Abstract
The current study examines the processing of upright and inverted faces in 3-year-old children (n = 35). Event-related potentials (ERPs) were recorded during a passive-looking paradigm including adult and newborn face stimuli. We observed three face-sensitive components, the P1, the N170 and the P400. Inverted faces elicited shorter P1 latency and larger P400 amplitude. P1 and N170 amplitudes were larger for adult faces. To examine the role of experience in the development of face processing, the processing of adult and newborn faces was compared for children with a younger sibling (n = 23) and children without a younger sibling (n = 12). Age of sibling at test correlated negatively with P1 amplitude for adult and newborn faces. This may indicate more efficient processing of different face ages in children with a younger sibling and potentially reflects a more flexible face representation.
Keywords: face processing, children, experience, event-related potentials
Introduction
Faces are a unique source of social and emotional information and are processed in a special way (compared to non-faces) by adults (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Farah, Tanaka, & Drain, 1995; Rousselet, Husk, Bennett, & Sekuler, 2008), children (Kuefner, de Heering, Jacques, Palmero-Soler, & Rossion, 2010; Taylor, Batty, & Itier, 2004) and infants (de Haan, Johnson, & Halit, 2003; Hoehl & Peykarjou, 2012). It has long been known that face stimuli are recognized faster than other visual stimuli, and that face inversion leads to a specific decrease in recognition accuracy (Hochberg & Galper, 1967). Electrophysiological studies have provided extensive evidence of face-specific processing in adults. In particular, event-related potential (ERP) studies have identified at least two visual components related to the early encoding stages of face processing, the N170 (Bentin et al., 1996; Rossion & Jacques, 2008; Tanaka & Curran, 2001) and the P1 (Dering, Martin, Moro, Pegna, & Thierry, 2011; Eimer, 1998; Melinder, Gredebäck, Westerlund, & Nelson, 2010). The N170 is a negative-going deflection over posterior-occipital cortices that is consistently modulated by stimulus category (face vs. non-face), orientation (upright vs. inverted) and expertise. The P1 is a positive visual ERP component occurring around 100 ms which is modulated by low-level stimulus characteristics (Rossion & Jacques, 2008) and, in some cases, by inversion (e. g., Itier & Taylor, 2004; Macchi Cassia, Kuefner, Westerlund, & Nelson, 2006; Melinder et al., 2010).
During infancy, the processing of faces has been reported to be distributed across two posterior-occipital ERP-components, the N290 and the P400 (de Haan et al., 2003). The N290 is a negative peak occurring around 250–350 ms, whereas the P400 is a positive peak around 300–600ms. Modulations of these components are associated with stimulus category, stimulus orientation and experience (de Haan et al., 2003; Halit, Csibra, Volein, & Johnson, 2004; Scott & Monesson, 2010). However, the specific properties (e.g., latency, amplitude, scalp distribution) of the N290 and P400 in response to faces are not consistent across age-groups and comparison categories, rendering a direct comparison with the N170 difficult (Balas et al., 2010; de Haan et al., 2003; Hoehl & Peykarjou, 2012).
How the N290 and P400 develop to become the N170 as observed in adolescents and adults is not clear as yet. Studies on the development of neural face processing during childhood are scarce, partly because visual ERP studies on toddlers and young children provide a special challenge given children's increasing motor ability, loss of interest in passive looking paradigms, and aversion to wearing the ERP sensor net (de Haan, 2007). However, both a negative peak identified as the N170 (Kuefner et al., 2010; Taylor et al., 2004) and a positive peak, referred to as P400 (Carver et al., 2003), have been observed in young children (ages 2–5). In addition, the P1 has also been shown to be face-sensitive (Taylor et al., 2004). The morphology of these components is slightly different compared to older children and adults (Carver et al., 2003; Kuefner et al., 2010). Concerning the inversion effect, in 4–5 year-old children the P1 was elicited with larger amplitude and longer latency and the N170 with larger amplitude to inverted than to upright faces (Taylor et al., 2004). In a study on the own-age bias in 5-year-old children, inverted faces elicited larger P1 amplitude and longer P1 latency as well as smaller N170 amplitude (Melinder et al., 2010). The P400 was not analyzed in these studies.
In order to determine how the face-specific responses observed during infancy relate to those observed in children and adults, more studies on face-processing in young children (aged 1–3) are needed. Particularly, the neural processing of upright and inverted faces and experience-related effects in young children require further examination. Therefore, the current study was conducted to elucidate the development of face processing by presenting upright and inverted faces to 3-year-old children. Based on previous studies (Taylor et al., 2004), we expected inversion effects on both the P1 and the N170. Regarding the P400, no clear predictions could be made given that no other study has yet analyzed the P400 in response to upright and inverted faces in early childhood.
