Abstract
AIM: To investigate the association between human papillomavirus (HPV) and esophageal squamous cell carcinoma (ESCC) in southern Brazil.
METHODS: We studied 189 esophageal samples from 125 patients from three different groups: (1) 102 biopsies from 51 patients with ESCC, with one sample from the tumor and another from normal esophageal mucosa distant from the tumor; (2) 50 esophageal biopsies from 37 patients with a previous diagnosis of head and neck squamous cell carcinoma (HNSCC); and (3) 37 biopsies from esophageal mucosa with normal appearance from 37 dyspeptic patients, not exposed to smoking or alcohol consumption. Nested-polymerase chain reaction (PCR) with the MY09/11 and GP5/6 L1 primers was used to detect HPV L1 in samples fixed in formalin and stored in paraffin blocks. All PCR reactions were performed with a positive control (cervicovaginal samples), with a negative control (Human Genomic DNA) and with a blank reaction containing all reagents except DNA. We took extreme care to prevent DNA contamination in sample collection, processing, and testing.
RESULTS: The histological biopsies confirmed the diagnosis of ESCC in 52 samples (51 from ESCC group and 1 from the HNSCC group) and classified as well differentiated (12/52, 23.1%), moderately differentiated (27/52, 51.9%) or poorly differentiated (7/52, 13.5%). One hundred twenty-eight esophageal biopsies were considered normal (51 from the ESCC group, 42 from the HNSCC group and 35 from dyspeptic patients). Nine had esophagitis (7 from the HNSCC and 2 from dyspeptic patients). Of a total of 189 samples, only 6 samples had insufficient material for PCR analysis: 1 from mucosa distant from the tumor in a patient with ESCC, 3 from patients with HNSCC and 2 from patients without cancer. In 183 samples (96.8%) GAPDH, G3PDH and/or β-globin were amplified, thus indicating the adequacy of the DNA in those samples. HPV DNA was negative in all the 183 samples tested: 52 with ESCC, 9 with esophagitis and 122 with normal esophageal mucosa.
CONCLUSION: There was no evidence of HPV infection in different ESCC from southern Brazil.
Keywords: Esophageal cancer, Esophageal squamous cell carcinoma, Human papillomavirus, Head and neck cancer, Polymerase chain reactions, Nested-polymerase chain reaction
Core tip: This paper gives additional evidence related to the controversy on the potential role of human papillomavirus (HPV) in the pathogenesis of esophageal squamous cell carcinoma (ESCC). Taking great care to avoid contamination by environmental HPV and using a very sensitive HPV DNA detection technique, we found no evidence of HPV neither in ESCC tumor tissue, nor in esophageal non-tumoral tissue from ESCC patients, or from head and neck squamous cell carcinoma patients or from dyspeptic controls without cancer. These data convincingly argue that when environmental contamination is carefully controlled, there is no evidence that HPV is involved in ESCC carcinogenesis in southern Brazil.
INTRODUCTION
Worldwide, esophageal cancer is the eighth most common cancer and the sixth most common cause of death from cancer, with an estimated incidence of 482000 new cases and 407000 deaths in 2008[1]. Esophageal squamous cell carcinoma (ESCC) is still the most common type worldwide and its known risk factors are smoking and excessive alcohol consumption, poor nutritional and socio-economic status, exposure to polycyclic aromatic hydrocarbons (PAH), low consumption of fruits and vegetables, ingestion of hot beverages, genetic factors, history of caustic injury in the esophagus, and history of head and neck squamous cell carcinoma (HNSCC)[2-4]. In Brazil, the highest rates of esophageal cancer occur in the country’s southernmost state, Rio Grande do Sul, where the rate of incidence is considered moderate with 18.01 cases per 100000 men and 6.60 cases per 100000 women[5]. In the south of Brazil the most important risk factors are the combination of smoking and excessive alcohol consumption[5], however, the consumption of a hot beverage made with the infusion of Ilex paraguayensis (also known as yerba mate) is also a risk factor[6]. This beverage is often consumed at high temperatures and contains high levels of PAH[7].
The role of human papillomavirus (HPV) in the development of ESCC remains controversial[8-12]. Two studies conducted in southern Brazil, each using a different technique, showed different results regarding the association between ESCC and HPV[13,14]. Therefore, to clarify the association between HPV infection and ESCC in southern Brazil, we looked for the presence of HPV DNA in esophageal biopsies from: (1) primary tumor and mucosa without neoplasia from patients with ESCC; (2) Lugol stained and unstained areas in patients with HNSCC; and (3) normal appearing mucosa from patients not exposed to tobacco or alcohol.
