Abstract
Mini invasive techniques are taking over conventional open liver resections in the setting of left lateral segmentectomy for living liver donation, and hydride procedure are being implemented for the living related right hepatectomy. Our center routinely performs laparoscopic left lateral segmentectomy for pediatric recipient and has been the first in the Europe performing an entirely robotic right hepatectomy. Great emphasis is posed on living donor safety which is the first priority during the entire operation, then the most majority of our procedures are still conventional open right hepatectomy (RHLD), defined as removal of a portion of liver corresponding to Couinaud segments 5-8, in order to obtain a graft for adult to adult living related liver transplant. During this 10 years period some changes, herein highlighted, have occurred to our surgical techniques. This study reports the largest Italian experience with RHLD, focused on surgical technique evolution over a 10 years period. Donor safety must be the first priority in right-lobe living-related donation: the categorization of complications of living donors, specially, after this “highly sensitive” procedure, reflects the need for prompt and detailed reports.
Keywords: Adult-to-adult living-related liver transplantation, Liver regeneration, Liver resections, Liver transplantation, Liver surgery
Core tip: A 12 years Italian single center experience is herein reported, focusing on the live donors who underwent conventional open right hepatectomies for adult to adult living related liver transplantations. In light of this experience we individualized three area of interest where we accomplished remarkable goals over this time period: donor nutritional status and rescue of steatotic donors; analysis of post hepatectomy liver regeneration; surgical technical developments.
INTRODUCTION
In July 2013 we have been communicated by the editorial board of the World Journal of Gastroenterology that our paper “Analysis of Surgical and Perioperative Complications in Seventy-five Right Hepatectomies for Living Donor Liver Transplantation” published in May 2008[1], had been cited, up to now, more than 27 times in the world English literature. This figure supported by data banks such as Scopus and ISI web of Science, made this scientific article entering in the 1% most cited papers of ever. Prompted by this achievement we reviewed our 12 years single Italian center experience with hepatic right lobe living donation. Indeed, we were particularly glad to contribute with this retrospective clinical report, to the special number of the World Journal of Gastroenterology, published to celebrate the 50th year’s anniversary of liver transplantation[2].
Although in the last 6 years the number of procedures performed each year has been dramatically fallen down, our center is still, by far, the busiest living related liver transplant program in Italy (Table 1).
Table 1.
Living liver donor potential candidates and type of surgical procedures performed at the Mediterranean Institute for Transplantation and Advanced Specialized Therapies, and the University of Pittsburgh Medical Center in Italy n (%)
| Statistics | |
| Exclusion criterion1 | |
| Donor-related reasons | 158 (44.0) |
| Donor withdrawal | 43 (12.0) |
| Donor death | 1 (0.3) |
| Exclusion at work-up: | |
| ABO incompatible | 11 (3.1) |
| Psychology | 18 (5.0) |
| Clinical/biochemistry | 27 (7.5) |
| At imaging | |
| CT scan | 30 (8.4) |
| MRCP scan | 10 (2.8) |
| At liver biopsy | 17 (4.7) |
| Other | 1 (0.3) |
| Recipient-related reasons | 93 (25.9) |
| Death awaiting LDLT | 21 (5.8) |
| Drop-out (HCC progression) | 21 (5.8) |
| LDLT refusal | 12 (3.3) |
| Unsuitable to LDLT | 10 (2.8) |
| Cadaveric OLT | 29 (8.1) |
| Total excluded | 251 (69.9) |
| Partial liver living graft2 | |
| For adult recipients | |
| Full left lobe | 2 (0.6) |
| Right lobe | 94 (26.2) |
| For pediatric recipients | 12 (3.3) |
| Left lateral segment | 12 (3.3) |
| Total included | 108 (30.1) |
| Overall | 359 (100) |
Potential donors;
Suitable donors. CT: Computed tomography; MRCP: Magnetic resonance cholangiopancreatography; HCC: Hepatocellular carcinoma; LDLT: Living donor liver transplant; OLT: Orthotopic liver transplant.