To further our understanding for the development of face processing, investigations should also take into account the effects of differential experience on face processing. Studies on the own-age bias (OAB) have employed the comparison of own- and other-age faces to determine whether own-age faces are processed in a special way (for a review, see Rhodes & Anastasi, 2012). A number of behavioural studies have shown that, in adults, recognition accuracy is higher for own- than other-age faces (Anastasi & Rhodes, 2006; Rhodes & Anastasi, 2012; Wiese, Komes, & Schweinberger, 2012). Although data are mixed, some studies have found a similar advantage in children older than 5 years (e.g., Anastasi & Rhodes, 2005; Crookes & McKone, 2009; Hills, 2012; Hills & Lewis, 2011). ERP responses to own- and other-age faces in both adults and children have been found to differ for several components in the time-range of 150–450ms (Ebner, He, Fichtenholtz, McCarthy, & Johnson, 2011; Melinder et al., 2010; Wiese, 2012; Wiese, Schweinberger, & Hansen, 2008). In 5-year-old children, the N170 was enhanced for child compared to young and older adult faces and P2 was smaller for child than older adult faces (Melinder et al., 2010). Moreover, in adults, ERPs starting around 200ms also distinguished more pronouncedly between hits and correct rejections for own-age than for other-age faces, thus mirroring the behavioural recognition advantage (Wiese, 2012; Wiese et al., 2008; Wolff, Wiese, & Schweinberger, 2012).
The comparison of own- and other-age faces is specific given the social and emotional relevance of own-age peers. Therefore, processing of faces belonging to different other-age groups should also be compared to examine the development of experience-dependent processing within the face domain. To this end, in the current study we compared ERP responses to upright and inverted adult and newborn faces in 3-year-old children with differing amounts of experience with infants, as provided by the presence of a younger sibling in their home. Newborn faces were chosen because, given that newborns are very infrequently present in our typical everyday environment, the amount of children's exposure to this specific face category is very limited and can be estimated rather well. In fact, newborns are not generally put into day-care, so young children without younger siblings rarely have extensive contact with a newborn child. This is important because evidence has been presented that social experience outside primary care may have a strong influence on face processing (Hills, 2012; Hills & Lewis, 2011). As a consequence, by comparing the processing of adult to newborn faces in children with and without a younger sibling, we were able to examine the effects of differential experience. Some recent behavioural studies have shown that intensive experience with a single infant face is sufficient to elicit perceptual expertise effects when gained in early childhood: 3-year-old children with a younger sibling show an inversion effect on perceptual discrimination accuracy for newborn faces compared to peers without a sibling (Macchi Cassia, Kuefner, Picozzi, & Vescovo, 2009). The current study sought to examine the neural basis for these experience effects by application of an ERP design. To this end, the processing of adult and newborn upright and inverted faces was examined in 3-year-old children with or without a younger sibling.
Methods
Participants
Participants included 35 children (13 males), with a mean age of 41.03 months (range 38.24–43.28, SD = 1.01). An additional 41 children were tested but could not be included in analyses for the following reasons: excessive eye and/or body movements that resulted in recording artifact (n = 23), too few trials were recorded for inclusion (n = 3), equipment failure or experimenter error (n = 7), or refusal to wear the sensor net (n = 8). In order to explore whether experience with infant faces influences neural processing, we included children with a younger sibling (n = 23) and children without a younger sibling and with limited current or past experience with infants, as indicated by a screening questionnaire (n = 12). Thus we were able to examine more specifically the influence that experience with infant faces may have on the processing of adult and newborn faces.
Stimuli and Procedure
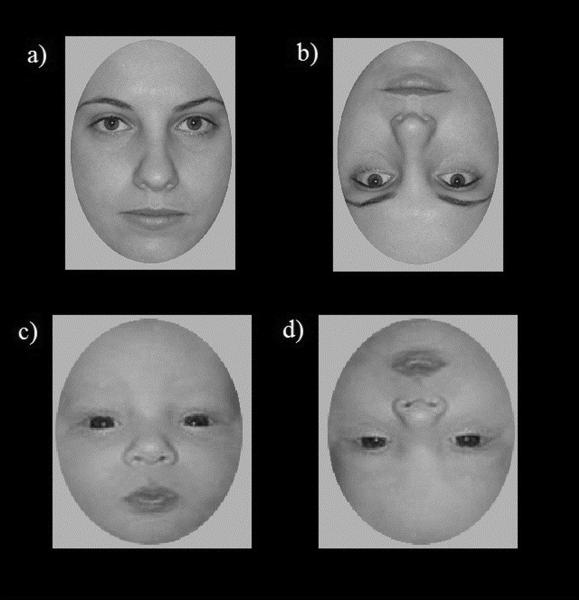
Children viewed grayscale photographic images of 21 adult female faces (20–30 year olds) and 21 newborn infant faces displaying a neutral expression (Macchi Cassia, et al., 2009). The faces were oval-cropped such that the external features of the face (i.e., hair and ears) were not visible. Adult and newborn faces were equalized for luminance and contrast using Photoshop. Natural differences between the two face ages were kept in terms of size and variability in the shape and distance of local features. Adult faces were slightly larger compared to newborn faces. To estimate variance in second-order relations, horizontal distance between pupils and vertical distance from pupil to mouth was measured. A ratio measure was created by dividing horizontal distance by vertical distance. The mean ratio (adult = .889, newborn = 1.196) and ratio SD (adult = .060, newborn = .074) was larger for newborn compared to adult faces, a fact that reflects real-life anthropometric norms (see also Farkas, 1988). The faces were presented on a gray square that appeared on a black background (see Figure 1). Stimuli were presented on a 22-inch computer monitor using E-Prime 1.2 software (Psychology Software Tools, Pittsburgh, PA) and subtended a visual angle of 9.1° × 7.2°.
Figure 1.
Examples of the infant and adult face stimuli used in the study. Top panel a) adult upright, b) adult inverted; bottom panel c) newborn upright, d) newborn inverted. Faces appeared successively in blocks by face age.