MATERIALS AND METHODS
Patients and tissues
We evaluated 189 consecutive esophageal samples, collected in Santa Maria, a city in the central region of Rio Grande do Sul, the most southern state in Brazil, from 2008 to 2011. We included 51 samples from esophageal tumors and 51 samples from non-tumoral areas of the esophagus of patients with ESCC. We analyzed 50 esophageal biopsies (32 from Lugol stained areas, 17 from unstained areas and 1 from tumor) from patients diagnosed with HNSCC. Neither the patients with ESCC nor those with HNSCC had received chemotherapy or radiotherapy before the sample collection. We also collected biopsies from the normal middle esophagus of thirty-seven non-smoking and non-alcohol drinking dyspeptic patients who underwent upper GI endoscopy.
We collected demographic data (sex, age, place of birth, occupation) and information regarding smoking habits, alcohol consumption and previous history of cancer. The samples were fixed in neutral buffered formalin, embedded in paraffin, cut and stained with Hematoxilin-Eosin. For DNA extraction, one slice at least 5 μm thick, as recommended for polymerase chain reaction (PCR) amplification, was cut from the paraffin-embedded tissues[15]. To minimize the risk of contamination, the materials used to process the sample stored in the paraffin block were completely disposable and used once per sample. In addition, the microtome sample holder was washed with absolute alcohol and the blade was replaced before each block was cut. Different rooms were used for DNA extraction, preparing the DNA solution, adding the DNA samples to the PCR solution and electrophoresis analysis. The rooms could only be accessed through antechambers with a single flow of material. All PCR reactions were performed with a positive control (cervicovaginal samples), with a negative control (Human Genomic DNA, Cat. No. G304A, Promega, Madison, WI) and with a blank reaction containing all reagents except DNA. The same method was also employed to test for the presence of HPV DNA in thirty-five samples of primary tumor from patients with HNSCC.
DNA Extraction and PCR
Once the samples were de-waxed, the DNA was extracted using the Qiagen QIAamp DNA Mini Kit (Valencia, CA) according to manufacturer’s instructions. DNA quantification and purity were determined by optical density in a spectrophotometer (Thermo Scientific NanoDrop 2000).
The DNA (25-80 ng) from each sample was amplified by PCR. The integrity of the DNA samples was observed by amplifying the human conserved genes GAPDH, G3PDH and β-globin. The sequences of the HPV L1 gene were amplified by nested-PCR using two general primer sets: MY09/MY11 (MY09: 5’-GTCCMARRGGAWACTGATC-3’, MY11: 5’-GCMCAGGGWCATAAYAATGG-3’) in the first amplification step to produce a 450 bp fragment; and GP5/GP6 (GP5: 5’-TTTGTTACTGTGGTAGATAC-3’, GP6: 5’-ACTAAATGTCAAATAAAAAG-3’) in the second step to produce 150 bp fragments of the PCR product. In the first step, PCR was performed with a reaction mixture containing 50 μL, including 5.0 μL of the genome from the extracted DNA sample, 5.0 μL 10 × PCR buffer, 5.0 μL of dNTP (2.5 mmol/L), 1.2 μL of MgCl2 (50 mmol/L), 0.5 μL of Taq DNA polymerase and 0.2 μL (500 pmol/μL) of the MY09 and MY11 primers. In the second step, which also had a final volume of 50 μL, 1.0 μL (500 pmol/μL) of the GP5 and GP6 primers was used. The PCR mixture was subjected to 40 amplification cycles, each consisting of an initial denaturation step at 94 °C for 30 s, annealment at 56 °C for 1 min and extension at 72 °C for 1 min. The PCR products were separated by eletrophoresis on 2% agarose gel and visualized by staining with ethidium bromide by electrophoresis (Figure 1).
Figure 1.
