sequence homology. Brugia and Wuchereria consistently form a monophyletic group in phylogenetic studies of the Onchocercinae, regardless of the genetic marker being analyzed (Casiraghi et al., 2001; Ferri et al., 2011; Fong et al., 2008; Huang et al., 2009; McNulty et al., 2012; Michalski et al., 2010; Xie et al., 1994; Yatawara et al., 2007). Alignments indicate that the mitochondrial genomes of B. malayi and W. bancrofti share approximately 88% sequence identity (Table 1) (Ghedin et al., 2007; McNulty et al., 2012; Ramesh et al., 2012). Using an assumed generation time of one year and a mutation rate comparable to that reported for Pristonchus pacificus and Caenorhabditis elegans, Ramesh et al estimated that Brugia and Wuchereria may have diverged some 675,000 years ago, a relatively recent split given the phylogenetic age of the superfamily Filarioidea (Ramesh et al., 2012).
Table 1.
Sequence identity shared between the mitochondrial genomes of lymphatic filarial parasites.
| Bm FR3 | Wb I | Wb WA | Wb PNG Ramesh | Wb PNG McNulty | |
|---|---|---|---|---|---|
| Bm FR3 | 100.0 | 88.3 | 88.5 | 88.6 | 88.5 |
| Wb I | 88.3 | 100.0 | 98.2 | 98.5 | 97.4 |
| Wb WA | 88.5 | 98.2 | 100.0 | 99.1 | 99.0 |
| Wb PNG Ramesh | 88.6 | 98.5 | 99.1 | 100.0 | 98.2 |
| Wb PNG McNulty | 88.5 | 97.4 | 99.0 | 98.2 | 100.0 |
Information was taken from the following sources: (McNulty et al., 2012; Ramesh et al., 2012). W. bancrofti strains from India (I), West Africa (WA) and Papua New Guinea (PNG) were described by Ramesh et al while a W. bancrofti strain from PNG was sequenced by McNulty et al. The abbreviations Bm and Wb were used for B. malayi and W. bancrofti, respectively.
b. Intra-Species Variation Among the Lymphatic-Dwelling Filariae
The few studies that have examined genetic diversity among populations of filarial parasites were performed prior to the advent of cost effective high throughput sequencing. These relied on random amplification of polymorphic DNA (RAPD). RAPD studies of B. malayi have detected minor differences in the structure of repetitive DNA elements in parasites from different strains or different geographical regions. A variable locus on the Y chromosome, TOY (Tag on Y), containing a microsatellite-like region with an 8 bp difference between the FR3 strain (originally from mainland Malaysia) and an isolate from Indonesia was described (Underwood and Bianco, 1999). Later studies of the same locus were able to detect differences in sub-periodic/zoophilic and nocturnally periodic/anthropophilic Indonesian strains as well (Underwood et al., 2000).
Several studies have reported the use of the RAPD technique for analyzing the genetic diversity of W. bancrofti populations in India. Phylogenetic trees based on RAPD profiles divide Indian W. bancrofti populations into two major strains that occur on the eastern and western sides of the Western Ghat mountain range (Patra et al., 2007; Thangadurai et al., 2006). Within each of these major strains, significant genetic variability was detected in parasite populations from different geographical regions, with the highest degrees of variability generally corresponding to urban areas with dense, dynamic human populations (Hoti et al., 2008). In studies focused on diurnally sub-periodic W. bancrofti in the Andaman and Nicobar Islands, phylogenetic trees constructed from RAPD data indicate that C. quinquefasciatus-transmitted parasites from Car Nicobar Island form a separate cluster from Ochlerotatus (Aedes) niveus-transmitted parasites from neighboring islands (Dhamodharan et al., 2008). The Car Nicobar parasites also showed a higher degree of genetic variability, indicating that these parasites might be in the process of adapting to the schedule of a night-biting vector (Dhamodharan et al., 2008). Outside of India, RAPD assays were developed to differentiate a nocturnally sub-periodic Thai strain from nocturnally periodic Myanmar strains (Nuchprayoon et al., 2007). Since elimination programs have significantly reduced the prevalence of W. bancrofti in Thailand, this approach could be used to determine whether future increases in filariasis rates in Thailand are due to a resurgence of Thai parasites or to an influx of parasites from neighboring regions.
A recent study was performed on intra-species variation in the mitochondrial genome of W. bancrofti (Ramesh et al., 2012). The mitochondrial genomes of three strains of W. bancrofti, from India, West Africa and Papua New Guinea (PNG), were sequenced. Sequence identity between all examined strains ranged from 97–99% (Table 1), and the West African and PNG strains appeared to share the greatest degree of homology. Unfortunately, no information was presented on the degree of variation within each population. The incomplete level of sequence identity shared between two different PNG strains highlight the significance of this issue (Table 1) (McNulty et al., 2012; Ramesh et al., 2012).
c. Variation Due to Selective Pressure from Drugs
The Global Program to Eliminate Lymphatic Filariasis relies on mass administration of combinations of anthelmintic drugs (albendazole plus ivermectin in areas co-endemic for onchocerciasis and albendazole plus diethylcarbamazine elsewhere) to block parasite transmission. More than three billion doses of these drugs have been administered to hundreds of millions of patients in more than 50 countries since 2000 (WHO, 2010, 2011). Macrocyclic lactones and benzimidazoles (e.g., ivermectin and albendazole, respectively) have also been used extensively in the veterinary setting, and resistance has been reported in nematode parasites of domestic animals (as reviewed by Kaminsky, 2003; Prichard, 1990; Wolstenholme et al., 2004). Although there is little hard evidence for the development of drug resistance in filarial nematodes, it stands to reason that selective pressure resulting from the use of these drugs could alter the genetic structure of parasite populations.