Faces were randomly presented in upright and inverted (rotated 180°) orientations with equal probability in trials blocked by face age (i.e., newborn, adult). The first two experimental blocks consisted of 48 trials each; the age category of the faces in the first block was counterbalanced across participants. The subsequent blocks of trials contained 26 trials in each block. The rationale for this was that we wanted children to view approximately equal numbers of trials from each face age category, but we anticipated that not all children would complete the entire experiment. The same faces were presented in upright and inverted orientation. Each face was repeated within the first block for adult or newborn faces. In subsequent blocks, faces from the first block were repeated. Orientation was varied randomly, so repetitions could be upright or inverted. Stimuli were presented for 500ms followed by a blank screen which appeared for 1000ms. The intertrial interval randomly varied from 500–1000ms, during which time a white fixation cross was presented on a black background. The experiment continued until children became too bored or fussy to attend, with a maximum of 200 trials. The average number of trials completed by children included in the final sample was 179 (range = 96–200, SD = 26.8).
Written informed consent was obtained from the parent of each child and children provided verbal assent before beginning the experiment. Children were seated on a small chair in a dimly-lit room in front of a computer monitor that was surrounded by a black screen in order to minimize distractions. An experimenter was seated next to the child in order to provide verbal instructions and re-direction as needed throughout the experiment. The passive viewing experiment was embedded within a child-friendly task that was designed to maintain children's attention. Children played a “Finding Nemo” game where an image of an orange clownfish (Nemo) was interspersed with the experimental images of faces (Nemo was programmed to appear after every 12 or so face trials). Nemo trials served as an experimental pause because the image stayed on the screen until the experimenter pressed a button to continue. The child “found” Nemo by pressing a button that was programmed to be inactive. Children were also given a sticker to place on a sticker chart that was used as a positive reinforcer throughout the experiment. Nemo trials were excluded from further data processing.
ERP Recording and Analysis
Prior to testing, a modified 128-channel 2.0 Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR) was placed on the child's head. Due to the young age of our participants, we removed four electrodes (channels 125, 126, 127 and 128) from the sensor net that children often find distracting because they are positioned on the face close to the eye region. A digital video of the child that was synchronized with the EEG recording was made for use in offline identification of eye movements. Electrode impedances were considered acceptable at or below 100 KΩ. Continuous electroencephalogram (EEG) signals were amplified using an EGI NetAmps 200 (Eugene, OR) and acquired using NetStation 4.3 software (EGI, Eugene, OR). Signals were referenced online to a single vertex electrode (Cz), a bandpass filter of .1 to 100 Hz was applied, and data were sampled at 250 Hz.
Subsequent data processing was performed offline using NetStation software. A lowpass filter of 30 Hz was applied. ERP trials were constructed using 100ms pre-stimulus, 500ms stimulus presentation, and 200ms post-stimulus. A baseline correction was applied using the average voltage of the 100ms prior to stimulus onset. An automated artifact detection algorithm was run to remove channels that showed signal changes greater than 150 μV within the segment period. Data were then visually inspected for electrooculogram (EOG) and motion artifacts not detected by the algorithm. Trials were rejected if they contained more than 12 bad channels. Of the remaining trials, individual bad channels were replaced using spherical spline interpolation. Individual subject averages were computed separately for each channel for the adult upright (AU), adult inverted (AI), newborn upright (NU) and newborn inverted (NI) conditions (M = 24 trials per condition, range = 11–44) and then re-referenced to the average reference 1.
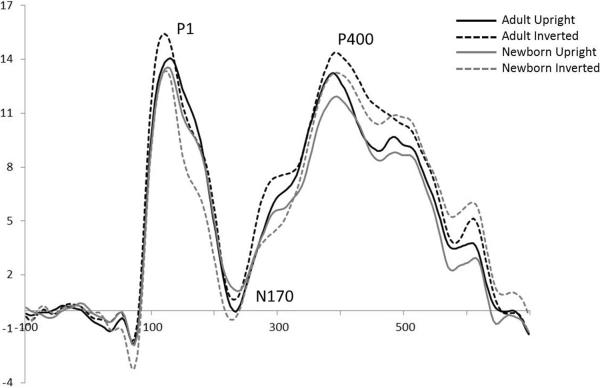
Based on previous literature (Carver et al., 2003; Taylor et al., 2004) we were interested in the P1, N170 and P400 components (see Figure 2). Electrode groupings and time windows were chosen based on previous literature as well as visual inspection of the grand-averaged and individually averaged waveforms. Time windows were selected (P1, 80–180ms; N170, 130–300ms, P400, 300–600ms) and electrode groupings were split into left (66, 67, 71 and 75), midline (72, 73, 76, 77), and right (78, 83, 84, 85) regions of interest for analysis. Peak amplitude and latency to peak were extracted for the P1 and N170. The absence of clear peaks in the individual averages for the P400 component rendered it necessary to extract mean amplitude and refrain from analyzing peak latency for this component.
Figure 2.
Grand-averaged ERP waveforms for the complete group of participants (n = 35). Adult upright (solid back line), adult inverted (dashed black line), newborn upright (solid gray line) and newborn inverted (dashed gray line) stimuli. The x-axis represents time in milliseconds (ms) and the y-axis represents amplitude in microvolts (μV).