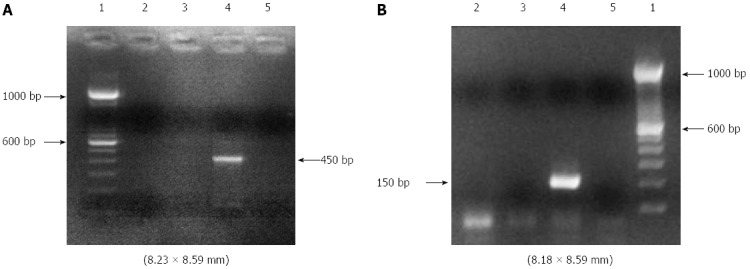
Analysis of DNA from esophageal tumor tissue for human papillomavirus using nested- polymerase chain reaction for human papillomavirus L1 gene. The MY09/11 primer pair was used in the first step (A) and the GP5/GP6 primer pair was used in the second step (B). 1: DNA size marker (100 bp DNA ladder); 2: Patient tumor DNA: [human papillomavirus (HPV)]-negative; 3: HPV DNA-negative control (Human Genomic DNA); 4: HPV DNA-positive control (cervicovaginal sample); 5: Negative control containing all polymerase chain reaction reagents except DNA.
Ethics
The study was approved by the Research Ethics Committee of the Federal University of Santa Maria and of the Federal University of Rio Grande do Sul, Rio Grande do Sul, Brazil. Informed consent was obtained from each participant before they underwent upper GI endoscopy.
Statistical analysis
The variables were expressed as mean and SD or numbers and percents. Associations would have been considered statistically significant when a two-sided P value was ≤ 0.05. All statistical analyzes were performed with the aid of SPSS 11 (Statistical Package for Social Sciences).
RESULTS
Patient characteristics
We included 125 individuals divided in three groups (Table 1): (1) 51 patients with ESCC, 43 male (84.3%) with a mean age of 60.1 ± 10.3 years; (2) 37 patients with previous diagnosis of HNSCC, 34 males (91.9%) with a mean age of 57.8 ± 8.4 years; and (3) 37 dyspeptic patients, 12 males (32.4%) with a mean age of 56.8 ± 17.7 years.
Table 1.
Clinical and demographic characteristics and risk factors of the patients n (%)
| ESCC (n = 51) | HNSCC (n = 37) | Not exposed (n = 37) | |
| Age (yr) | |||
| Range | 42-79 | 41-78 | 19-87 |
| mean ± SD | 60.1 ± 10.3 | 57.8 ± 8.4 | 56.8 ± 17.7 |
| Sex | |||
| Male | 43 (84.3) | 34 (91.9) | 12 (32.4) |
| Female | 8 (15.7) | 3 (8.1) | 25 (67.6) |
| Smoking | |||
| Current | 42 (82.4) | 35 (94.6) | - |
| Ex-smokers (> 10 yr) | 5 (9.8) | 2 (5.4) | 10 (27.0) |
| Never smoked | 4 (7.8) | 0 (0) | 27 (73.0) |
| Alcohol use | |||
| Current | 27 (53.0) | 31 (83.8) | - |
| Ex-alcohol users (> 10 yr) | 8 (15.7) | 2 (5.4) | 2 (5.4) |
| Never used alcohol | 16 (31.4) | 4 (10.8) | 35 (94.6) |
| Active smokers and alcohol users | 18 (35.3) | 18 (48.6) | - |
| Never smoked or drunk alcohol | 3 (5.9) | 0 (0) | 26 (70.3) |
ESCC: Esophageal squamous cell carcinoma; HNSCC: Head and neck squamous cell carcinoma.
Histology
We studied 189 esophageal biopsy samples. All fifty-two biopsies from tumoral areas (51 from the ESCC group and 1 from the HNSCC group) were diagnosed as squamous cell carcinoma and classified as well differentiated (12/52, 23.1%), moderately differentiated (27/52, 51.9%) or poorly differentiated (7/52, 13.5%). None of the 51 samples of esophageal mucosa distant from the tumor in the ESCC group showed malignancy. In the patients with HNSCC, we performed 55 esophageal biopsies. Their histological analysis showed the following findings: 42 were normal (76.4%), 7 had esophagitis (12.7%), 1 contained ESCC (1.8%) and five had insufficient tissue for histological analysis (9.1%). In the biopsies of normal esophagus in the dyspeptic patients, 35 were considered normal and 2 had mild esophagitis.
PCR analysis
The average DNA concentration in the samples was 214.68 ng/μL (range: 8-1313), with a mean ratio between the absorbance readings at 260 nm and 280 nm wavelengths of 2.12 (range 1.15-5.95). GAPDH, G3PDH and/or β-globin were amplified in 183 (96.8%) esophageal biopsies, showing that the DNA was adequate for analysis. These conserved human genes were not amplified in only six samples, all with normal esophageal mucosa (1 from mucosa distant from the tumor in a patient with ESCC, 3 from patients with HNSCC and 2 from patients without cancer). The PCR results were negative for HPV DNA in all the esophageal biopsies (52 with ESCC, 9 with esophagitis and 122 with normal esophageal mucosa), as well as in all biopsies from the primary tumor of head and neck cancer.