Herein, we will focus our attention on live donor of conventional right lobe (Coineaud segment 5-8) and only marginally on live donor of open left lateral segments (Coineaud segments 2-3)[3,4]. To further improve the outcome of these complex procedures, refinements in the surgical technique and better comprehension of the interrelations between post resectional liver regeneration, and donor nutritional status is, in our opinion, needed.
We individualized three area of interest where we accomplished remarkable goals over a 12 years period: nutritional status and rescue of steatotic donors[5]; analysis of liver regeneration in donors[1]; technical developments in conventional open resection for living donation[6].
PATIENTS POPULATION
At the “Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione” in Palermo, Italy, from January 2002 to April 2013, we performed an overall of 107 live donor hepatectomy of which in details: 95 for adult patients and 12 for pediatric recipients. One additional case of potential right hepatectomy was aborted after the beginning of the surgery because of an abnormal venous outflow discovered at the intra-operative ultrasound and not previously detected at the pre operative imaging.
In the early phase of our experience two of the adult patients received a full left lobe (Coineaud segment 2-4), while the others adult recipients received a right lobe (Coineaud segments 5-8); in one case the right hepatic graft was harvested using a totally robotic procedure of retrieval. This case, the first performed in Europe and the second in the world, will not be treated here.
The pediatric recipients always received an anatomical left lateral segmentectomy (Coineaud segments 2, 3); in 8 cases we have performed entirely laparoscopic harvesting procedures and details of these procedures will not be discussed here[7].
No living donor mortality, neither administration of heterologous blood transfusion were reported; 25 (27.1%) living donors presented 1 or more episodes of complication in the post-operative period.
One case of HCV infection was revealed after donation[8,9].
Regarding recipients, the patient and graft survival at 1, 3 and 5 year after right hepatectomy (RHLD) were 89.3%, 83.2%, 77.8% and 83.4%, 77.3%, 71.9% respectively.
NUTRITIONAL STATUS AND RESCUE OF STEATOTIC DONORS
In our center a comprehensive step-by-step donor work-up protocol is designed to ensure donor and recipient safety.
Donors with hepatic steatosis > 30% are excluded from donation, due to reported impairment of both graft and patient survival after living donor liver transplantation (LDLT)[10].
Donors with body mass index (BMI) ≥ 30 kg/m², a significant correlation between BMI and overall grade of steatosis is well known[11] and/or steatosis at imaging (ultrasonography, computed tomography or magnetic resonance imaging) or at histology underwent dietician consult and then re-evaluated within 3 mo.
After nutritional assessment [nutritional and dietary anamnesis, life-style evaluation and resting energy expenditure (REE) calculation] the dietician arranged a personal diet (carbohydrates 55%-57%, proteins 17%-19%, and lipids 24%-27%) and encouraged the donor to do physical activity.
Dietetic compliance of the donor was monthly re-assessed.
Acceptable monthly weight loss was considered 2-4 kg, with a final gained BMI of < 30 kg/m².
Eighteen potential living donors (age 27-59 years, male/female 14/4) were treated with diet. Nine out of 18 donors didn’t complete the 3-mo dietary follow-up for donor-unrelated reasons. In 9 donors who successfully completed the 3-mo treatment a liver biopsy was performed. In all cases a hepatic steatosis degree < 30% was found and they became eligible as donors (Figure 1).
Figure 1.
