Abstract
AIM: To investigate expression of stem cell marker Musashi-1 (Msi-1) in relationship to tumorigenesis and progression of intestinal-type gastric cancer (GC).
METHODS: Endoscopic biopsy specimens and surgical specimens were obtained, including 54 cases of intestinal-type GC, 41 high-grade intraepithelial neoplasia, 57 low-grade intraepithelial neoplasia, 31 intestinal metaplasia, and 36 normal gastric mucosa. Specimens were fixed in 10% paraformaldehyde, conventionally dehydrated, embedded in paraffin, and sliced in 4-μm-thick serial sections. Two-step immunohistochemical staining was used to detect Msi-1 and proliferating cell nuclear antigen (PCNA) expression. Correlation analysis was conducted between Msi-1 and PCNA expression. The relationship between Msi-1 expression and clinicopathological parameters of GC was analyzed statistically.
RESULTS: There were significant differences in Msi-1 and PCNA expression in different pathological tissues (χ2 = 15.37, P < 0.01; χ2 = 115.36, P < 0.01). Msi-1 and PCNA-positive cells were restricted to the isthmus of normal gastric glands. Expression levels of Msi-1 and PCNA in intestinal metaplasia were significantly higher than in normal mucosa (U = 392.0, P < 0.05; U = 40.50, P < 0.01), whereas there was no significant difference compared to low or high-grade intraepithelial neoplasia. Msi-1 and PCNA expression in intestinal-type GC was higher than in high-grade intraepithelial neoplasia (U = 798.0, P < 0.05; U = 688.0, P < 0.01). There was a significantly positive correlation between Msi-1 and PCNA expression (rs = 0.20, P < 0.01). Msi-1 expression in GC tissues was correlated with their lymph node metastasis and tumor node metastasis stage (χ2 = 12.62, P < 0.01; χ2 = 11.24, P < 0.05), but not with depth of invasion and the presence of distant metastasis.
CONCLUSION: Msi-1-positive cells may play a key role in the early events of gastric carcinogenesis and may be involved in invasion and metastasis of GC.
Keywords: Musashi-1, Stem cells, Gastric cancer, Precancerous lesions, Immunohistochemistry.
Core tip: Gastric cancer (GC) is currently thought to be a disease originating in stem cells. We detected expression of stem cell marker Musashi-1 (Msi-1) and proliferating cell nuclear antigen (PCNA) in intestinal-type GC and precancerous lesions. Expression of Msi-1 and PCNA in precancerous lesions was significantly higher than in normal mucosa, but lower than in intestinal-type GC. Msi-1 expression in GC tissues was correlated with lymph node metastasis and tumor node metastasis stage. These results suggest that expansion of Msi-1-positive cells is an early event in gastric carcinogenesis and may be involved in invasion and metastasis of GC.
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related death worldwide. Intestinal-type GC is believed to arise from an intestinal metaplasia to intraepithelial neoplasia sequence in gastric epithelial cells. The cancer stem cell theory indicates that cancers contain tumor-initiating stem cells possess the capacity for self-renewal and can cause the heterogeneous lineages of cancer cells constituting the tumor[1]. There is consensus that all gastric mucosal cells originate from stem cells[2]. The classical multistep model of gastric carcinogenesis requires a cell to receive multiple ‘‘hits’’ before it is transformed. Stem cells are long-lived; therefore, they are more likely to acquire these multiple hits and become transformed[3]. It was proposed that tumor-initiating cancer stem cells may be derived from normal stem cells; however, the lack of useful markers has made it difficult to characterize the stem cells in the human stomach and has hindered the study of the origin of GC.
Musashi-1 (Msi-1), an RNA-binding protein isolated as a mammalian homolog of a Drosophila protein, is selectively expressed in murine and human neural progenitor cells, including neural stem cells, can be used as a neural stem/progenitor cell marker, and plays an important role in the asymmetric division of neural stem cells[4]. In recent years, Msi-1 was also found to be expressed in tissues outside the nervous system. It has been confirmed that Msi-1 is preferentially expressed in the predicted stem cell regions of mouse and human intestinal crypts, suggesting that it can serve as a potential marker for intestinal stem/progenitor cells[5,6]. Msi-1 has also been observed in the stomach of chickens[7], mice[7], rats[8] and humans[9]. Akasaka et al[9] reported that Msi-1 is mostly expressed in the antrum and Murata et al[10] found that it is expressed in both the antrum and corpus of the human stomach. For the past several years, the functional role of Msi-1 in tumors has attracted increasing interest. Msi-1 overexpression has been reported in tumor tissues and cell lines, such as medulloblastoma[4], astrocytoma[11], retinoblastoma[12] and endometrial carcinoma[13].