DISCUSSION
The current study used a nested primer-based PCR test to identify HPV DNA in esophageal samples from individuals from a moderate risk area for ESCC in southern Brazil. Our results showed no evidence of HPV DNA in any of the samples of the ESCC and from non-tumoral areas of the esophagus, in the esophageal mucosa of patients with HNSCC, or in the esophageal mucosa from patients without risk factors for ESCC.
Some studies using a bovine model have found that the bovine papillomavirus is essential in the early stages of carcinogenesis of the upper digestive tract, but is not required for progression to the status of malignancy[8,16,17]. Considering the possibility of this “hit-and-run” mechanism to induce oncogenesis in the esophagus in humans, we included samples with and without cancer from the esophageal mucosa of patients with ESCC and esophageal mucosal samples from patients with HNSCC. In order to enhance the detection of ESCC precursor lesions, all patients with HNSCC underwent upper endoscopy with mucosal iodine staining and biopsy of stained and unstained areas[18-21]. However, all samples were negative for HPV DNA, both in the primary tumors and in the esophageal mucosa without neoplasia of patients with ESCC or HSNCC and in the group without cancer who were not exposed to tobacco or alcohol. The consistent presence of the GAPDH, G3PDH or β-globin genes in the samples indicated that the specimens were suitable for DNA analysis.
We used the nested primer-based PCR system with the MY11-MY09 consensus primers in combination with another general primer pair, GP5-GP6, positioned within the former. According to Evander et al[22], this two-step PCR amplification is able to detect 1 to 10 copies of the HPV 16 genome, while the use of isolated pairs of primers MY11-MY09 or GP5-GP6 detect 100 and 10 copies, respectively. Thus, we used a technique with good yield for samples with low viral load. In addition, the MY11-MY09 primer is highly capable of detecting multiple HPV types within a given sample[23]. Furthermore, the inner primer pair GP5-GP6 spans a shorter region (about 150 bp), which is useful when the DNA is isolated from paraffin-embedded tissue fixed with formalin[24-27]. More sensitive techniques can be used to detect HPV DNA, but as Ha et al[28] suggest, without at least one copy of the viral genome per cell, a clonal relationship cannot be established; therefore, a sufficient number of copies of HPV affecting most of the cells in the lesion is required. Hence, we believe that our technique was sufficiently sensitive to accurately detect the presence of HPV DNA in tissue samples from the aero-digestive tract.
In southern Brazil, Weston and Prolla[13] analyzed 40 ESCC samples and 10 benign esophageal biopsies from patients without cancer. Using the Hybrid Capture II test, they detected HPV DNA in only one case of ESCC and in one benign specimen. Subsequently, Souto Damin et al[14] reported detecting HPV in 15.75% of ESCC patients (26/165) and in none of the specimens of benign esophagus (0/26) using auto-nested PCR with the GP5+/GP6+ consensus primer pair. Some features of our study that differed from these two previous HPV studies conducted in southern Brazil are: (1) the extreme care we took to prevent DNA contamination throughout the specimen processing and testing; (2) the use of a more highly sensitive HPV detection method; and (3) the assessment of possible precursor lesions in the non-tumoral mucosa of esophagi with cancer and in the esophageal mucosa of patients with head and neck cancer.
A tissue study similar to ours was recently reported from a high-risk area of China[29]. The authors analyzed tumor samples from 272 patients with ESCC who underwent esophagectomy at the Yaocun Commune Hospital in Linxian, in north-central China. The patients came from various regions of China, with different incidence rates of ESCC and different mortality rates for cervical cancer. HPV DNA was tested on fresh frozen tumor tissue and tumor samples fixed with formalin and stored in paraffin using PCR with the PGMY L1 and SPF10 L1 consensus primers, respectively. Adopting careful measures to avoid contamination of the samples, similar to those used in the present study, these authors also found no cases with convincing evidence of carcinogenic HPV activity.
There are some limitations to the present study, such as the lack of information regarding sexual habits, socio-economic characteristics and the consumption of the beverage yerba mate, but there is no reason to think that having such information would have changed the PCR results of this study.