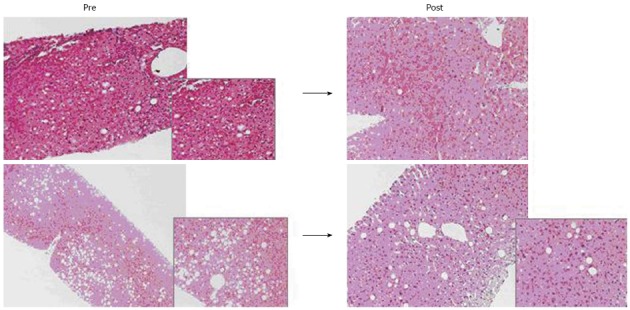
Two histologic examinations of liver parenchyma with steatosis, before and after intensive dietary treatment in the same donor. 10/18 donors successfully completed the 3-mo treatment (body mass index < 30 kg/m²). In all 10 treated donors, hepatic macrosteatosis < 30% was found (range: 2%-15%) and therefore they were considered eligible for donation.
After LDLT, none of them experienced life-threatening complications or died. Liver function in both remnant liver donors and transplanted grafts showed a good outcome, with no differences (in terms of hospital length of stay, liver function parameter normalization after resection, and liver regeneration) between them and other LDLT without hepatic steatosis.
Surprisingly, in a very recent multicentric study, according to data maintained in the LiverMetSurvey database, a paradoxical survival advantage was observed in patients with steatosis undergoing liver resection for colorectal liver metastases (CLM)[12].
This data has generated a fascinating hypothesis that of excess body adiposity has a survival protective effect, concept which warrants further research.
DONOR LIVER REGENERATION
Based on the evidence that an exposure of a small graft to persisting hyperdynamic circulation and high portal blood inflow may induce impairment of liver regeneration, and hepatic dysfunction[13,14], we have translated in a population of 70 donors who underwent right hepatectomy the analysis of the impact of the donor regeneration predictors on post hepatectomy outcomes[1].
Liver regeneration was evaluated with multidetector computed tomography (MDCT) at a mean of 61.07 d after surgery.
We have examined the possible impact of pre-surgical variables (e.g., age, weight, height, BMI, liver function tests, creatinine levels, platelet counts, international normalized ratio, and glucose levels) and variables detected with preoperative MDCT imaging [e.g., main portal vein diameter, steatosis, original liver volume, and spleen volume (SV)]. The future remnant liver volume (FRLV) was preoperatively calculated with a virtual surgical cut. Donor BMI was 23.7 ± 2.9 kg/m2, and was used to physiologically assess nutritional status.
In 26 of the 70 donors analyzed (37.14%), 100% or greater hepatic regeneration had occurred at 2 mo.
There was no association between the clinical outcome and the liver regeneration rate. A stepwise multiple regression analysis showed that a higher BMI (coefficient = 0.035, P < 0.0001) and preoperative parameters such as a smaller FRLV (coefficient = -0.002, P < 0.0001) and a greater SV/FRLV ratio (coefficient = 1.196, P < 0.0001) were predictors of greater liver regeneration (Figure 2).
Figure 2.
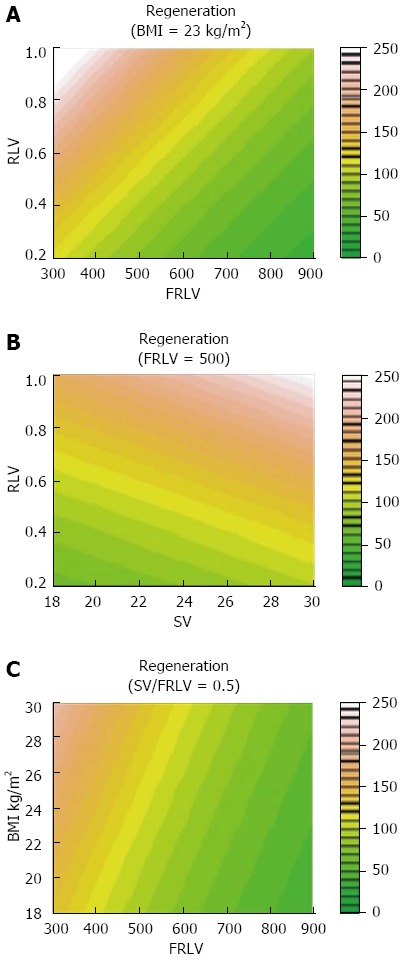
Locally Weighted Scatterplot Smoother graphics of the overall population, evidencing the association of percentage of liver regeneration with levels of body mass index (A), future remnant liver volume (B) and spleen volume/future remnant liver volume (C). FRLV: Future remnant liver volume; SV: Spleen volume; BMI: Body mass index.