It is difficult to specifically test for drug resistance in species like W. bancrofti with no laboratory animal model, but suboptimal responses to diethylcarbamazine (DEC) had been reported prior to the initiation of the GPELF (Eberhard et al., 1991; Eberhard et al., 1988). The molecular target of DEC is unknown, and this makes it hard to assess the impact of treatment on parasite genetics. Conversely, albendazole is known to interfere with microtubule polymerization through the binding of beta-tubulin (Kohler and Bachmann, 1981; Lubega and Prichard, 1990). A phenylalanine to tyrosine substitution at position 200 of the beta-tubulin gene associated with albendazole resistance in animal parasites was detected in higher frequencies in albendazole/ivermectin treated W. bancrofti populations in West Africa as compared to untreated populations (Schwab et al., 2005). Mathematical models predict that MDA treatments should select an increased frequency of this allele (Schwab et al., 2006, 2007), and PCR-based assays have been developed to track the frequencies of resistance alleles in treated parasite populations (Hoti et al., 2009). However, no parasitological evidence for albendazole resistance in W. bancrofti has been noted (Bisht et al., 2006). Similarly, there have been no reports of drug resistance in Brugia species either in nature or under experimental conditions.
VII. Genetic Variation in Wolbachia Genomes
It is well known that Wolbachia endobacteria play a vital role in the biology of many filarial species, including B. malayi, B. timori and W. bancrofti. Not only do these endosymbionts support the growth and reproduction of dependent filarial species; they are also believed to play a role in pathogenesis, and they may serve as a practical anti-filarial drug target (Slatko et al., 2010; Taylor et al., 2005; Taylor et al., 2010). Similar to the lymphatic filariae themselves, only one filarial Wolbachia genome (the endosymbiont of the B. malayi FR3 strain) has been fully sequenced, annotated and published (Foster et al., 2005). Therefore, little is known about genetic variation among the Wolbachia endobacteria of the lymphatic filariae. In light of the agreement between phylogenies of Wolbachia and their filarial nematode hosts (Casiraghi et al., 2004; Ferri et al., 2011), it is likely that the Wolbachia strains carried by the lymphatic filariae will be more similar to one another than to those carried by more distantly related filarial species, but the exact degree of inter- and/or intra-strain variation among filarial Wolbachia cannot be predicted. With the genomic sequences of the Wolbachia containing filarial parasites W. bancrofti, O. volvulus, O. ochengi and D. immitis either published or in progress, further Wolbachia genomes are being co-sequenced (Darby et al., 2012; Godel et al., 2012). The first results suggest that the Wolbachia genomes are very similar in some species (e.g., O. volvulus and O. ochengi) but that others differ slightly in size and in the gene number (Brindley et al., 2009; Darby et al., 2012). Additionally, crossing experiments among cytoplasmic incompatibility inducing Wolbachia strains in C. pipiens indicate a high degree of incompatibility even in strains that are genetically indistinguishable using typical multi locus strain typing genes (Baldo et al., 2006; Guillemaud et al., 1997). This means that a small amount of sequence variation in Wolbachia can lead to significant biological consequences.
VIII. Discussion
In insect vectors of filariae, such as mosquitoes and blackflies, advances in cytotaxonomy and molecular taxonomy have led to the discovery of a large number of new, sibling species that could not be differentiated by external morphology (Krueger, 2006; Norris, 2002; Sharakhov et al., 2002; Wondji et al., 2005). A similar situation could present itself in the case of the lymphatic dwelling filariae. Each of the species discussed in this review comprise a collection of biologically distinct strains that differ in their geographical distribution, host and vector affinities, periodicity, pathogenicity, response to diagnostic tests, and potentially, to drug treatments. Given the large geographic range of lymphatic dwelling filariae, the isolation of some populations, and the absence of robust population genetic data, it is possible that the lymphatic–dwelling filariae comprise more than three taxa.
The classification and organization of the lymphatic dwelling filariae clearly deserves to be reconsidered in the genomic era. One biological criterion for setting a species boundary is to determine whether individuals from different groups are capable of mating to produce viable offspring. Unfortunately, we cannot determine whether nocturnally periodic W. bancrofti from mainland India are compatible with diurnally subperiodic W. bancrofti from the Andaman and Nicobar island ranges, since they do not share a common vector and cannot be maintained in the laboratory. It may be that this union is feasible (as are experimental unions between certain strains of B. malayi, B. pahangi and B. patei discussed previously) and that it does not occur in nature simply because of geographical or other barriers. On the other hand, cross-mating may be impossible and it may be more appropriate to consider the two strains as different species.