Our results confirm previous observations in other regions that report the absence of an association between esophageal mucosa infection by HPV and ESCC, and suggest that, in southern Brazil, this virus is not an important risk factor for squamous cell carcinoma of the esophagus.
ACKNOWLEDGMENTS
The authors thank the following individuals for their assistance: Patrícia Chaves Brites for useful suggestions in the human papillomavirus analysis, and Drs Leandro Bizarro Muller, Eduardo Buzatti Souto, Daniela Costa, Stela Maria Motta, Alexandre Rampazzo and João Carlos Cantarelli Jr for their collaboration in the upper endoscopy. Special acknowledgment is given to Dr Sanford M. Dawsey for his helpful comments on the manuscript and for his help in the English revision.
COMMENTS
Background
The high geographical variation in esophageal squamous cell carcinoma (ESCC) incidence observed worldwide reflects the exposure to specific environmental factors that are not completely understood. High-risk human papillomavirus (HPV) infections are present in almost all cervical cancers, beside other neoplasms in squamous epithelial-lined tissues, probably being involved in their genesis. The etiologic role of HPV in ESCC remains highly controversial.
Research frontiers
A large number of controversial manuscripts have been published investigating the possible role of HPV in the etiology of esophageal cancer. In this study the authors used a very sensitive HPV DNA detection technique, and evaluated the HPV presence in ESCC tumor tissue and esophageal non-tumoral tissue.
Innovations and breakthroughs
Previous studies have suggested that the HPV would have an essential role in the early stages of the ESCC. Besides ESCC tumor tissue, they also examined esophageal non-tumoral tissue from the same ESCC patients. The authors also analysed esophageal biopsies from patients with head and neck squamous cell carcinoma and from dyspeptic controls without cancer. They used a highly sensitive technique for detection of HPV with nested-polymerase chain reaction (PCR), taking the utmost care to avoid false positive results caused by samples contamination.
Applications
This study adds more evidence that HPV is not involved in esophageal carcinogenesis, and it also suggests the high prevalence of HPV in some ESCC studies can be due to contamination. Therefore, the results of this study reinforce the need of utmost care to avoid contamination in further protocols looking for HPV in tissue samples.
Terminology
Nested PCR uses two sequential sets of primers. The first pair of PCR primers amplifies a fragment similar to a standard PCR. The second primer set binds to sequences in the target DNA that are within the portion amplified by the first set. Thus, the second set of primers will bind and amplify target DNA within the products of the first reaction. The advantage of nested PCR is that if the wrong PCR fragment was amplified, the probability is quite low that the region would be amplified a second time by the second set of primers.
Peer review
Overall, it is a well written and easy to follow paper that reports a hot topic about the involvement of HPV infection in the development of ESCC. The technique and the sampling procedure used are correct. The topic of the contribution of HPV to ESCC is an important one since there is a vaccine for HPV that if related to squamous cell carcinoma could have a significant impact on the public health implications of this disease. This is a well prepared manuscript that reads well.
Footnotes
P- Reviewers Darwich L, McQuillan GM S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro U, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174–1185. [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57, vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamangar F INCA. Estimativa 2012: Incidência de câncer no Brasil. Rio de Janeiro: INCA;; 2011. p. 118. Available from: http: //www.inca.gov.br/estimativa/2012/ estimativa20122111.pdf. [Google Scholar]
- 6.Fagundes RB, Abnet CC, Strickland PT, Kamangar F, Roth MJ, Taylor PR, Dawsey SM. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking maté in healthy subjects from Rio Grande do Sul, Brazil. BMC Cancer. 2006;6:139. doi: 10.1186/1471-2407-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamangar F, Schantz MM, Abnet CC, Fagundes RB, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev. 2008;17:1262–1268. doi: 10.1158/1055-9965.EPI-08-0025. [DOI] [PubMed] [Google Scholar]