Visual inspection of waveforms revealed that potential differences at the N170 may be driven by differences at the preceding P1 component. In order to control for the differences at the P1 in the analyses of the N170, a peak-to-trough subtraction was computed for amplitude and a difference score was calculated for latency (see also Kuefner et al., 2010). The adjusted values for the N170 amplitude and latency were entered in the analyses of variance for this component. A similar approach was used for the P400 analyses. As mean amplitude was analyzed for the P400, the baseline for P400 analyses was provided by a difference score which was computed using the 200–300ms time-window directly preceding the P400.
Experience variables
To explore possible relations between experience with infant faces and processing of adult and newborn faces, we calculated two experience variables. Age of sibling at test (m.d) quantifies the duration of experience with faces younger than the child, as well as the time that has passed since the child's sibling was a newborn. In our sample, there were no participants with a sibling younger than 3 months of age at test, therefore none of the children had current extensive experience with a newborn face. The second experience variable included was age of participant at birth of sibling (m.d). This variable can be useful in exploring effects of a sensitive period in the sense that earlier exposure to newborn faces may lead to stronger expertise effects than later exposure. For both experience variables, participants from the no-sibling group were assigned zero values.
Statistical Analysis
Amplitude and latency for each component were analyzed using repeated-measures analyses of variance (ANOVA). Within-subjects factors included face age (adult, newborn), orientation (upright, inverted), and region of interest (left, midline, right). Greenhouse-Geisser corrections were applied when the assumption of sphericity was violated. When significant (p ≤ .05) main or interaction effects emerged, post hoc comparisons were conducted and Bonferroni corrected.
Results
P1
Amplitude
Analyses revealed a main effect of face age for P1 amplitude, F(1, 34) = 4.37, p = .044, η2 = .114, whereby adult faces elicited larger P1 amplitude (M = 13.93 μV, SD = 6.4) than newborn faces (M = 12.70 μV, SD = 6.5). There was also a main effect of region, F(2, 33) = 18.56, p < .001, η2 = .529, in that the P1 was largest over the midline region (M = 15.38 μV, SD = 7.4) compared to the right (M = 13.22 μV, SD = 6.6) and left regions (M = 11.35 μV, SD = 5.9).
Latency
For P1 latency there was a main effect of orientation, F(1, 34) = 12.85, p = .001, η2= .274, in that the response to inverted faces (M = 122.67ms, SD = 13.6) was faster than to upright faces (M = 126.8ms, SD = 15.8). In addition, there was a main effect of region, F(2, 33) = 6.54, p < .01, η2 = .284, with shorter latencies recorded at the midline (M = 123.20ms, SD = 14.6) compared to the right region (M = 126.2ms, SD = 15.8).
N170
Amplitude
Analyses revealed a main effect of face age, F(1, 34) = 6.89, p < .05, η2 = .169, in that adult faces (M = −14.89 μV, SD = 7.6) elicited significantly larger N170 amplitude than newborn faces (M = −13.52 μV, SD = 7.1). There was also a main effect of region (F(2, 33) = 15.21, p < .001, η2 = .480), which was qualified by a region x orientation interaction, F(2, 33) = 4.01, p < .05, η2 = .195. When comparing N170 amplitude for upright and inverted stimuli post-hoc separately for the three regions of interest, all tests failed to reach significance.
Latency
Analyses revealed a main effect of region, F(2, 33) = 3.28, p = .05, η2 = .166. Follow-up tests failed to reach significance.
P400
Amplitude
For P400 amplitude, there was a main effect of orientation, F(1, 34) = 5.25, p < .05, η2 = .134, with larger amplitude for inverted (M = 13.39 μV, SD = 6.1) than upright (M = 12.04 μV, SD = 5.5) faces. This effect was qualified by a face age x orientation interaction F(1, 34) = 8.72, p < .01, η2 = .204. Post-hoc comparisons indicated larger amplitude for inverted stimuli compared to upright stimuli in response to newborn (upright: M = 10.96 μV, SD = 5.0, inverted: M = 13.88 μV, SD = 6.6), but not to adult faces (upright: M = 13.12 μV, SD = 6.9, inverted: M = 12.92 μV, SD = 6.5).
Correlation analyses
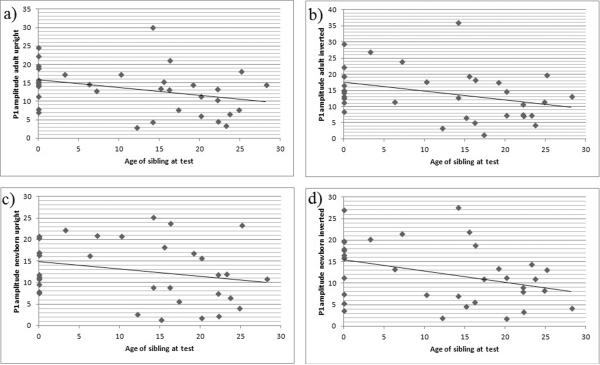
Pearson correlations were conducted to examine relations between age at birth of sibling and age of sibling at test with the amplitude and latency of the P1 and N170 and the amplitude of the P400. For the complete group, significant relations were observed between age of sibling at test and P1 amplitude in response to adult upright (r = −.339, p = .046), adult inverted (r = −.350, p = .039) and newborn inverted (r = −.369, p = .029) stimuli (see Table 1). All other correlations failed to reach significance. We also conducted Pearson correlations for the sibling group alone. Again, relations were observed for P1 amplitude with age of sibling at test, but due to the smaller sample size (N = 23) these were only marginally significant. Relations were observed for adult inverted (r = −.377, p = .078), newborn upright (r = −.377, p = .078) and newborn inverted (r = −.378, p = .078).