Today’s sequencing methods could facilitate a reclassification of the lymphatic filariae based on genetic rather than phenotypic or other biological characters (e.g., cross-mating). High throughput sequencing technologies (introduced since 2005) are ever evolving, as reviewed by Mardis in 2008 and 2011. Rapid declines in cost coupled with advances in molecular methods for isolating parasite material allow researchers to generate cDNA and whole genome sequence libraries for deep sequencing from nanograms of starting material. Despite its minor role in lymphatic filarial disease, zoophilic B. malayi was the obvious choice for genome sequencing 20 years ago because it can be maintained in the laboratory (Blaxter, 1995; Unnasch, 1994). Less material is required for genome sequencing these days; a few dozens of Mf should be sufficient for full genome sequencing. Mf can be obtained from finger-prick volumes of infected blood, and these volumes are routinely collected in ongoing mapping and epidemiological studies. Sequencing of such clinical strains would enhance our understanding of the genetic variation of LF parasites and consequently contribute to:
Identification of genetic markers suitable for fingerprinting of nematodes in order to differentiate persistent or reintroduced infections;
Determine polymorphic genes/gene families that might affect drug or vaccine development;
Determine genetic differences and similarities between phenotypically different strains (e.g. Nocturnally periodic and subperiodic W. bancrofti; W. bancrofti strains transmitted by different vectors; subperiodic, zoophilic, Mansonia-transmitted B. malayi and nocturnally periodic, anthropophilic, Anopheles-transmitted B. malayi and B. timori;
Identify genes/gene families that appear to have been affected by the various MDA drug regimens to look for development of resistance.
Although technical advances will facilitate genomic studies of lymphatic-dwelling filarial parasites, questions will arise regarding the definition of standard taxonomical terms. However, these questions should not discourage the scientific community from attempting to address filarial nematode taxonomy in a more rigorous manner. After all, no set criteria were laid out for the degree of phenotypic variation required for distinguishing species when the filariae were originally described (Grove, 1990). It was only after years of study and data collection that a satisfying consensus was reached based on the information that was available at the time. Now that more data have been obtained and even more data are within reach, we face the same task. There is no reason to believe that we will be unable to come to similarly satisfying conclusions in the genomic era.
A more accurate classification of the lymphatic-dwelling filariae is not only interesting from a basic biological or taxonomical perspective. It could also have practical importance for the global effort to eliminate LF. In order to combat this disabling disease, it is necessary to understand the infecting agents. Are we engaged in an effort to eliminate three species or a complex of many species? Are there logical reasons to go about the elimination effort differently in different geographical locations or in the presence of different species or strains? If LF resurgence is observed following MDA, is this due to a resumption of transmission of the endemic strain or caused by importation of parasites from another area? Clearly, a better understanding of the intra- and inter-species diversity of the lymphatic dwelling filarial parasites would assist in answering these important questions.
Three filarial species cause lymphatic filariasis and encompass various ecological variants.
Filarial parasites react differently regarding diagnostics, pathogenesis and chemotherapy.
Available genomic data provide scarce knowledge of their inter- and intra- species variation.
Technological advances will facilitate the study of inter- and intra-species variation in the future.
Genome sequencing of clinical isolates will provide insight into the impact of intervention on parasite genetics.
Acknowledgments
This review was supported by the Barnes Jewish Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah WO, et al. Detection of circulating antigens and parasite specific antibodies in filariasis. The Southeast Asian journal of tropical medicine and public health. 1993;24(Suppl 2):31–36. [PubMed] [Google Scholar]
- Ash LR, Riley JM. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. The Journal of parasitology. 1970;56:969–973. [PubMed] [Google Scholar]
- Ash LR, Schacher JF. Early life cycle and larval morphogenesis of Wuchereria bancrofti in the jird, Meriones unguiculatus. The Journal of parasitology. 1971;57:1043–1051. [PubMed] [Google Scholar]
- Atmosoedjono S, et al. Anopheles balabacensis (Diptera: Culicidae), a vector of Wuchereria kalimantani (Nematoda: Onchocercidae) in east Kalimantan (Borneo), Indonesia. Medical and veterinary entomology. 1993;7:390–392. doi: 10.1111/j.1365-2915.1993.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Bain O, Chabaud AG. Atlas of infective larvae of filariae. Trop Med Parasitol. 1986;37:301–340. [PubMed] [Google Scholar]
- Baldo L, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Applied and environmental microbiology. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennuru S, et al. Stage-specific proteomic expression patterns of the human filarial parasite Brugia malayi and its endosymbiont Wolbachia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9649–9654. doi: 10.1073/pnas.1011481108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennuru S, et al. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis. 2009;3:e410. doi: 10.1371/journal.pntd.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht R, et al. Isolation of Wuchereria bancrofti microfilariae from archived stained blood slides for use in genetic studies and amplification of parasite and endosymbiont genes. Acta tropica. 2006;99:1–5. doi: 10.1016/j.actatropica.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Blaxter ML. The filarial genome network. Parasitology Today. 1995;11:441–442. [Google Scholar]