TECHNICAL UPDATES
The most majority of our procedures are still conventional open RHLD, defined as removal of a portion of liver corresponding to Couinaud segments 5-8, in order to obtain a graft for adult to adult living related liver transplant.
While in the setting of the left lateral segment procurement open procedures are reserved only to rare cases presenting vascular anomalies.
During this 12 years period some changes, herein highlighted, have occurred to our surgical techniques. In particular we fell that the modifications we adopted concerning the transection of the accessories veins and final severing of the vascular stumps contributed to fasten, ameliorate and make safer the entire procedure.
Those technical development were possible using tools, strategies and experiences gained in laparoscopic surgery.
The operation is lately (after March 2008) being performed (last 10 cases, 9.2%) with a right renal flap incision while we were used to start with a bilateral subcostal incision, with upper midline extension (Mercedes incision). In the setting of the left lateral donation even an upper midline incision has been employed. For the right hepatectomy our original technique has been described elsewhere[15].
We later adapted our technique to all type of anatomic variants. In the case of a right dominant hepatic vein, when a tributary of the hepatic venous system larger than 5 mm was encountered in the transection plane a test clamp was performed in order to see whether the liver parenchyma became dusky, after which a decision was made as to whether to preserve the branch.
This practice was lately substituted by the preoperative use of the MEVIS Hepavision® system in order to obtain a more detailed analysis of the outflow venous drainage.
In case of preservation we severed the accessory hepatic vein, regardless was anterior or posterior, with the Endopath vascular staples (35 mm long, 12.3 mm wide; Ethicon Inc., Somerville, NJ) (Figure 3).
Figure 3.
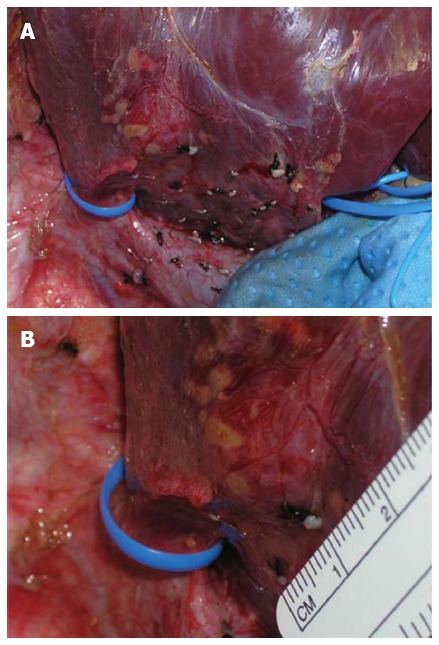
A large accessory hepatic vein draining the right lobe along with the right hepatic vein is encircled using a vessel loop (A). The measurement of the accessory hepatic vein [major of 5 mm, (B)] was performed for decision making to transect it with a vascular stapler, only when transections of liver parenchyma and of the structures of hepatic pedicle were almost complete.
This step was originally taken using straight pediatric Pott vascular clamp and then suturing the two stumps of the vein.
This ultimate practice allowed us to avoid a complex manual suture especially in case of medial tributary when the parenchyma is not yet completely transected. Subsequently, once the parenchyma had been completely divided, the vascular stumps of the right branch of the portal vein, of the right hepatic vein and of the ipsilateral hepatic artery were sectioned with the Endopath vascular staples (35 mm long, 12.3 mm wide; Ethicon Inc., Somerville, NJ), while before the traditional Satinsky and Pott vascular clamps with subsequent manual suture were used.