Recently, Wang et al[14] selected 10 cases of intestinal-type GC taken from the transitional area between malignancy and adjacent normal mucosa for full section analysis. They found that Msi-1 was frequently expressed in both premalignant gastric lesions and invasive GC; however, the number of patients with premalignant gastric lesions in this study was low. Proliferating cell nuclear antigen (PCNA) is the auxiliary protein of DNA polymerase δ and can be used as a good indicator of GC cell proliferation and prognosis[15]. In the present study, to explore proliferation activity diversity of Msi-1-positive cells in the development of GC, we investigated the expression of Msi-1 and PCNA in intestinal-type GC and precancerous lesions, including 41 high-grade intraepithelial neoplasia, 57 low-grade intraepithelial neoplasia, and 31 intestinal metaplasia. The correlation between Msi-1 expression and various clinicopathological parameters was also studied. Additionally, we aimed to determine whether Msi-1 is a candidate marker of intestinal-type GC stem cells because this would be an important aspect of the function of Msi-1 in gastric carcinogenesis.
MATERIALS AND METHODS
Clinical and pathological features
Endoscopic biopsy antral specimens were obtained from September 2008 to January 2011 at Qianfoshan Hospital Affiliated to Shandong University, including: 36 cases of normal gastric mucosa (21 males and 15 females; aged 39-60 years, mean 57 ± 7.2 years); 31 cases of intestinal metaplasia (17 males and 14 females; aged 43-70 years, mean 54 ± 8.4 years); 57 cases of low-grade intraepithelial neoplasia (33 males and 24 females; aged 45-79 years, mean 58 ± 8.2 years); and 41 cases of high-grade intraepithelial neoplasia (23 males and 18 females; aged 46-80 years, mean 57 ± 9.6 years). The other 54 cases were intestinal-type GC, including 29 males and 25 females, aged 40-72 years, mean age 57 ± 9.5 years. All the pathological specimens were diagnosed and reviewed by three veteran pathologists.
According to tumor node metastasis (TNM) stage of the Union for International Cancer Control in 1997, GC is divided into I a, I b, II, III a, III b and IV stage. Twenty-seven cases of I a and I b stage were considered as the early clinical GC (I stage), including 17 male and 10 female patients, with a mean age of 56 ± 9.4 years. Twenty-seven cases of II-IV stage GC merged in the late stage GC (II-IV stage), including 12 male and 15 female patients, with a mean age of 57 ± 9.8 years. On the basis of the depth of cancer invasion, 20 cases were limited to mucosa and submucosa (≤ T1) and 34 cases broke through the submucosa (T2-4). In GC metastasis, there were 27 patients with lymph node metastasis and 27 without, or 14 patients with distant metastasis and 40 without. No significant difference in age and sex existed among all the groups of patients.
Immunohistochemistry
Specimens were fixed in 10% paraformaldehyde, conventionally dehydrated, embedded in paraffin, and sliced into 4-μm-thick serial sections. The microwave ethylene diamine tetraacetic acid (EDTA) (pH 9.0) antigen retrieval method was used to retrieve the antigen. A two-step immunohistochemical method was used to observe the antigen expression. Rabbit polyclonal anti-human Msi-1 antibody (ab52685; 1:300 dilution) and mouse anti-human PCNA monoclonal antibody (ab29; 1:1000 dilution) were purchased from Abcam Company (Cambridge, MA, United States).
Assessment of cell staining
Cells were considered positive for Msi-1 when the cytoplasm contained evenly stained yellow or brown granules. PCNA positivity was adjudged by the appearance of brown or yellow granules in the nucleus. Tissue sections were observed microscopically at × 400 magnification. Five representative regions were selected with 200 cells each. Msi-1 staining intensity was defined as follows: no staining or < 10% of cells stained, negative (-); 10%-30% stained cells, weakly positive (+); 31%-50% stained cells, positive (++); and > 50% stained cells, strongly positive (+++). PCNA staining intensity was defined as follows: no staining or < 25% of cells stained, negative (-); 25%-50% stained cells, weakly positive (+); 51%-75% stained cells, positive (++); and > 75% stained cells, strongly positive (+++).
Statistical analysis
All statistical analyses were performed using SPSS version 13.0. Measurement data were analyzed using Student’s t test and categorical data were studied using the χ2 test, Fisher’s exact test or nonparametric rank sum test. The Spearman correlation test was used to determine the correlation between expression of Msi-1 and PCNA. Correlations between Msi-1 expression and clinicopathological parameters were also statistically analyzed. Statistical significance was taken as P < 0.05.