- Blaxter ML, et al. Genes expressed in Brugia malayi infective third stage larvae. Molecular and biochemical parasitology. 1996;77:77–93. doi: 10.1016/0166-6851(96)02571-6. [DOI] [PubMed] [Google Scholar]
- Bockarie MJ, et al. Role of vector control in the global program to eliminate lymphatic filariasis. Annual review of entomology. 2009;54:469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, et al. Helminth genomics: The implications for human health. PLoS Negl Trop Dis. 2009;3:e538. doi: 10.1371/journal.pntd.0000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan JH, et al. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 3. Uptake and damage to ingested microfilariae by Anopheles gambiae, An. arabiensis, An. merus and An. funestus in east Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:265–268. doi: 10.1016/0035-9203(90)90281-i. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Southgate BA. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 1. Uptake of microfilariae. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988a;82:128–137. doi: 10.1016/0035-9203(88)90286-6. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Southgate BA. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 2. Damage to ingested microfilariae by mosquito foregut armatures and development of filarial larvae in mosquitoes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988b;82:138–145. doi: 10.1016/0035-9203(88)90288-x. [DOI] [PubMed] [Google Scholar]
- Buse E, Kuhlow F. Scanning microscopical observations on the foregut structures o mosquitoes and their role for the ingestion of microfilariae (author’s transl) Tropenmedizin und Parasitologie. 1979;30:446–454. [PubMed] [Google Scholar]
- Casiraghi M, et al. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122(Pt 1):93–103. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. International journal for parasitology. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Choi YJ, et al. A deep sequencing approach to comparatively analyze the transcriptome of lifecycle stages of the filarial worm, Brugia malayi. PLoS Negl Trop Dis. 2011;5:e1409. doi: 10.1371/journal.pntd.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JH, et al. Experimental transmission of Wuchereria bancrofti to monkeys. The American journal of tropical medicine and hygiene. 1979;28:56–66. doi: 10.4269/ajtmh.1979.28.56. [DOI] [PubMed] [Google Scholar]
- Cross JH, et al. Further studies on the development of Wuchereria bancrofti in laboratory animals. The Southeast Asian journal of tropical medicine and public health. 1981;12:114–122. [PubMed] [Google Scholar]
- Darby AC, et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome research. 2012 doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamodharan R, et al. Genetic variability of diurnally sub-periodic Wuchereria bancrofti in Nicobarese tribe of Nicobar group of Islands, Andaman and Nicobar Islands, India. Parasitology research. 2008;103:59–66. doi: 10.1007/s00436-008-0927-2. [DOI] [PubMed] [Google Scholar]
- Dhamodharan R, et al. Characterization of cofactor-independent phosphoglycerate mutase isoform-1 (Wb-iPGM) gene: a drug and diagnostic target from human lymphatic filarial parasite, Wuchereria bancrofti. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:957–965. doi: 10.1016/j.meegid.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Dissanaike AS, et al. Recovery of a species of Brugia, probably B. ceylonensis, from the conjunctiva of a patient in Sri Lanka. Annals of tropical medicine and parasitology. 2000;94:83–86. [PubMed] [Google Scholar]
- Dissanaike AS, Niles WJ. Attempts to Transmit Wuchereria Bancrofti to Cats and to a Toque Monkey. Annals of tropical medicine and parasitology. 1965;59:189–192. doi: 10.1080/00034983.1965.11686298. [DOI] [PubMed] [Google Scholar]
- Dreyer G, et al. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16:544–548. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- Eberhard ML, et al. Evidence of nonsusceptibility to diethylcarbamazine in Wuchereria bancrofti. The Journal of infectious diseases. 1991;163:1157–1160. doi: 10.1093/infdis/163.5.1157. [DOI] [PubMed] [Google Scholar]
- Eberhard ML, et al. Persistence of microfilaremia in bancroftian filariasis after diethylcarbamazine citrate therapy. Trop Med Parasitol. 1988;39:128–130. [PubMed] [Google Scholar]
- Eberhard ML, et al. A survey of knowledge, attitudes, and perceptions (KAPs) of lymphatic filariasis, elephantiasis, and hydrocele among residents in an endemic area in Haiti. The American journal of tropical medicine and hygiene. 1996;54:299–303. doi: 10.4269/ajtmh.1996.54.299. [DOI] [PubMed] [Google Scholar]
- Ferri E, et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PloS one. 2011;6:e20843. doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, et al. Development of a quantitative, competitive polymerase chain reaction--enzyme-linked immunosorbent assay for the detection of Wuchereria bancrofti DNA. Parasitology research. 1999;85:176–183. doi: 10.1007/s004360050531. [DOI] [PubMed] [Google Scholar]
- Fischer P, et al. Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA. The American journal of tropical medicine and hygiene. 2000;62:291–296. doi: 10.4269/ajtmh.2000.62.291. [DOI] [PubMed] [Google Scholar]