Table 1.
Uncorrected Means and Standard Deviations (SDs) for PI, N170 and P400 recorded from children in the two groups (No-sibling, Sibling).2
| P1 | |||||
|---|---|---|---|---|---|
| Condition | No-sibling group | Sibling group | |||
|
|
|||||
| Means | SDs | Means | SDs | ||
| P1 amplitude | Adult upright | 15.62 | 5.3 | 12.30 | 6.4 |
| Adult inverted | 16.53 | 5.6 | 13.31 | 8.4 | |
| Newborn upright | 13.56 | 4.6 | 12.59 | 7.9 | |
| Newborn inverted | 14.83 | 6.8 | 11.26 | 6.6 | |
|
| |||||
| P1 latency | Adult upright | 128.83 | 19.8 | 129.10 | 17.5 |
| Adult inverted | 124.77 | 16.0 | 122.10 | 17.2 | |
| Newborn upright | 123.75 | 21.8 | 125.02 | 11.3 | |
| Newborn inverted | 123.78 | 20.8 | 121.56 | 8.4 | |
| N170 | |||||
|---|---|---|---|---|---|
| Condition | No-sibling group | Sibling group | |||
|
|
|||||
| Means | SDs | Means | SDs | ||
| N170 amplitude | Adult upright | 1.00 | 7.1 | −2.27 | 5.5 |
| Adult inverted | 2.03 | 6.4 | −1.60 | 5.2 | |
| Newborn upright | 2.10 | 6.2 | −1.40 | 5.9 | |
| Newborn inverted | 0.82 | 8.0 | −2.42 | 4.6 | |
|
| |||||
| N170 latency | Adult upright | 211.10 | 33.5 | 218.83 | 30.4 |
| Adult inverted | 210.89 | 32.9 | 216.61 | 32.5 | |
| Newborn upright | 212.80 | 39.6 | 220.76 | 37.4 | |
| Newborn inverted | 209.35 | 29.0 | 213.77 | 32.9 | |
| P400 | |||||
|---|---|---|---|---|---|
| Condition | No-sibling group | Sibling group | |||
|
|
|||||
| Means | SDs | Means | SDs | ||
| P400 amplitude | Adult upright | 15.95 | 5.3 | 14.41 | 6.2 |
| Adult inverted | 16.84 | 5.1 | 15.28 | 7.6 | |
| Newborn upright | 15.85 | 5.6 | 11.83 | 5.9 | |
| Newborn inverted | 18.41 | 8.0 | 13.87 | 6.7 | |
Analyses of variance
ANCOVAs were run for the complete group using both age at birth of sibling and age of sibling at test as covariates. Age of sibling at test had a significant influence on P1 amplitude, F(3,32) = 5.087, p < .05, η2 = .137. In addition, we also conducted ANOVAs with group (sibling, no-sibling) as between-subjects factor. Despite the relation between P1 amplitude and age of sibling at test, all ANOVAs failed to reach significance.
General Discussion
We examined the processing of adult and newborn upright and inverted faces in 3-year-old children. Three face-sensitive ERP components, the P1, the N170, and the P400, were observed and analyzed. For inverted faces, shorter P1 latency and enhanced P400 amplitude were observed. P1 and N170 amplitude were larger in response to adult faces.
Inversion effects on the P1 are in line with other studies looking at processing of upright and inverted faces in 4–5-year-old children (Melinder et al., 2010; Taylor et al., 2004). Unlike these studies, however, 3-year-old children in the current study processed inverted faces faster than upright faces. Moreover, we did not observe inversion effects on P1 or N170 amplitude. The N170 has been found to be sensitive to face inversion in 4–5-year-old children (Melinder et al., 2010; Taylor et al., 2004) and face inversion effects on the N290 have been found in 12-month-olds (Halit, de Haan, & Johnson, 2003). Thus, the absence of orientation effects for the N170 in the current study may result from a change in functional properties of this component across childhood. Alternatively, discrepancies in the results may reflect methodological disparities between studies. For example, in the study by Melinder and colleagues (2010), faces were not cropped, so there was larger variance between stimuli than in our set. Moreover, in contrast with former studies (Halit et al., 2003; Taylor et al., 2004), we computed N170 peak-to-trough values in order to distinguish N170 from P1 orientation effects.
We found an orientation effect on the P400, which was driven by the newborn face stimuli. The P400 is observed in response to faces in infants (de Haan et al., 2003) and young children (Melinder et al., 2010), but it has not been reported in older children or adults. Our findings add to this evidence suggesting that P400 face sensitivity may gradually decline throughout development: Whereas it may be sensitive to inversion of all human faces in infants, it may be sensitive to inversion of less familiar face categories (e. g., newborn faces) in young children and completely lose its sensitivity in older children. Future studies may test this hypothesis by contrasting processing of highly familiar face categories (own-age, own-race) with unfamiliar face categories (other-age, other-race) during development.
The comparison between the processing of adult and newborn faces allowed us a) to compare processing of adult faces to a face category for which experience can be quantified rather precisely, and b) to examine effects of face-age in two other-age categories. Inclusion of a third category, own-age faces, would have been desirable but was not possible because of 3-year-olds' limited attention span. We observed face-age effects on the amplitude of the P1 and the N170, with adult faces eliciting larger amplitudes. These face-age effects may reflect structural differences between newborn and adult faces. For example, the N170 is to some extent sensitive to the presence of the eyes (Eimer, Kiss, & Nicholas, 2010; Itier, Latinus, & Taylor, 2006). The eyes are wider in adult faces than in newborn faces, and more salient because of the contrast between the iris and the sclera, which is not visible in newborns. Thus, larger P1 and N170 amplitude to adult faces may result from greater saliency of the eyes.