- 8.Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721–728. doi: 10.1136/jcp.55.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200–1203. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao GF, Roth MJ, Wei WQ, Abnet CC, Chen F, Lu N, Zhao FH, Li XQ, Wang GQ, Taylor PR, et al. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. Int J Cancer. 2006;119:1354–1359. doi: 10.1002/ijc.21980. [DOI] [PubMed] [Google Scholar]
- 11.Koh JS, Lee SS, Baek HJ, Kim YI. No association of high-risk human papillomavirus with esophageal squamous cell carcinomas among Koreans, as determined by polymerase chain reaction. Dis Esophagus. 2008;21:114–117. doi: 10.1111/j.1442-2050.2007.00726.x. [DOI] [PubMed] [Google Scholar]
- 12.Koshiol J, Kreimer AR. Lessons from Australia: human papillomavirus is not a major risk factor for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:1889–1892. doi: 10.1158/1055-9965.EPI-10-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weston AC, Prolla JC. Association between esophageal squamous cell carcinoma and human papillomavirus detected by Hybrid Capture II assay. Dis Esophagus. 2003;16:224–228. doi: 10.1046/j.1442-2050.2003.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Souto Damin AP, Guedes Frazzon AP, de Carvalho Damin D, Beck Biehl H, Abruzzi de Oliveira L, Auler R, Marroni C, Alexandre CO. Detection of human papillomavirus DNA in squamous cell carcinoma of the esophagus by auto-nested PCR. Dis Esophagus. 2006;19:64–68. doi: 10.1111/j.1442-2050.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 15.Greer CE, Wheeler CM, Manos MM. Sample preparation and PCR amplification from paraffin-embedded tissues. PCR Methods Appl. 1994;3:S113–S122. doi: 10.1101/gr.3.6.s113. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett WF, McNeil PE, Grimshaw WT, Selman IE, McIntyre WI. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978;274:215–217. doi: 10.1038/274215a0. [DOI] [PubMed] [Google Scholar]
- 17.Saveria Campo M, Moar MH, Jarrett WF, Laird HM. A new papillomavirus associated with alimentary cancer in cattle. Nature. 1980;286:180–182. doi: 10.1038/286180a0. [DOI] [PubMed] [Google Scholar]
- 18.Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, Yano T, Oku K, Miyata M, Nishiyama K. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068–2071. doi: 10.1002/1097-0142(19901115)66:10<2068::aid-cncr2820661005>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Makuuchi H, Machimura T, Shimada H, Mizutani K, Chino O, Kise Y, Nishi T, Tanaka H, Mitomi T, Horiuchi M, et al. Endoscopic screening for esophageal cancer in 788 patients with head and neck cancers. Tokai J Exp Clin Med. 1996;21:139–145. [PubMed] [Google Scholar]
- 20.Tincani AJ, Brandalise N, Altemani A, Scanavini RC, Valério JB, Lage HT, Molina G, Martins AS. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck. 2000;22:170–174. doi: 10.1002/(sici)1097-0347(200003)22:2<170::aid-hed9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Scherübl H, von Lampe B, Faiss S, Däubler P, Bohlmann P, Plath T, Foss HD, Scherer H, Strunz A, Hoffmeister B, et al. Screening for oesophageal neoplasia in patients with head and neck cancer. Br J Cancer. 2002;86:239–243. doi: 10.1038/sj.bjc.6600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evander M, Edlund K, Bodén E, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Comparison of a one-step and a two-step polymerase chain reaction with degenerate general primers in a population-based study of human papillomavirus infection in young Swedish women. J Clin Microbiol. 1992;30:987–992. doi: 10.1128/jcm.30.4.987-992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, Burk RD. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35:1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Ezra J, Johnson DA, Rossi J, Cook N, Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991;39:351–354. doi: 10.1177/39.3.1704393. [DOI] [PubMed] [Google Scholar]
- 25.Karlsen F, Kalantari M, Chitemerere M, Johansson B, Hagmar B. Modifications of human and viral deoxyribonucleic acid by formaldehyde fixation. Lab Invest. 1994;71:604–611. [PubMed] [Google Scholar]
- 26.Thompson CH, Rose BR. Deleterious effects of formalin/acetic acid/alcohol (FAA) fixation on the detection of HPV DNA by in situ hybridization and the polymerase chain reaction. Pathology. 1991;23:327–330. doi: 10.3109/00313029109063598. [DOI] [PubMed] [Google Scholar]
- 27.Coura R, Prolla JC, Meurer L, Ashton-Prolla P. An alternative protocol for DNA extraction from formalin fixed and paraffin wax embedded tissue. J Clin Pathol. 2005;58:894–895. doi: 10.1136/jcp.2004.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, Califano JA. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 29.Koshiol J, Wei WQ, Kreimer AR, Chen W, Gravitt P, Ren JS, Abnet CC, Wang JB, Kamangar F, Lin DM, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]