RESULTS
Expression of Msi-1 and PCNA in different pathological tissues
There were significant differences in Msi-1 and PCNA expression in different pathological tissues (χ2 = 15.37, P < 0.01; χ2 = 115.36, P < 0.01). Msi-1 and PCNA-positive cells were restricted to the isthmus of gastric glands in normal gastric mucosa (Figures 1A and 2A). The expression levels of Msi-1 and PCNA in intestinal metaplasia were higher than in normal mucosa (U = 392.0, P < 0.05; U = 40.50, P < 0.01) and the distribution of Msi-1 and PCNA-positive cells was more diffuse (Figures 1B and 2B). Expression of Msi-1 and PCNA showed no significant difference among intestinal metaplasia, low and high-grade intraepithelial neoplasia (Figures 1C, 1D, 2C and 2D) (χ2 = 2.72, P > 0.05; χ2 = 1.64, P > 0.05). Msi-1 expression in intestinal-type GC was higher than in high-grade intraepithelial neoplasia (Figure 1E) (U = 798.0, P < 0.05) and PCNA expression in GC was also higher than in high-grade intraepithelial neoplasia (Figure 2E) (U = 688.0, P < 0.01) (Table 1).
Figure 1.
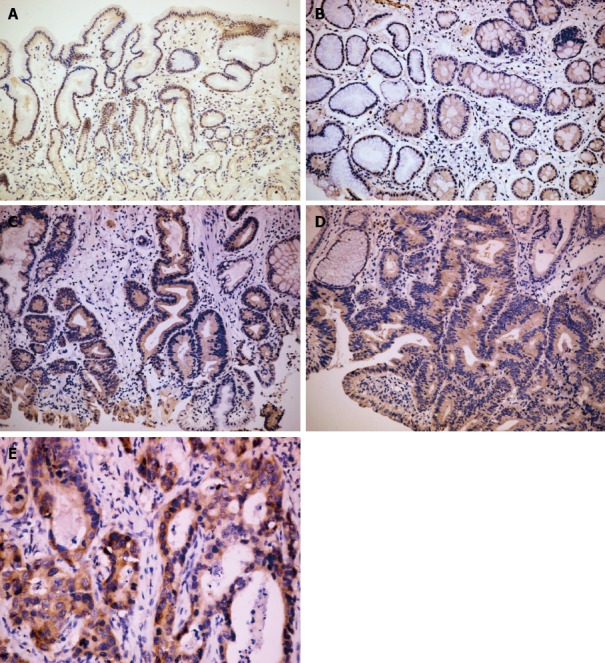
Immunohistochemical staining for Musashi-1 in different gastric tissues. A: A weak expression of Musashi-1 (Msi-1) was observed in the isthmus of normal gastric glands (× 100); B: The expression of Msi-1 was significantly increased in intestinal metaplastic mucosa (× 100); C: Msi-1 expression showed no significant difference between in low grade intraepithelial neoplasia (× 100); D: high grade intraepithelial neoplasia (× 100); E: The expression of Msi-1 was increased again in the intestinal type gastric cancer (× 400).
Figure 2.
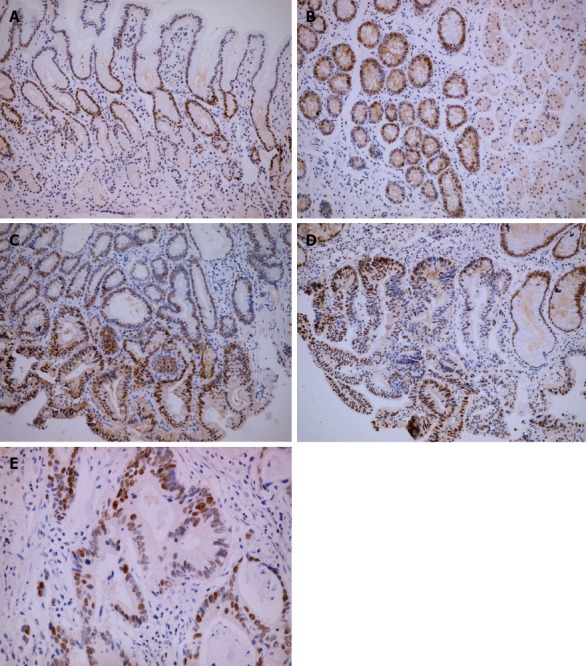
Immunohistochemical staining for proliferating cell nuclear antigen was increased along with the development of gastric carcinogenesis. A: The staining of proliferating cell nuclear antigen (PCNA) in the isthmus of normal gastric glands (× 100); B: Intestinal metaplastic mucosa (× 100); C: Low grade intraepithelial neoplasia (× 100); D: High grade intraepithelial neoplasia (× 100); E: Strong staining of PCNA in intestinal type gastric cancer (× 400).