- Fischer P, et al. PCR-based detection and identification of the filarial parasite Brugia timori from Alor Island, Indonesia. Annals of tropical medicine and parasitology. 2002;96:809–821. doi: 10.1179/000349802125002239. [DOI] [PubMed] [Google Scholar]
- Fong MY, et al. Inferring the phylogenetic position of Brugia pahangi using 18S ribosomal RNA (18S rRNA) gene sequence. Tropical biomedicine. 2008;25:87–92. [PubMed] [Google Scholar]
- Fong MY, et al. Comparative analysis of ITS1 nucleotide sequence reveals distinct genetic difference between Brugia malayi from Northeast Borneo and Thailand. Parasitology. 2012:1–7. doi: 10.1017/S0031182012001242. [DOI] [PubMed] [Google Scholar]
- Foster J, et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS biology. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghedin E, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godel C, et al. The genome of the heartworm, Dirofilaria immitis, reveals drug and vaccine targets. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.12-205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory WF, et al. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Molecular and biochemical parasitology. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Grove DI. Wuchereria bancrofti, Brugia species and Filariasis, A History of Human Helminthology. C.A.B. International; Wallingford, UK: 1990. pp. 579–640. [Google Scholar]
- Guillemaud T, et al. Contrasting levels of variability between cytoplasmic genomes and incompatibility types in the mosquito Culex pipiens. Proceedings. Biological sciences/The Royal Society. 1997;264:245–251. doi: 10.1098/rspb.1997.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyapong JO, et al. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother. 2005;6:179–200. doi: 10.1517/14656566.6.2.179. [DOI] [PubMed] [Google Scholar]
- Hawking F. The distribution of Bancroftian filariasis in Africa. Bulletin of the World Health Organization. 1957;16:581–592. [PMC free article] [PubMed] [Google Scholar]
- Hewitson JP, et al. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Molecular and biochemical parasitology. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Hii J, et al. The epidemiology and control of lymphatic filariasis on Lihir Island, New Ireland Province. Papua and New Guinea medical journal. 2000;43:188–195. [PubMed] [Google Scholar]
- Hoti SL, et al. An allele specific PCR assay for screening for drug resistance among Wuchereria bancrofti populations in India. The Indian journal of medical research. 2009;130:193–199. [PubMed] [Google Scholar]
- Hoti SL, et al. Genetic heterogeneity of Wuchereria bancrofti populations at spatially hierarchical levels in Pondicherry and surrounding areas, south India. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2008;8:644–652. doi: 10.1016/j.meegid.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. Molecular characterization and phylogenetic analysis of Dirofilaria immitis of China based on COI and 12S rDNA genes. Veterinary parasitology. 2009;160:175–179. doi: 10.1016/j.vetpar.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Jayasekera N, et al. The susceptibility of Liberian Culex quinquefasciatus to Wuchereria bancrofti in Sri Lanka. Tropenmedizin und Parasitologie. 1980;31:507–511. [PubMed] [Google Scholar]
- Jitpakdi A, et al. Variation in microfilariae and infective stages of two types of Wuchereria bancrofti from the Thai-Myanmar border. Journal of helminthology. 1999;73:317–321. [PubMed] [Google Scholar]
- Kalra N. Filariasis among aborigines of Andaman and Nicobar islands. Journal of Communicable Diseases. 1974:40–65. [Google Scholar]
- Kaminsky R. Drug resistance in nematodes: a paper tiger or a real problem? Current opinion in infectious diseases. 2003;16:559–564. doi: 10.1097/00001432-200312000-00008. [DOI] [PubMed] [Google Scholar]
- Kaushal S, et al. Morphological variations in microfilaria of Wuchereria bancrofti in cytology smears: a morphometric study of 32 cases. Acta cytologica. 2012;56:431–438. doi: 10.1159/000337446. [DOI] [PubMed] [Google Scholar]
- Kohler P, Bachmann R. Intestinal tubulin as possible target for the chemotherapeutic action of mebendazole in parasitic nematodes. Molecular and biochemical parasitology. 1981;4:325–336. doi: 10.1016/0166-6851(81)90064-5. [DOI] [PubMed] [Google Scholar]
- Krueger A. Guide to blackflies of the Simulium damnosum complex in eastern and southern Africa. Medical and veterinary entomology. 2006;20:60–75. doi: 10.1111/j.1365-2915.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- Lalitha P, et al. Development of antigen detection ELISA for the diagnosis of brugian and bancroftian filariasis using antibodies to recombinant filarial antigens Bm-SXP-1 and Wb-SXP-1. Microbiology and immunology. 2002;46:327–332. doi: 10.1111/j.1348-0421.2002.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Li BW, et al. Brugia malayi: effects of radiation and culture on gene expression in infective larvae. Molecular and biochemical parasitology. 2006;149:201–207. doi: 10.1016/j.molbiopara.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Li BW, et al. Transcription profiling reveals stage- and function dependent expression patterns in the filarial nematode Brugia malayi. BMC genomics. 2012;13:184. doi: 10.1186/1471-2164-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubega GW, Prichard RK. Specific interaction of benzimidazole anthelmintics with tubulin: high-affinity binding and benzimidazole resistance in Haemonchus contortus. Molecular and biochemical parasitology. 1990;38:221–232. doi: 10.1016/0166-6851(90)90025-h. [DOI] [PubMed] [Google Scholar]