In the setting of the left lateral segmentectomy, after mobilization of the left anatomic lobe and after performing the parenchymal transection as described above for the right counterpart, we lately substituted the conventional use of vascular Satinsky clamps with endo- vascular stapler.
In particular, while the vascular stumps of the left portal vein and of the left hepatic artery were treated with the Endopath vascular staples (35 mm long, 12.3 mm; Ethicon Inc., Somerville, NJ), the left or the common middle and left stumps of the hepatic veins was treated with Endo GIA™ Universal 12 mm vascular stapler (Covidien, New Haven, CT).
Eventually we like to report on the utility of vascular endo-stapler into the open transplantation surgery, in the setting of the use of vascular graft. Indeed in few cases, the usage of several vascular staplers were crucial to shapes and employees a heterologous venous conduit, previously harvested from a deceased donors, for a back-table reconstruction of a large accessory hepatic vein which was connected to the recipient inferior vena cava of a LRLT (Figure 4).
Figure 4.
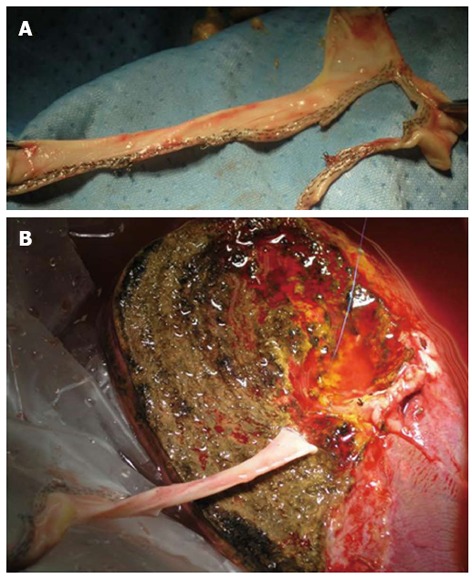
Usage of several vascular staplers were crucial to shapes and employees a heterologous venous conduit (A), previously harvested from a deceased donors, for a back-table reconstruction of a large accessory hepatic vein (B) to be connected to the recipient inferior vena cava in the LERLT.
DISCUSSION
Deceased donor quality is worsening in Italy, the average of donor age in our center is much better in the living counterpart (Figure 5). However living donation carries a special risk and donor safety must be the first priority in liver living-related donation.
Figure 5.
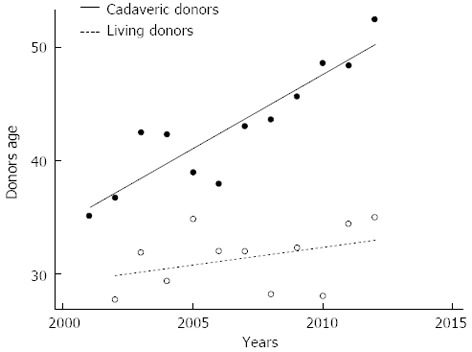
Linear regression models were used for explaining differences in terms of age between deceased and living liver donors over the last decade at the Mediterranean Institute for Transplantation and Advanced Specialized Therapies, and the University of Pittsburgh Medical Center in Italy. In our series of 677 cadaveric liver transplantation and 105 living donor liver transplantation, the deceased donor age has been increasing with a significative difference of average values between deceased and living liver donor age (95%CI: 0.9-1.3), P < 0.0001.
Indeed, the categorization of complications, the developments of new surgical tips, the changes matured over time in terms of donor selection and match needs to be promptly reported in details[16].
Herein, we separately analyzed three area of interest in live donor hepatectomy that have been exposed to some changes and ameliorations since the beginning of our practice.
We found that the reduction of hepatic steatosis to a values of < 30%, could be obtained with a strategic nutritional assessment and arranging an adequate personal diet. This concept is guiding our strategy in order to expand the live donor pool without affecting donor and recipient safety.