Table 1.
Expression intensity of Musashi-1 and proliferating cell nuclear antigen in different pathological tissues
| Pathological tissues |
Msi-1 protein staining intensity (n) |
PCNA protein staining intensity (n) |
||||||
| - | + | ++ | +++ | - | + | ++ | +++ | |
| Normal gastric mucosa | 17 | 19 | 0 | 0 | 27 | 9 | 0 | 0 |
| Intestinal metaplasiaab | 7 | 21 | 3 | 0 | 0 | 9 | 10 | 12 |
| Low grade intraepithelial neoplasia | 21 | 32 | 4 | 0 | 0 | 9 | 29 | 19 |
| High grade intraepithelial neoplasia | 19 | 17 | 4 | 1 | 0 | 6 | 16 | 19 |
| Intestinal-type GCcd | 12 | 29 | 9 | 4 | 0 | 0 | 10 | 44 |
P < 0.05,
P < 0.01, significantly different vs normal gastric mucosa;
P < 0.05,
P < 0.01, significantly different vs high-grade intraepithelial neoplasia. PCNA: Proliferating cell nuclear antigen; Msi-1: Musashi-1; GC: Gastric cancer.
Relationship between Msi-1 expression and GC invasion and metastasis
No significant difference existed in Msi-1 expression between tumor invasion depth ≤ T1 and T2-4 (χ2 = 7.37, P > 0.05). Msi-1 expression was higher in GC with than without lymph node metastasis (χ2 = 12.62, P < 0.01). There was no significant difference between Msi-1 expression in GC with and without distant metastasis (χ2 = 7.06, P > 0.05). Msi-1 expression was higher in GC II -IV stage group than in stage I group (χ2 = 11.24, P < 0.05) (Figure 3, Table 2).
Figure 3.
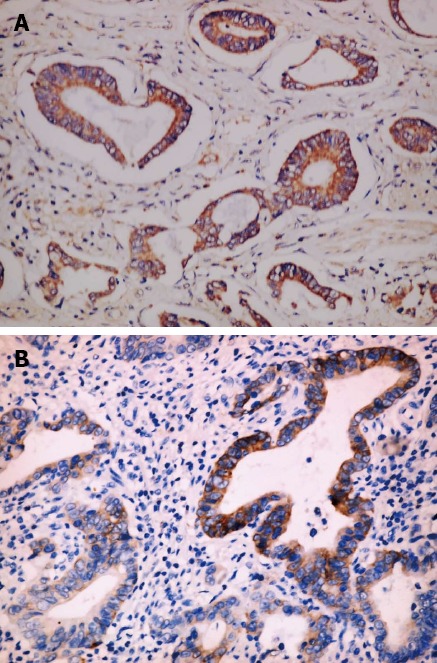
Musashi-1 expresses higher in intestinal type gastric cancer classified as stage II-IV. A: Type gastric cancer classified as stage II-IV; B: Those classified as stage I (× 400).
Table 2.
Relationship between Musashi-1 expression and clinicopathological parameters
| Variable | Group | No. |
Msi-1 staining intensity |
|||
| – | + | ++ | +++ | |||
| Infiltration depth | ≤ T1 | 20 | 8 | 9 | 3 | 0 |
| T2-4 | 34 | 4 | 20 | 6 | 4 | |
| Lymph node metastasisa | NO | 27 | 11 | 9 | 5 | 2 |
| YES | 27 | 1 | 20 | 4 | 2 | |
| Distant metastasis | NO | 40 | 12 | 21 | 5 | 2 |
| YES | 14 | 0 | 8 | 4 | 2 | |
| TNM stagingb | Stage I | 27 | 11 | 10 | 4 | 2 |
| Stage II-IV | 27 | 1 | 19 | 5 | 2 | |
There was a significant difference between Musashi-1 (Msi-1) expression and clinicopathological parameters in Gastric cancer.
P < 0.01 and
P < 0.05. TNM: Tumor node metastasis.
Correlation between expression of Msi-1 and PCNA
Spearman’s bivariate correlation analysis was made between Msi-1 and PCNA expression in different pathological tissues. Msi-1 and PCNA expression showed a significant positive correlation (rs = 0.20, P < 0.01) (Table 3).
Table 3.
Relationship between Musashi-1 and proliferating cell nuclear antigen expression
| Msi-1 protein staining intensity |
PCNA protein staining intensity |
Total | |||
| Negative | Weak positive | Positive | Strong positive | ||
| Negative | 10 | 23 | 17 | 26 | 76 |
| Weak positive | 17 | 7 | 40 | 54 | 118 |
| Positive | 0 | 2 | 7 | 11 | 20 |
| Strong positive | 0 | 1 | 1 | 3 | 5 |
| Total | 27 | 33 | 65 | 94 | 219 |
PCNA: Proliferating cell nuclear antigen; Msi-1: Musashi-1.