Moreover, the larger a component's amplitude, the greater the neuronal population involved in generating this component may be, so larger amplitudes may reflect stronger engagement. In this vein, adult faces may have been perceived as more salient than newborn faces. Particularly, the amplitude difference may relate to the social relevance of the faces. In a comparable ERP study examining the processing of child, young adult and older adult faces (Melinder et al., 2010), smaller N170 amplitude was observed for adult than child faces. In that study, same-age children may have been perceived as potentially more interesting social partners than adults, leading to an increase in attentional engagement to child faces (see also Macchi Cassia, 2011). The comparison of adult and newborn faces in the current study may possibly have rendered the adults the more interesting potential social partners. In fact, it would be possible that young children are motivated more strongly to attend to faces of individuals whose perceptual characteristics resemble those of the caregiver (see Macchi Cassia, 2011; Scherf & Scott, 2012).
With regard to the effects of experience on face processing, P1 amplitude to adult (upright and inverted) as well as newborn (inverted) faces negatively correlated with age of sibling at test. Assuming that smaller amplitudes reflect ease of processing, it may be argued that longer experience with the sibling's face progressively broadened children's face representation. The contact with different aged faces, namely adults' as well as a newborn face that progressively becomes an infant face, may cause the face space (Valentine, 1991) to develop dimensions along which the processing and the categorization of different face-ages is easier than when there is mainly contact with one age-group. Thus, the face representation of children with a younger sibling may become increasingly flexible compared to the representation of children without younger siblings. Moreover, the younger a child's sibling, the more the sibling may have structurally resembled the newborn faces presented here. Thus, it may be that P1 amplitude in response to newborn faces varied as a function of attentional engagement based on similarity between the sibling and the newborn faces.
P1 amplitude is thought to reflect the processing of low-level stimulus properties which, of course, contribute to the identification of a face as a face (Rossion & Caharel, 2011). In the current study, low-level differences in terms of stimulus brightness and contrast were controlled for, so it is unlikely that these factors contributed to the P1 effects. However, natural differences between face ages, e.g. size and variability, were preserved.
A concurrent explanation would be that children with a younger sibling generally have more contact with faces of different ages than children without younger siblings, thereby enhancing the flexibility of the face space. Regarding the impact of experience on overt behaviour, 3-year-old children showed an inversion effect on discrimination accuracy for newborn faces only if they had a younger sibling (Macchi Cassia et al., 2009). Similarly, first-time mothers only showed this inversion effect if they had gained experience with newborn faces via a younger sibling during their own childhood. An inversion effect for own-age faces in 3-year-olds was only observed if children had an older sibling (Macchi Cassia, Pisacane, & Gava, 2012). The inversion effect is an index of holistic/ configural processing (McKone & Yovel, 2009), so it can be concluded that early experience with a certain face age enhances holistic processing for this face category.
This is the first study to report on neural expertise effects from experience with different-aged faces in childhood, but past studies have not focused on this hypothesis. To further test the hypothesis that longer experience leads to greater flexibility of the face representation, participants with and without experience along a certain face dimension (e. g. age) should be compared regarding their processing properties for a certain face category (e. g. older adults) with which none of them has had direct experience.
To conclude, the current study is the first to show that 3-year-old children show an inversion effect for adult and newborn faces as inferred from patterns of brain activity, irrespective of experience However, daily experience with infants, indicated by the existence of a younger sibling, did affect the processing of adult and newborn faces via the moderating influence of age of sibling at test. It seems that the exposure to the face of a younger sibling has subtle, but relevant effects on young children's neural face processing template. Still, many questions remain unsolved. Why is the inversion effect, though consistently elicited, observed on different components in different studies (de Haan et al., 2003; Halit et al., 2003; Peykarjou & Hoehl, under revision; Taylor et al., 2004)? Do these differences actually reflect development of the face processing system, or do they depend more on task demands than assumed so far? Future studies will have to address these questions to further our understanding of the role that experience plays in generating these face-specific responses.
Figure 3.
Scatterplots for the relation of P1 amplitude and age of sibling at test in the complete group of participants (n = 35). The x-axis represents age of sibling at test in months, the y-axis P1 amplitude in microvolts (μV) in response to a) adult upright, b) adult inverted, c) newborn upright, d) newborn inverted faces.
Table 2.
Correlations between experience variables and ERP responses for the complete group.