At this regard, the paradoxical survival advantage observed in patients with steatosis undergoing liver resection for CLM might create a new fascinating scenario, in which overweight living donor could be suitable for transplantation[12].
However it is too early to conclude that peri-diagnosis overweight is a good prognosticator after a major liver resection, because its impact upon long-term survival is less well documented.
Further clinical studies with large series, comparing patients and potential live donors with different BMI, grade of liver steatosis and nutritional marker, will be necessary to obtain convincing evidence.
Assuming that preoperative nutritional status is one of the key points for successful resection in living-related liver donors, we have recently put emphasis not only on the evaluation of the ratio between donor and recipient liver volume but also on the predictors of optimal early liver regeneration in the donors.
Historical series suggested that in adult-to-adult living related liver transplantation one of the most challenging tasks is to match an optimal size graft, balancing the clinical condition of the sick recipient and the safety of the healthy donor. In this setting, particularly care must be taken in the pre operative imaging evaluation of liver and spleen volume[17-19].
Eventually, in the scenario of the surgical refinements obtained over a 12 years period the adoption into the open conventional surgery of tools created for laparoscopic surgery such as the endo-mechanical stapler for vascular structures allowed us to make safer a unique surgical operation such as the living donor hepatectomy.
At this regard, we like to mention that traditionally one of the most stress full point of the operation is the positioning of the vascular clamp around the right hepatic vein or the common trunk of the middle and left hepatic vein and the subsequent manual suture of a potentially long vascular stump. Indeed, no matter how safely the clamp is placed the length of remnant vein to be sutured in the donor side might be short, and any moment the clamps could be displaced with detrimental consequences.
On the other hand, using the vascular stapler will make this step faster and safer with no consequence on the recipient side, once the stumps are opened on the back-table and the graft is flushed.
Adult-to-Adult Living-related Donor Liver Transplantation remains the greatest most recent and challenging evolution of liver transplantation, both from a technical and ethical point of view, which has contributed to reduce donor shortage[20]. Stringent criteria of donor selection criteria and peri-operative care were implemented following one of the first reported case of living donor death in 2002, and a smaller number of centers with large experience refined the surgical technique, selection and clinical management of both donors and recipients[21].
Footnotes
P- Reviewers Dragoteanu M, Gheonea DI S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Gruttadauria S, Parikh V, Pagano D, Tuzzolino F, Cintorino D, Miraglia R, Spada M, Vizzini G, Luca A, Gridelli B. Early regeneration of the remnant liver volume after right hepatectomy for living donation: a multiple regression analysis. Liver Transpl. 2012;18:907–913. doi: 10.1002/lt.23450. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lortat-Jacob JL, ROBERT HG. [Well defined technic for right hepatectomy] Presse Med. 1952;60:549–551. [PubMed] [Google Scholar]
- 4.Gruttadauria S, Vasta F, Minervini MI, Piazza T, Arcadipane A, Marcos A, Gridelli B. Significance of the effective remnant liver volume in major hepatectomies. Am Surg. 2005;71:235–240. [PubMed] [Google Scholar]
- 5.Pagano D, Spada M, Grosso G, Cintorino D, Li Petri S, Luca A, Vizzini GB, Volpes R, Gridelli BG, Gruttadauria S. Impact of Donor’s Nutritional Status on Recipient Survival in a Consecutive Series of 87 Living-Related Liver Transplants. Liver Transpl. 2013;19:S273–S274. [Google Scholar]