DISCUSSION
The epithelial stem cells of the adult stomach are thought to be present in the proliferative region of the isthmus of the gastric glands. They constantly regenerate and migrate bidirectionally, up to the mucosal surface and down to the gland base, as they differentiate into mature cells of the gastric unit[16]. In the present study, immunohistochemistry demonstrated the presence of small numbers of PCNA-positive cells concentrated in the isthmus region of normal gastric glands, suggesting that this region is the proliferative zone of the gastric glands. We found that Msi-1 expression was also limited to the isthmus region of the gastric glands where adult putative stem cells are located. The positive correlation between the distribution of Msi-1-positive cells and that of the proliferative regions in the gastric glands suggests that Msi-1-positive cells have stem cell characteristics in the human stomach. Akasaka et al[9] have reported a similar expression pattern of Msi-1 in the human antrum, suggesting that Msi-1 can be useful for identifying stem cells in the human stomach.
Our observations indicate that Msi-1 expression can correlate positively with proliferative activity in the stomach, but the correlation coefficient is low. All the cells in the gastrointestinal epithelium are renewed within a short time; thus, it is possible that precursor cells, not stem cells, in the proliferative zone produce a large number of mature cells within a short time. Indeed, it has been proposed that the proliferative zone has a pyramid-like structure, consisting of various cell layers with stem cells on top, and that the number of stem cells produced is small[17]. In our study, PCNA expression showed proliferative activity in all the cells in the gastric tissue. We believe that the expression of Msi-1 in the human stomach is associated with cell proliferation; however, because the number of stem cells is small, the correlation is not close.
Intestinal metaplasia is the precancerous lesion of intestinal-type GC[18]. Akasaka et al[9] reported that Msi-1 expression was markedly decreased in intestinal metaplasia cells of the human stomach. They believe that intestinal metaplasia is a consequence of abnormal differentiation of stem cells, in which tissue-specific stem cells in the stomach may not differentiate into any of the normal intestinal epithelial phenotypes. In our study, however, more Msi-1-positive cells were observed in the intestinal metaplasia than in the normal gastric mucosa and the distribution was also more diffuse. It is known that Msi-1 is not a tissue-specific marker of epithelial stem cells. We believe that Msi-1 can be expressed in epithelial stem cells in both gastric mucosa and intestinal metaplasia, but proliferation of stem cells is different, which may be due to the mode of fission. In normally growing gastric tissues, stem cells undergo a slow but constant self-renewal by asymmetric division and give rise to all the various gastric epithelial cell types by differentiation[19]. These properties are essential to their normal role in gastric tissues because stem cells maintain tissue homeostasis by regulating cell turnover, depending on the demand at any given time. Surprisingly, experimental and mathematical modeling studies have indicated that intestinal crypt stem cells divide symmetrically and stochastically, and not with the asymmetry observed in stem cell division in some other tissues[20,21]. Similarly, symmetric division among intestinal metaplasia stem cells probably leads to accelerated division of stem cells, adapting to high proliferative activity. Murata et al[10] demonstrated that Msi-1 was expressed in gastric glands with intestinal metaplasia where proliferative activity was relatively high. Similar results were also obtained in the study of Wang et al[14]. Therefore, by influencing dividing cells, Msi-1 might regulate metaplastic changes.
We found that there was no significant difference between intestinal metaplasia and low and high-grade intraepithelial neoplasia for the expression of PCNA and Msi-1, suggesting that there was similar proliferation of epithelial stem cells in the gastric precancerous lesions. An in vitro study in primary cultures of intestinal epithelium showed that Msi-1 overexpression promotes the proliferation of stem cells and activates Wnt and Notch pathways. Moreover, Msi-1-overexpressing cells exhibited tumorigenic properties in xenograft experiments[22]. Using mitochondrial DNA mutations as a marker of clonal expansion, Mcdonald et al[23] investigated how mutations expand in gastric mucosa showing signs of intestinal metaplasia. They have shown that intestinal metaplastic crypts are clonal, possess multiple stem cells, and that fission is a mechanism by which intestinal metaplasia spreads. The expansion and spread of mutated gastric stem cells is one mechanism of carcinogenesis in the human stomach. Our findings suggest that expansion of Msi-1-positive cells in the intestinal metaplasia mucosa may be an early event in initiating the intestinal metaplasia - intraepithelial neoplasia - adenocarcinoma cascade. Stem cell amplification caused by deregulation of this self-renewal process may play a key role in the early events of gastric carcinogenesis.