- Mardis ER. The impact of next-generation sequencing technology on genetics. Trends in genetics: TIG. 2008;24:133–141. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Mardis ER. A decade’s perspective on DNA sequencing technology. Nature. 2011;470:198–203. doi: 10.1038/nature09796. [DOI] [PubMed] [Google Scholar]
- McCarthy JS, et al. Evaluation of a polymerase chain reaction-based assay for diagnosis of Wuchereria bancrofti infection. The Journal of infectious diseases. 1996;173:1510–1514. doi: 10.1093/infdis/173.6.1510. [DOI] [PubMed] [Google Scholar]
- McNulty SN, et al. Comparing the Mitochondrial Genomes of Wolbachia-Dependent and Independent Filarial Nematode Species. BMC genomics. 2012;13:145. doi: 10.1186/1471-2164-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds LA, et al. Cloning and comparison of repeated DNA sequences from the human filarial parasite Brugia malayi and the animal parasite Brugia pahangi. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:797–801. doi: 10.1073/pnas.83.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael E, Bundy DA. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–476. doi: 10.1016/s0169-4758(97)01151-4. [DOI] [PubMed] [Google Scholar]
- Michalski ML, et al. Identification and phylogenetic analysis of Dirofilaria ursi (Nematoda: Filarioidea) from Wisconsin black bears (Ursus americanus) and its Wolbachia endosymbiont. The Journal of parasitology. 2010;96:412–419. doi: 10.1645/GE-2208.1. [DOI] [PubMed] [Google Scholar]
- Michalski ML, et al. The NIH-NIAID Filariasis Research Reagent Resource Center. PLoS Negl Trop Dis. 2011;5:e1261. doi: 10.1371/journal.pntd.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SJ, Copeman DB. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol. 1990;41:403–406. [PubMed] [Google Scholar]
- Moreno Y, Geary TG. Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis. 2008;2:e326. doi: 10.1371/journal.pntd.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulia-Pelat JP, et al. Periodicity of Wuchereria bancrofti var. pacifica filariasis in French Polynesia. Trop Med Parasitol. 1993;44:83–85. [PubMed] [Google Scholar]
- Norris DE. Genetic markers for study of the anopheline vectors of human malaria. International journal for parasitology. 2002;32:1607–1615. doi: 10.1016/s0020-7519(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Nuchprayoon S, et al. Random amplified polymorphic DNA (RAPD) for differentiation between Thai and Myanmar strains of Wuchereria bancrofti. Filaria journal. 2007;6:6. doi: 10.1186/1475-2883-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchprayoon S, et al. Detection and differentiation of filarial parasites by universal primers and polymerase chain reaction-restriction fragment length polymorphism analysis. The American journal of tropical medicine and hygiene. 2005;73:895–900. [PubMed] [Google Scholar]
- Nutman TB. Experimental infection of humans with filariae. Reviews of infectious diseases. 1991;13:1018–1022. doi: 10.1093/clinids/13.5.1018. [DOI] [PubMed] [Google Scholar]
- Orihel TC, Beaver PC. Zoonotic Brugia infections in North and South America. The American journal of tropical medicine and hygiene. 1989;40:638–647. doi: 10.4269/ajtmh.1989.40.638. [DOI] [PubMed] [Google Scholar]
- Orihel TC, Eberhard ML. Zoonotic filariasis. Clinical microbiology reviews. 1998;11:366–381. doi: 10.1128/cmr.11.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- Paily KP, et al. A review of the complexity of biology of lymphatic filarial parasites. Journal of Parasitic Diseases. 2009;33:2–13. doi: 10.1007/s12639-009-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri JR, et al. Filarid parasites of South Kalimantan (Borneo) Indonesia. Wuchereria kalimantani sp. n. (Nematoda: Filarioidea) from the silvered leaf monkey, Presbytis cristatus Eschscholtz 1921. The Journal of parasitology. 1980;66:645–651. [PubMed] [Google Scholar]
- Pandey V, et al. Antigen detection assay with parasite specific monoclonal antibodies for diagnosis of lymphatic filariasis. Clinica chimica acta; international journal of clinical chemistry. 2011;412:1867–1873. doi: 10.1016/j.cca.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Parra G, et al. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- Partono F, et al. Malayan filariasis in Central Sulawesi (Celebes), Indonesia. The Southeast Asian journal of tropical medicine and public health. 1977;8:452–458. [PubMed] [Google Scholar]
- Partono F, et al. Epidemiological and clinical features of Brugia timori in a newly established village. Karakuak, West Flores, Indonesia. The American journal of tropical medicine and hygiene. 1978;27:910–915. doi: 10.4269/ajtmh.1978.27.910. [DOI] [PubMed] [Google Scholar]
- Partono F, Purnomo Clinical features of timorian filariasis among immigrants to an endemic area in West Flores, Indonesia. The Southeast Asian journal of tropical medicine and public health. 1978;9:338–343. [PubMed] [Google Scholar]
- Partono F, Purnomo Periodicity studies of Brugia malayi in Indonesia: recent findings and a modified classification of the parasite. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:657–662. doi: 10.1016/0035-9203(87)90445-7. [DOI] [PubMed] [Google Scholar]
- Patra KP, et al. Identification of a molecular marker for genotyping human lymphatic filarial nematode parasite Wuchereria bancrofti. Experimental parasitology. 2007;116:59–65. doi: 10.1016/j.exppara.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Annals of tropical medicine and parasitology. 2002;96(Suppl 2):S143–152. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- Pothikasikorn J, et al. Susceptibility of various mosquitoes of Thailand to nocturnal subperiodic Wuchereria bancrofti. Journal of vector ecology: journal of the Society for Vector Ecology. 2008;33:313–320. doi: 10.3376/1081-1710-33.2.313. [DOI] [PubMed] [Google Scholar]