| P1 | |||
|---|---|---|---|
| Condition | Age of sibling at test | Age of child at birth of sibling | |
| P1 amplitude | Adult upright | −.339* | −.244 |
| Adult inverted | −.350* | −.090 | |
| Newborn upright | −.242 | −.001 | |
| Newborn inverted | −.369* | −.142 | |
|
| |||
| P1 latency | Adult upright | .046 | −.058 |
| Adult inverted | .015 | −.167 | |
| Newborn upright | .105 | −.065 | |
| Newborn inverted | −.016 | −.089 | |
| N170 | |||
|---|---|---|---|
| Condition | Age of sibling at test | Age of child at birth of sibling | |
| N170 amplitude | Adult upright | .102 | −.058 |
| Adult inverted | .037 | −.113 | |
| Newborn upright | −.097 | −.206 | |
| Newborn inverted | .080 | −.060 | |
|
| |||
| N170 latency | Adult upright | .061 | .163 |
| Adult inverted | .130 | .187 | |
| Newborn upright | .084 | .140 | |
| Newborn inverted | .028 | .127 | |
| P400 | |||
|---|---|---|---|
| Condition | Age of sibling at test | Age of child at birth of sibling | |
| P400 amplitude | Adult upright | .053 | .064 |
| Adult inverted | −.007 | .153 | |
| Newborn upright | −.025 | −.028 | |
| Newborn inverted | .052 | −.121 |
Acknowledgements
The research reported in this manuscript was supported by the NIMH (R01MH078829) (to CAN). The authors are indebted to the many children and families who have graciously given of their time, and to Katherine Hung and Eliza Congdon for their assistance with experimental programming and data collection.
Footnotes
In addition to the 4 EOG sensors that were removed from the nets, 4 additional sensors (1, 8, 26, and 33) were excluded from the computation of the average reference due to their heightened susceptibility to EOG and movement artifacts. Therefore, the average reference was calculated from 117 channels.
Here, uncorrected values for N170 amplitude and latency and P400 amplitude are reported. For statistical analyses, values were corrected in order to control for potential differences at the preceding component. N170 amplitude was corrected via a peak-to-trough subtraction using the P1. For N170 latency, a difference score was calculated in which P1 latency was subtracted from N170 latency. For P400 amplitude, a difference score was calculated using the using the 200–300ms time-window directly preceding the component.
References
- Anastasi JS, Rhodes MG. An own-age bias in face recognition for children and older adults. Psychonomic Bulletin & Review. 2005;12(6):1043–1047. doi: 10.3758/bf03206441. doi: 10.3758/bf03206441. [DOI] [PubMed] [Google Scholar]
- Anastasi JS, Rhodes MG. Evidence for an Own-Age Bias in Face Recognition. North American Journal of Psychology. 2006;8(2):237–252. [Google Scholar]
- Balas BJ, Nelson CA, III, Westerlund A, Vogel-Farley V, Riggins T, Kuefner D. Personal familiarity influences the processing of upright and inverted faces in infants. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/neuro.09.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, Munson J. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42(2):148–159. doi: 10.1002/dev.10078. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookes K, McKone E. Early maturity of face recognition: no childhood development of holistic processing, novel face encoding, or face-space. Cognition. 2009;111(2):219–247. doi: 10.1016/j.cognition.2009.02.004. doi: S0010-0277(09)00045-6 [pii]10.1016/j.cognition.2009.02.004. [DOI] [PubMed] [Google Scholar]
- de Haan M, editor. Infant EEG and Event-Related Potentials. Psychology Press; Hove, England: 2007. [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. doi: S0167876003001521 [pii] [DOI] [PubMed] [Google Scholar]
- Dering B, Martin CD, Moro S, Pegna AJ, Thierry G. Face-sensitive processes one hundred milliseconds after picture onset. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00093. doi: 10.3389/fnhum.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, He Y, Fichtenholtz HM, McCarthy G, Johnson MK. Electrophysiological correlates of processing faces of younger and older individuals. Social Cognitive and Affective Neuroscience. 2011;6(4):526–535. doi: 10.1093/scan/nsq074. doi: 10.1093/scan/nsq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. Does the face-specific N170 component reflect the activity of a specialized eye processor? Neuroreport. 1998;9(13):2945–2948. doi: 10.1097/00001756-199809140-00005. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M, Nicholas S. Response profile of the face-sensitive N170 component: a rapid adaptation study. Cerebral Cortex. 2010;20(10):2442–2452. doi: 10.1093/cercor/bhp312. doi: bhp312 [pii]10.1093/cercor/bhp312. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? Journal of Experimental Psychology: Human Perception and Performance. 1995;21(3):628–634. doi: 10.1037//0096-1523.21.3.628. doi: 10.1037/0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Farkas LG. Age- and sex-related changes in facial proportions. In: Farkas LG, Munro IR, editors. Anthropometric proportions in medicine. Charles C. Thomas; Springfield: 1988. pp. 29–56. [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry. 2004;45(7):1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. doi: 10.1111/j.1469-7610.2004.00321.xJCPP321 [pii] [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19(3):1180–1193. doi: 10.1016/s1053-8119(03)00076-4. doi: S1053811903000764 [pii] [DOI] [PubMed] [Google Scholar]
- Hills PJ. A developmental study of the own-age face recognition bias in children. Developmental Psychology. 2012;48(2):499–508. doi: 10.1037/a0026524. doi: 10.1037/a0026524. [DOI] [PubMed] [Google Scholar]
- Hills PJ, Lewis MB. The own-age face recognition bias in children and adults. The Quarterly Journal of Experimental Psychology. 2011;64(1):17–23. doi: 10.1080/17470218.2010.537926. doi: 10.1080/17470218.2010.537926. [DOI] [PubMed] [Google Scholar]
- Hochberg J, Galper RE. RECOGNITION OF FACES: I. AN EXPLORATORY STUDY. Psychonomic Science. 1967;9(12):619–620. [Google Scholar]