We found that there were significantly more PCNA-positive and Msi-1 positive cells in intestinal-type GC than in gastric precancerous lesions, suggesting that Msi-1 could be used as a potential marker for intestinal-type GC stem cells, and that cancer stem cells have stronger proliferation compared to stem cells in precancerous lesions. Cancer stem cells are able to sustain and propagate tumors and give rise to invasive lesions and metastases[24]. In agreement with our data, Msi-1-positive cells may be involved in tumor invasion and metastasis because Msi-1 expression correlated with lymph node metastasis and TNM stage of GC. Msi-1 plays a fundamental role in maintaining stem cells in an undifferentiated state and it has been implicated in proliferative pathology in various tissues where Msi-1 may be acting to promote self-renewal of tumor cells with stem cell-like properties[25-27]. Small cell pulmonary carcinoma is usually an aggressive neoplasm with high metastatic potential and recurrence. Moreira et al[28] reported that small cell carcinomas demonstrate diffuse expression of Msi-1, whereas focal or isolated positivity is seen in most other types of pulmonary carcinoma. In human colorectal cancer, Msi-1 protein expression is significantly higher in tissue samples classified as stage III than stage I or II[29]. It was demonstrated that siRNA-mediated knockdown of Msi-1 in the HCT116 colon adenocarcinoma xenografts resulted in the arrest of tumor growth[30]. Knockdown of Msi-1 expression by siRNA induced apoptosis and a severe decline in cell numbers in 5637 bladder carcinoma cells[31]. These results suggest that Msi-1 plays an important role in tumorigenesis and tumor progression. We speculate that Msi-1 overexpression contributes to maintain cancer stem cells in an undifferentiated state, increasing their capacity for self-renewal or proliferation, thus promoting GC invasion and metastasis.
Shen et al[32] proposed that cancer may arise from a long development process of tumor-initiating cells - precancerous stem cells - cancer stem cells - cancer, which is in parallel to histological changes of hyperplasia - precancer - carcinoma, accompanied by clonal evolutionary epigenetic and genetic alterations. In the present study, we demonstrated that expansion of Msi-1-positive cells appeared to be an early event in the process of gastric carcinogenesis and proliferation of Msi-1-positive cells was different in GC and precancerous lesions. The precancerous stem cells, representing the early stage of developing cancer stem cells, have the potential for both benign and malignant differentiation, depending on environmental cues[33]. Therefore, a broad therapeutic approach to GC may be achievable through rational targeting of precancerous stem cells. More work is needed to investigate the differentiation of Msi-1-positive cells between GC and precancerous lesions. However, Msi-1 may eventually represent a biomarker that can be used for early diagnosis of GC or to monitor cancer progression and response to therapy.
COMMENTS
Background
Gastric cancer (GC) remains one of the leading causes of global cancer mortality. The gastric epithelium is continuously regenerated by gastric stem cells, which give rise to various kinds of epithelial cells. GC is currently thought to be a disease originating in stem cells and it is hypothesized that cancer stem cells drive cancer growth and metastasis. However, the lack of useful markers has made it difficult to characterize the stem cells in the human gastric mucosa and has hindered study of the origin of GC. The RNA binding protein Musashi-1 (Msi-1) is a putative stem cell marker. However, the role of Msi-1 in GC and precancerous lesions is not fully understood.
Research frontiers
Msi-1, a neural RNA-binding protein, was initially identified as a neuronal stem cell marker. In recent years, Msi-1 has been found in tissues outside the nervous system and identified as a putative intestinal and gastric stem cell marker. For the past several years, overexpression of Msi-1 has been reported in many tumor tissues and cell lines. High levels of Msi-1 expression in glioma and astrocytoma indicate a poor prognosis. Knockdown of Msi-1 in the HCT116 colon adenocarcinoma xenografts resulted in the arrest of tumor growth. These results suggest that Msi-1 plays an important role in tumorigenesis and tumor progression.
Innovations and breakthroughs
Msi-1 has been proposed as a putative stem cell marker in the mouse intestine and human stomach but little is known about the role of such markers in the overall pathway of gastric carcinogenesis. Moreover, the results of previous studies in intestinal metaplasia are different. In this study, the authors explored proliferation diversity of Msi-1-positive cells in the development of GC and found that the expression levels of Msi-1 and proliferating cell nuclear antigen (PCNA) in intestinal metaplasia were significantly higher than in normal mucosa, while there were no significant differences compared with intraepithelial neoplasia. Msi-1 and PCNA expression in intestinal-type GC was higher than in intraepithelial neoplasia. Msi-1 expression in GC tissues correlated with their lymph node metastasis and TNM stage. There was a significantly positive relationship between Msi-1 and PCNA expression. These results suggest that Msi-1-positive cells may play a key role in the early events of gastric carcinogenesis, and may be involved in invasion and metastasis of GC.