- Prichard RK. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. International journal for parasitology. 1990;20:515–523. doi: 10.1016/0020-7519(90)90199-w. [DOI] [PubMed] [Google Scholar]
- Rahmah N, et al. Multicentre laboratory evaluation of Brugia Rapid dipstick test for detection of brugian filariasis. Trop Med Int Health. 2003;8:895–900. doi: 10.1046/j.1365-3156.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran CP. Biological aspects in the transmission of Brugia malayi by Aedes aegypti in the laboratory. Journal of medical entomology. 1966;3:239–252. doi: 10.1093/jmedent/3.3-4.239. [DOI] [PubMed] [Google Scholar]
- Ramachandran CP, et al. Aedes aegypti as an experimental vector of Brugia malayi. Annals of tropical medicine and parasitology. 1960;54:371–375. doi: 10.1080/00034983.1960.11685999. [DOI] [PubMed] [Google Scholar]
- Ramachandran CP, et al. Early stages in the development of Brugia malayi in different species. Ann Soc Belg Med Trop (1920) 1961;41:285–289. [PubMed] [Google Scholar]
- Ramachandran CP, Zaini MA. Studies on the transmission of sub-periodic Brugia malayi by Aedes (Finlaya) togoi in the laboratory. I. The intake and migration of microfilariae. The Medical journal of Malaya. 1967;22:136–144. [PubMed] [Google Scholar]
- Ramachandran CP, Zaini MA. Studies on the transmission of sub-periodic Brugia malayi by Aedes (Finlaya) togoi in the laboratory. 3. The survival of infected mosquitoes under laboratory conditions. The Medical journal of Malaya. 1968a;23:323–329. concl. [PubMed] [Google Scholar]
- Ramachandran CP, Zaini MA. Studies on the transmission of sub-periodic Brugia malayi by Aedes (Finlaya) togoi in the laboratory. II. The development of the parasite to the infective form; the relationship between concentration of microfilariae in the vertebrate host and infection in the mosquitoes. The Medical journal of Malaya. 1968b;22:198–203. [PubMed] [Google Scholar]
- Ramesh A, et al. The complete mitochondrial genome sequence of the filarial nematode Wuchereria bancrofti from three geographic isolates provides evidence of complex demographic history. Molecular and biochemical parasitology. 2012;183:32–41. doi: 10.1016/j.molbiopara.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RU, et al. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. The American journal of tropical medicine and hygiene. 2006a;74:826–832. [PMC free article] [PubMed] [Google Scholar]
- Rao RU, et al. Detection of Brugia parasite DNA in human blood by real-time PCR. Journal of clinical microbiology. 2006b;44:3887–3893. doi: 10.1128/JCM.00969-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthidevi M, et al. Lymphatic filarial species differentiation using evolutionarily modified tandem repeats: generation of new genetic markers. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2010;10:591–594. doi: 10.1016/j.meegid.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Sasa M. Human Filariasis: A Global Survey of Epidemiology and Control. University of Tokyo Press; Japan: 1976. Filariasis Due to Wuchereria and Brugia. [Google Scholar]
- Schwab AE, et al. Detection of benzimidazole resistance-associated mutations in the filarial nematode Wuchereria bancrofti and evidence for selection by albendazole and ivermectin combination treatment. The American journal of tropical medicine and hygiene. 2005;73:234–238. [PubMed] [Google Scholar]
- Schwab AE, et al. Population genetics of concurrent selection with albendazole and ivermectin or diethylcarbamazine on the possible spread of albendazole resistance in Wuchereria bancrofti. Parasitology. 2006;133:589–601. doi: 10.1017/S003118200600076X. [DOI] [PubMed] [Google Scholar]
- Schwab AE, et al. An analysis of the population genetics of potential multi-drug resistance in Wuchereria bancrofti due to combination chemotherapy. Parasitology. 2007;134:1025–1040. doi: 10.1017/S0031182007002363. [DOI] [PubMed] [Google Scholar]
- Schweinfurth U. Filarial diseases in Ceylon: a geographic and historical analysis. Ecology of disease. 1983;2:309–319. [PubMed] [Google Scholar]
- Scott AL, Ghedin E. The genome of Brugia malayi - all worms are not created equal. Parasitology international. 2009;58:6–11. doi: 10.1016/j.parint.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharakhov IV, et al. Inversions and gene order shuffling in Anopheles gambiae and A. funestus. Science. 2002;298:182–185. doi: 10.1126/science.1076803. [DOI] [PubMed] [Google Scholar]
- Slatko BE, et al. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51:55–65. doi: 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow LC, et al. Transmission dynamics of lymphatic filariasis: vector-specific density dependence in the development of Wuchereria bancrofti infective larvae in mosquitoes. Medical and veterinary entomology. 2006;20:261–272. doi: 10.1111/j.1365-2915.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- Southgate BA, Bryan JH. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:523–530. doi: 10.1016/0035-9203(92)90096-u. [DOI] [PubMed] [Google Scholar]
- Supali T, et al. Detection of filaria-specific IgG4 antibodies using Brugia Rapid test in individuals from an area highly endemic for Brugia timori. Acta tropica. 2004;90:255–261. doi: 10.1016/j.actatropica.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Supali T, et al. High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. The American journal of tropical medicine and hygiene. 2002;66:560–565. doi: 10.4269/ajtmh.2002.66.560. [DOI] [PubMed] [Google Scholar]