- Hoehl S, Peykarjou S. The early development of face processing — What makes faces special? Neuroscience Bulletin. 2012:1–24. doi: 10.1007/s12264-012-1280-0. doi: 10.1007/s12264-012-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Latinus M, Taylor MJ. Face, eye and object early processing: what is the face specificity? Neuroimage. 2006;29(2):667–676. doi: 10.1016/j.neuroimage.2005.07.041. doi: S1053-8119(05)00565-3 [pii]10.1016/j.neuroimage.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Effects of repetition and configural changes on the development of face recognition processes. Dev Sci. 2004;7(4):469–487. doi: 10.1111/j.1467-7687.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Kuefner D, de Heering A, Jacques C, Palmero-Soler E, Rossion B. Early Visually Evoked Electrophysiological Responses Over the Human Brain (P1, N170) Show Stable Patterns of Face-Sensitivity from 4 years to Adulthood. Frontiers in Human Neuroscience. 2010;3:67. doi: 10.3389/neuro.09.067.2009. doi: 10.3389/neuro.09.067.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi Cassia V. Age biases in face processing: The effects of experience across development. British Journal of Psychology. 2011;102(4):816–829. doi: 10.1111/j.2044-8295.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V, Kuefner D, Picozzi M, Vescovo E. Early experience predicts later plasticity for face processing: Evidence for the reactivation of dormant effects. Psychological Science. 2009;20(7):853–859. doi: 10.1111/j.1467-9280.2009.02376.x. doi: 10.1111/j.1467-9280.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V, Kuefner D, Westerlund A, Nelson CA. Modulation of face-sensitive event-related potentials by canonical and distorted human faces: the role of vertical symmetry and up-down featural arrangement. Journal of Cognitive Neuroscience. 2006;18(8):1343–1358. doi: 10.1162/jocn.2006.18.8.1343. doi: 10.1162/jocn.2006.18.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi Cassia V, Pisacane A, Gava L. No own-age bias in 3-year-old children: More evidence for the role of early experience in building face-processing biases. Journal of Experimental Child Psychology. 2012 doi: 10.1016/j.jecp.2012.06.014. doi: 10.1016/j.jecp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- McKone E, Yovel G. Why does picture-plane inversion sometimes dissociate perception of features and spacing in faces, and sometimes not? Toward a new theory of holistic processing. Psychonomic Bulletin & Review. 2009;16(5):778–797. doi: 10.3758/PBR.16.5.778. doi: 16/5/778 [pii]10.3758/PBR.16.5.778. [DOI] [PubMed] [Google Scholar]
- Melinder A, Gredebäck G, Westerlund A, Nelson CA. Brain activation during upright and inverted encoding of own- and other-age faces: Erp evidence for an own-age bias. Developmental Science. 2010;13(4):588–598. doi: 10.1111/j.1467-7687.2009.00910.x. doi: 10.1111/j.1467-7687.2009.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peykarjou S, Hoehl S. Three-month-olds' brain responses to upright and inverted faces and cars. Developmental Neuropsychology. doi: 10.1080/87565641.2013.786719. under revision. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, Anastasi JS. The own-age bias in face recognition: A meta-analytic and theoretical review. Psychological Bulletin. 2012;138(1):146–174. doi: 10.1037/a0025750. doi: 10.1037/a0025750. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception. Vision Research. 2011;51(12):1297–1311. doi: 10.1016/j.visres.2011.04.003. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage. 2008;39(4):1959–1979. doi: 10.1016/j.neuroimage.2007.10.011. doi: S1053-8119(07)00936-6 [pii]10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Bennett PJ, Sekuler AB. Time course and robustness of ERP object and face differences. Journal of Vision. 2008;8(12):1–18. doi: 10.1167/8.12.3. doi: 10.1167/8.12.3. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Scott LS. Connecting developmental trajectories: Biases in face processing from infancy to adulthood. Developmental Psychobiology. 2012;54(6):643–663. doi: 10.1002/dev.21013. doi: 10.1002/dev.21013. [DOI] [PubMed] [Google Scholar]
- Scott LS, Monesson A. Experience-dependent neural specialization during infancy. Neuropsychologia. 2010;48(6):1857–1861. doi: 10.1016/j.neuropsychologia.2010.02.008. doi: S0028-3932(10)00052-7 [pii]10.1016/j.neuropsychologia.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. J Cogn Neurosci. 2004;16(8):1426–1442. doi: 10.1162/0898929042304732. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Valentine T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1991;43A(2):161–204. doi: 10.1080/14640749108400966. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Wiese H. The role of age and ethnic group in face recognition memory: ERP evidence from a combined own-age and own-race bias study. Biological Psychology. 2012;89(1):137–147. doi: 10.1016/j.biopsycho.2011.10.002. doi: 10.1016/j.biopsycho.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Wiese H, Komes J, Schweinberger SR. Daily-life contact affects the own-age bias and neural correlates of face memory in elderly participants. Neuropsychologia. 2012;50(14):3496–3508. doi: 10.1016/j.neuropsychologia.2012.09.022. doi: 10.1016/j.neuropsychologia.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Wiese H, Schweinberger SR, Hansen K. The age of the beholder: ERP evidence of an own-age bias in face memory. Neuropsychologia. 2008;46(12):2973–2985. doi: 10.1016/j.neuropsychologia.2008.06.007. doi: 10.1016/j.neuropsychologia.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wolff N, Wiese H, Schweinberger SR. Face recognition memory across the adult life span: Event-related potential evidence from the own-age bias. Psychology and Aging. 2012;27(4):1066–1081. doi: 10.1037/a0029112. doi: 10.1037/a0029112. [DOI] [PubMed] [Google Scholar]