Applications
The study results contribute to a better understanding of the associations between putative stem cell marker expression and tumorigenesis and progression of GC. The knowledge gained by studying cancer stem cells in gastric mucosa will support the development of novel therapeutic strategies for GC. Msi-1 may eventually represent a biomarker that can be used for early diagnosis of GC or to monitor cancer progression and response to therapy.
Terminology
Cancer stem cells are defined as the unique subpopulation in tumors that possess the ability to initiate tumor growth and sustain self-renewal, as well as metastatic potential. Musashi is an evolutionarily conserved family of RNA-binding proteins that is preferentially expressed in the nervous system. The first member of the Musashi family was identified in Drosophila. Its mammalian homolog, Msi-1, is a neural RNA-binding protein that is selectively expressed in murine and human neural stem cells, can be used as a neural stem cell marker, and plays important roles in the maintenance of stem cell states, as well as in the regulation of differentiation of stem cells.
Peer review
The study was well designed and performed. The authors detected stem cell marker Msi-1 and PCNA expression in the multistep process of gastric carcinogenesis. The results are interesting and suggest that the expansion of Msi-1-positive cells is an early event in the process of gastric carcinogenesis and may be involved in invasion and metastasis of GC.
Footnotes
Supported by Jinan Science and Technology Bureau for Independent Innovation Projects of Universities and Research Institutes in Jinan city, China, No. 201102060
P- Reviewers Dubois A, Huang ZH, Tsianos EV S- Editor Qi Y L- Editor Roemmele A E- Editor Ma S
References
- 1.Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: the ‘heartbeat’ of gastric cancer. J Gastroenterol. 2013;48:781–797. doi: 10.1007/s00535-012-0712-y. [DOI] [PubMed] [Google Scholar]
- 2.Han ME, Oh SO. Gastric stem cells and gastric cancer stem cells. Anat Cell Biol. 2013;46:8–18. doi: 10.5115/acb.2013.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang S, Yan YM, Mao F, Gu HB, Cao HL, et al. Oct4, a novel marker for human gastric cancer. J Surg Oncol. 2009;99:414–419. doi: 10.1002/jso.21270. [DOI] [PubMed] [Google Scholar]
- 4.Nakano A, Kanemura Y, Mori K, Kodama E, Yamamoto A, Sakamoto H, Nakamura Y, Okano H, Yamasaki M, Arita N. Expression of the Neural RNA-binding protein Musashi1 in pediatric brain tumors. Pediatr Neurosurg. 2007;43:279–284. doi: 10.1159/000103307. [DOI] [PubMed] [Google Scholar]
- 5.Yu T, Chen QK, Gong Y, Xia ZS, Royal CR, Huang KH. Higher expression patterns of the intestinal stem cell markers Musashi-1 and hairy and enhancer of split 1 and their correspondence with proliferation patterns in the mouse jejunum. Med Sci Monit. 2010;16:BR68–BR74. [PubMed] [Google Scholar]
- 6.Samuel S, Walsh R, Webb J, Robins A, Potten C, Mahida YR. Characterization of putative stem cells in isolated human colonic crypt epithelial cells and their interactions with myofibroblasts. Am J Physiol Cell Physiol. 2009;296:C296–C305. doi: 10.1152/ajpcell.00383.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai R, Okano H, Yasugi S. Correlation between Musashi-1 and c-hairy-1 expression and cell proliferation activity in the developing intestine and stomach of both chicken and mouse. Dev Growth Differ. 2005;47:501–510. doi: 10.1111/j.1440-169X.2005.00825.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagata H, Akiba Y, Suzuki H, Okano H, Hibi T. Expression of Musashi-1 in the rat stomach and changes during mucosal injury and restitution. FEBS Lett. 2006;580:27–33. doi: 10.1016/j.febslet.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Akasaka Y, Saikawa Y, Fujita K, Kubota T, Ishikawa Y, Fujimoto A, Ishii T, Okano H, Kitajima M. Expression of a candidate marker for progenitor cells, Musashi-1, in the proliferative regions of human antrum and its decreased expression in intestinal metaplasia. Histopathology. 2005;47:348–356. doi: 10.1111/j.1365-2559.2005.02223.x. [DOI] [PubMed] [Google Scholar]
- 10.Murata H, Tsuji S, Tsujii M, Nakamura T, Fu HY, Eguchi H, Asahi K, Okano H, Kawano S, Hayashi N. Helicobacter pylori infection induces candidate stem cell marker Musashi-1 in the human gastric epithelium. Dig Dis Sci. 2008;53:363–369. doi: 10.1007/s10620-007-9858-5. [DOI] [PubMed] [Google Scholar]