- Suswillo RR, et al. Hybridization between Brugia patei, B. pahangi and sub-periodic B. malayi. Parasitology. 1978;77:153–160. doi: 10.1017/s0031182000049350. [DOI] [PubMed] [Google Scholar]
- Tan LH, et al. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitology international. 2011;60:111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, et al. Wolbachia bacterial endosymbionts of filarial nematodes. Advances in parasitology. 2005;60:245–284. doi: 10.1016/S0065-308X(05)60004-8. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, et al. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Thangadurai R, et al. Phylogeography of human lymphatic filarial parasite, Wuchereria bancrofti in India. Acta tropica. 2006;98:297–304. doi: 10.1016/j.actatropica.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Thanomsub BW, et al. Differential diagnosis of human lymphatic filariasis using PCR-RFLP. Molecular and cellular probes. 2000;14:41–46. doi: 10.1006/mcpr.1999.0283. [DOI] [PubMed] [Google Scholar]
- Toumanoff C. Human filariasis and its transmission in Lower Guinea (estuary of the Rio Nunez) Bulletin de la Societe de pathologie exotique et de ses filiales. 1958;51:908–912. [PubMed] [Google Scholar]
- Triteeraprapab S, et al. Lymphatic filariasis caused by Brugia malayi in an endemic area of Narathiwat Province, southern of Thailand. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2001;84(Suppl 1):S182–188. [PubMed] [Google Scholar]
- Trpis M, et al. Non-Mendelian inheritance of mosquito susceptibility to infection with Brugia malayi and Brugia pahangi. Science. 1981;211:1435–1437. doi: 10.1126/science.7466401. [DOI] [PubMed] [Google Scholar]
- Ughasi J, et al. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasites & vectors. 2012;5:89. doi: 10.1186/1756-3305-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood AP, Bianco AE. Identification of a molecular marker for the Y chromosome of Brugia malayi. Molecular and biochemical parasitology. 1999;99:1–10. doi: 10.1016/s0166-6851(98)00180-7. [DOI] [PubMed] [Google Scholar]
- Underwood AP, et al. Two microsatellite loci from Brugia malayi show polymorphisms among isolates from Indonesia and Malaysia. Molecular and biochemical parasitology. 2000;106:299–302. doi: 10.1016/s0166-6851(99)00214-5. [DOI] [PubMed] [Google Scholar]
- Unnasch TR. The filarial genome project. Parsitology Today. 1994;10:415–416. [Google Scholar]
- Vythilingam I, et al. Anopheles donaldi incriminated as a vector of periodic Brugia malayi in Grik, Perak, Malaysia. The Southeast Asian journal of tropical medicine and public health. 1996;27:637–641. [PubMed] [Google Scholar]
- Weil GJ, et al. A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia malayi antigen Bm-14. Acta tropica. 2011;120(Suppl 1):S19–22. doi: 10.1016/j.actatropica.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil GJ, Liftis F. Identification and partial characterization of a parasite antigen in sera from humans infected with Wuchereria bancrofti. J Immunol. 1987;138:3035–3041. [PubMed] [Google Scholar]
- Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends in parasitology. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Whitton C, et al. A genome sequence survey of the filarial nematode Brugia malayi: repeats, gene discovery, and comparative genomics. Molecular and biochemical parasitology. 2004;137:215–227. doi: 10.1016/j.molbiopara.2004.05.013. [DOI] [PubMed] [Google Scholar]
- WHO. Global Programme to Eliminate Lymphatic Filariasis. Wkly Epidemiol Rec. 2006;81:221–232. [PubMed] [Google Scholar]
- WHO. Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2009;84:437–444. [PubMed] [Google Scholar]
- WHO. Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis; Geneva, Switzerland. 2010. [Google Scholar]
- WHO. Global Programme to eliminate lymphatic filariasis: progress report on mass drug administration, 2010. Wkly Epidemiol Rec. 2011;86:377–388. [PubMed] [Google Scholar]
- Williams SA, et al. A polymerase chain reaction assay for the detection of Wuchereria bancrofti in blood samples from French Polynesia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90:384–387. doi: 10.1016/s0035-9203(96)90515-5. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, et al. Drug resistance in veterinary helminths. Trends in parasitology. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wondji C, et al. Species and populations of the Anopheles gambiae complex in Cameroon with special emphasis on chromosomal and molecular forms of Anopheles gambiae s.s. Journal of medical entomology. 2005;42:998–1005. doi: 10.1093/jmedent/42.6.998. [DOI] [PubMed] [Google Scholar]
- Wongkamchai S, et al. An antigen detection assay for diagnosing filariasis. Asian Pacific journal of allergy and immunology/launched by the Allergy and Immunology Society of Thailand. 2003;21:241–251. [PubMed] [Google Scholar]
- Xie H, et al. Molecular phylogenetic studies on filarial parasites based on 5S ribosomal spacer sequences. Parasite. 1994;1:141–151. doi: 10.1051/parasite/1994012141. [DOI] [PubMed] [Google Scholar]
- Yatawara L, et al. Molecular characterization and phylogenetic analysis of Setaria digitata of Sri Lanka based on CO1 and 12S rDNA genes. Veterinary parasitology. 2007;148:161–165. doi: 10.1016/j.vetpar.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Zhong M, et al. A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples. The American journal of tropical medicine and hygiene. 1996;54:357–363. doi: 10.4269/ajtmh.1996.54.357. [DOI] [PubMed] [Google Scholar]
- Zielke E, Kuhlow F. On the inheritance of susceptibility for infection with Wuchereria bancrofti in Culex pipiens fatigans. Tropenmedizin und Parasitologie. 1977;28:68–70. [PubMed] [Google Scholar]