- 6.Gruttadauria S, Pagano D, Cintorino D, Li Petri S, Ricotta C, Bonsignore P, Romano M, Caruso S, Gridelli BG, Spada M. Evolution of surgical technique in conventional open right hepatectomy for living liver donation over a 10 years period in a single center. Liver Transpl. 2013;19:S151–S152. doi: 10.1016/j.transproceed.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Spada M, Boggi U, Pagano D, Echeverri GJ, Bartoccelli C, Catalano P, Ricotta C, Cintorino D, Li Petri S, di Francesco F, et al. Laparoscopic Left Liver Sectionectomy ([LLLS) for Pediatric Living Related Liver Transplantation (LRLT) Am J Transpl. 2011;11:85. [Google Scholar]
- 8.Gruttadauria S, Pagano D, Petridis I, Vizzini G, Volpes R, Grossi PA, Gridelli B. Hepatitis C virus infection in a living-related liver donor. Am J Transplant. 2010;10:191. doi: 10.1111/j.1600-6143.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertino G, Ardiri A, Boemi PM, Calvagno GS, Ruggeri IM, Speranza A, Santonocito MM, Ierna D, Bruno CM, Valenti M, et al. Epoetin alpha improves the response to antiviral treatment in HCV-related chronic hepatitis. Eur J Clin Pharmacol. 2010;66:1055–1063. doi: 10.1007/s00228-010-0868-4. [DOI] [PubMed] [Google Scholar]
- 10.Gruttadauria S, Marsh JW, Bartlett DL, Gridelli B, Marcos A. Ex situ resection techniques and liver autotransplantation: last resource for otherwise unresectable malignancy. Dig Dis Sci. 2005;50:1829–1835. doi: 10.1007/s10620-005-2946-5. [DOI] [PubMed] [Google Scholar]
- 11.Volpes R, Randisi L, Gruttadauria S, Rubino M, Minervini M, Vizzini GB, Gridelli BG. Usage of donors with steatosis after an intensive dietary treatment in living donor liver transplantation in adults. Hepatology. 2007;46:245A. [Google Scholar]
- 12.Parkin E, O’Reilly DA, Adam R, Kaiser GM, Laurent C, Elias D, Capussotti L, Renehan AG. The effect of hepatic steatosis on survival following resection of colorectal liver metastases in patients without preoperative chemotherapy. HPB (Oxford) 2013;15:463–472. doi: 10.1111/hpb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruttadauria S, Pagano D, Luca A, Gridelli B. Small-for-size syndrome in adult-to-adult living-related liver transplantation. World J Gastroenterol. 2010;16:5011–5015. doi: 10.3748/wjg.v16.i40.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A, et al. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761–766. doi: 10.1111/j.1399-0012.2007.00735.x. [DOI] [PubMed] [Google Scholar]
- 15.Gruttadauria S, Mandalà L, Vasta F, Cintorino D, Musumeci A, Marsh W, Marcos A, Gridelli B. Improvements in hepatic parenchymal transection for living related liver donor. Transplant Proc. 2005;37:2589–2591. doi: 10.1016/j.transproceed.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 16.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 17.Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697–1703. doi: 10.1097/00007890-200012270-00006. [DOI] [PubMed] [Google Scholar]
- 18.Humar A, Beissel J, Crotteau S, Cohen M, Lake J, Payne WD. Delayed splenic artery occlusion for treatment of established small-for-size syndrome after partial liver transplantation. Liver Transpl. 2009;15:163–168. doi: 10.1002/lt.21636. [DOI] [PubMed] [Google Scholar]
- 19.Luca A, Miraglia R, Caruso S, Milazzo M, Gidelli B, Bosch J. Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transpl. 2006;12:1237–1243. doi: 10.1002/lt.20762. [DOI] [PubMed] [Google Scholar]
- 20.Gruttadauria S, di Francesco F, Vizzini GB, Luca A, Spada M, Cintorino D, Li Petri S, Pietrosi G, Pagano D, Gridelli B. Early graft dysfunction following adult-to-adult living-related liver transplantation: predictive factors and outcomes. World J Gastroenterol. 2009;15:4556–4560. doi: 10.3748/wjg.15.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C, Smith ML, Fujiki M, Uso TD, Quintini C. Preparing for the inevitable: the death of a living liver donor. Liver Transpl. 2013;19:656–660. doi: 10.1002/lt.23637. [DOI] [PubMed] [Google Scholar]