- 11.Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J. Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol. 2008;86:31–45. doi: 10.1007/s11060-007-9439-7. [DOI] [PubMed] [Google Scholar]
- 12.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–832. [PMC free article] [PubMed] [Google Scholar]
- 13.Götte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schüring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–329. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, et al. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658–665. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czyzewska J, Guzińska-Ustymowicz K, Pryczynicz A, Kemona A, Bandurski R. Immunohistochemical evaluation of Ki-67, PCNA and MCM2 proteins proliferation index (PI) in advanced gastric cancer. Folia Histochem Cytobiol. 2009;47:289–296. doi: 10.2478/v10042-009-0042-y. [DOI] [PubMed] [Google Scholar]
- 16.Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11–24. doi: 10.1007/s10120-009-0537-4. [DOI] [PubMed] [Google Scholar]
- 17.Fukui T, Takeda H, Shu HJ, Ishihama K, Otake S, Suzuki Y, Nishise S, Ito N, Sato T, Togashi H, et al. Investigation of Musashi-1 expressing cells in the murine model of dextran sodium sulfate-induced colitis. Dig Dis Sci. 2006;51:1260–1268. doi: 10.1007/s10620-006-8046-3. [DOI] [PubMed] [Google Scholar]
- 18.Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193–201. doi: 10.1111/j.1440-1746.2008.05774.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei B, Chen L, Li R, Tian J. Stem cells in gastrointestinal cancers: a matter of choice in cell fate determination. Expert Rev Anticancer Ther. 2010;10:1621–1633. doi: 10.1586/era.10.52. [DOI] [PubMed] [Google Scholar]
- 20.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 22.Rezza A, Skah S, Roche C, Nadjar J, Samarut J, Plateroti M. The overexpression of the putative gut stem cell marker Musashi-1 induces tumorigenesis through Wnt and Notch activation. J Cell Sci. 2010;123:3256–3265. doi: 10.1242/jcs.065284. [DOI] [PubMed] [Google Scholar]
- 23.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Vries RG, Huch M, Clevers H. Stem cells and cancer of the stomach and intestine. Mol Oncol. 2010;4:373–384. doi: 10.1016/j.molonc.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Diaz PC, Burton TL, Burns SC, Hung JY, Penalva LO. Musashi1 modulates cell proliferation genes in the medulloblastoma cell line Daoy. BMC Cancer. 2008;8:280. doi: 10.1186/1471-2407-8-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, Zhao C, Lagoo A, Gerrard G, Foroni L, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–768. doi: 10.1038/nature09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobryshev YV, Freeman AK, Botelho NK, Tran D, Levert-Mignon AJ, Lord RV. Expression of the putative stem cell marker Musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23:580–589. doi: 10.1111/j.1442-2050.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 28.Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol. 2010;23:889–895. doi: 10.1038/modpathol.2010.68. [DOI] [PubMed] [Google Scholar]
- 29.Fan LF, Dong WG, Jiang CQ, Xia D, Liao F, Yu QF. Expression of putative stem cell genes Musashi-1 and beta1-integrin in human colorectal adenomas and adenocarcinomas. Int J Colorectal Dis. 2010;25:17–23. doi: 10.1007/s00384-009-0791-2. [DOI] [PubMed] [Google Scholar]
- 30.Sureban SM, May R, George RJ, Dieckgraefe BK, McLeod HL, Ramalingam S, Bishnupuri KS, Natarajan G, Anant S, Houchen CW. Knockdown of RNA binding protein musashi-1 leads to tumor regression in vivo. Gastroenterology. 2008;134:1448–1458. doi: 10.1053/j.gastro.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Nikpour P, Baygi ME, Steinhoff C, Hader C, Luca AC, Mowla SJ, Schulz WA. The RNA binding protein Musashi1 regulates apoptosis, gene expression and stress granule formation in urothelial carcinoma cells. J Cell Mol Med. 2011;15:1210–1224. doi: 10.1111/j.1582-4934.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen R, Tao L, Xu Y, Chang S, Van Brocklyn J, Gao JX. Reversibility of aberrant global DNA and estrogen receptor-alpha gene methylation distinguishes colorectal precancer from cancer. Int J Clin Exp Pathol. 2009;2:21–33. [PMC free article] [PubMed] [Google Scholar]
- 33.Shen R, Ye Y, Chen L, Yan Q, Barsky SH, Gao JX. Precancerous stem cells can serve as tumor vasculogenic progenitors. PLoS One. 2008;3:e1652. doi: 10.1371/journal.pone.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]


