Summary
Proteomic analyses of protein-electrophile adducts generally employ affinity capture of the adduct moiety, which enables global analyses, but is poorly suited to targeted studies of specific proteins. We describe a targeted molecular-probe approach to study modifications of the molecular chaperone heat-shock protein 90 (Hsp90), which regulates diverse client proteins. Noncovalent affinity capture with a biotinyl analog of the HSP90 inhibitor geldanamycin enables detection of the native protein isoforms Hsp90α and Hsp90β and their phosphorylated forms. We applied this probe to map and quantify adducts formed on Hsp90 by 4-hydroxynonenal (HNE) in RKO cells. This approach was also applied to measure the kinetics of site-specific adduction of selected Hsp90 residues. A protein-selective affinity capture approach is broadly applicable for targeted analysis of electrophile adducts and their biological effects.
Keywords: electrophile, Hsp90, affinity probe, geldanamycin, kinetic analysis
1. Introduction
Covalent modification of proteins by electrophilic metabolites is a key step in drug toxicity mediated by cytochrome P-450 enzymes. A growing body of literature describes the identification of proteins that are modified by electrophiles (1). Progress in this field has been driven by the combination of affinity capture for electrophile adducts and mass spectrometry (MS) for identification of modified proteins (2–5). This strategy requires affinity-labeling chemistry to efficiently capture adducts and typically identifies a broad range of modified proteins. On the other hand, mechanistic studies often target a particular protein or a small family of functionally related proteins, where the analytical challenge is to selectively capture these proteins and their modified forms. This may be achieved by immunoprecipitation, but satisfactory reagents are often lacking and covalent adducts may interfere with antibody binding.
We have approached the problem of targeted protein adduct analysis by employing an affinity-tagged, small-molecule inhibitor to capture target proteins (6). In our studies, we focused on the heat-shock protein 90 (Hsp90) family proteins, which are regulators of diverse cellular functions and are targets of electrophiles (2,4,7). The inhibitor is a derivative of geldanamycin, a natural product that selectively targets Hsp90 and binds to the N-terminal ATPase domain (8). Geldanamycin induces a conformational change that releases Hsp90 client proteins and co-chaperones. Here we describe the use of the commercially available reagent geldanamycin-PEG-biotin (9) to isolate both cytosolic forms of Hsp90, for analysis of Hsp90 adducts by liquid chromatography-tandem mass spectrometry (LC-MS/MS). We characterized adduction sites on Hsp90 captured from cells and treated in vitro with HNE. We also identified additional adduction sites on both Hsp90α and Hsp90β isolated from HNE-treated RKO cells. Reaction rates for HNE adduction at several sites on both isoforms of Hsp90 both in vitro and in intact cells were characterized by combining geldanamycin-biotin capture with targeted, label-free LC-MS/MS quantification. Our results demonstrate the utility of protein-selective affinity capture for targeted analysis of electrophile adducts and their biological effects.
2. Materials
2.1. RKO cell culture, HNE treatment and preparation of cellular lysate
McCoy’s 5A medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Atlas Biologicals, Fort Collins, CO).
Solution of trypsin (0.25%) (Gibco/BRL, Bethesda, MD).
The lipid electrophile 4-hydroxy-2-nonenal (HNE) (Cayman Chemical, Ann Arbor, MI) is dissolved in ethanol (64 mM), stored in aliquots at −80°C, and added to tissue-culture dishes as required.
NETN lysis buffer: 50 mM HEPES, pH 7.5, 150 mM NaCl, 0.5% Igepal. Can be prepared as a stock solution and be stored at 4°C.
Ethyl Alcohol 200 Proof, Absolute, Anhydrous (EtOH).
Sodium borohydride (NaBH4). A 2M stock solution is stored under the hood at room temperature. The final concentration of sodium borohydride in the experimental samples is 2mM.
Ice cold phosphate-buffered saline 1×, pH 7.2 (1× PBS).
Protease inhibitor cocktail: 1.0 mM phenylmethylsulfonylfluoride, 1.0 mM N-ethylmaleimide, leupeptin (10 µg/mL), aprotinin (10 µg/mL), pepstatin (10 µg/mL).
Phosphatase inhibitor cocktail: 1.0 mM sodium fluoride, 1.0 mM sodium molybdate, 1.0 mM sodium orthovanadate, 10.0 mM β-glycerophosphate.
BCA Protein Assay Kit (Pierce, Rockford, IL).
Distilled H2O.
2.2. Geldanamycin-Biotin Protein Capture
Geldanamycin and Geldanamycin-Biotin (Enzo Life Sciences, Plymouth Meeting, PA).
High-capacity Neutravidin agarose resin beads (Thermo Scientific, Rockford, IL).
1M sodium chloride prepared in 1× PBS.
Dithiothreitol (DTT). A 1M stock solution is stored at −20°C. The working concentration of DTT is 50mM.
NuPAGE® LDS Sample Buffer (4×) (Invitrogen, Carlsbad, CA).
2.3. Western Blot Procedure
NuPAGE® Bis-Tris 10% SDS-PAGE gels (Invitrogen, Carlsbad, CA).
Precision Plus Protein Standard Kaleidoscop Molecular Weight Marker (Bio-Rad Laboratories, Hercules, CA).
20×-MOPS running buffer: 50 mM MOPS, 50 mM Tris Base, 0.1% SDS, 1 mM EDTA, pH 7.7 (Invitrogen, Carlsbad, CA).
20× NuPAGE® Transfer Buffer (Invitrogen, Carlsbad, CA).
Polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA).
4× NuPAGE® LDS Sample Buffer (Invitrogen, Carlsbad, CA).
Methanol (Sigma-Aldrich, St. Louis, MO).
1× TBS-Tween (25 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.5).
Blocking Buffer for Near Infra Red Fluorescent Western Blotting (Rockland, Gilbertsville, PA).
Anti-HNE-Michel reduced rabbit polyclonal antibody (EMD biosciences, San Diego, CA).
Anti-heat-shock protein 90 (Hsp90) mouse monoclonal antibody, used for western blotting (BD Biosciences, San Jose, CA).
Anti-heat-shock protein 90 (Hsp90) rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and protein G-agarose (Roche Applied Science, Indianapolis, IN), used for immunoprecipitation of Hsp90 protein.
AlexaFluor®680-conjugated fluorescent secondary antibodies (Molecular Probes, Eugene, OR).
Odyssey™ Infrared Imaging System and Odyssey software (Li-Cor, Lincoln, NE).
2.4. LC-MS-/MS procedures
Trypsin Gold, Mass Spectrometry Grade (Promega, Madison, WI), is reconstituted in 50 mM acetic acid to a final concentration of 1 mg/mL. Aliquots are stored frozen at −20°C. Trypsin is diluted in 25 mM ammonium bicarbonate to 0.01 mg/mL and used at a ratio of 1:50 (trypsin:protein).
Safe Stain Blue (Invitrogen, Carlsbad, CA) is used for 1h staining followed by distaining in water overnight.
Iodoacetamide (IAM); DTT; ammonium bicarbonate, acetonitrile, trifluoroacetic acid (TFA) (Sigma-Aldrich, St. Louis, MO).
All reagents are prepared immediately before use (See Note 1).
3. Methods
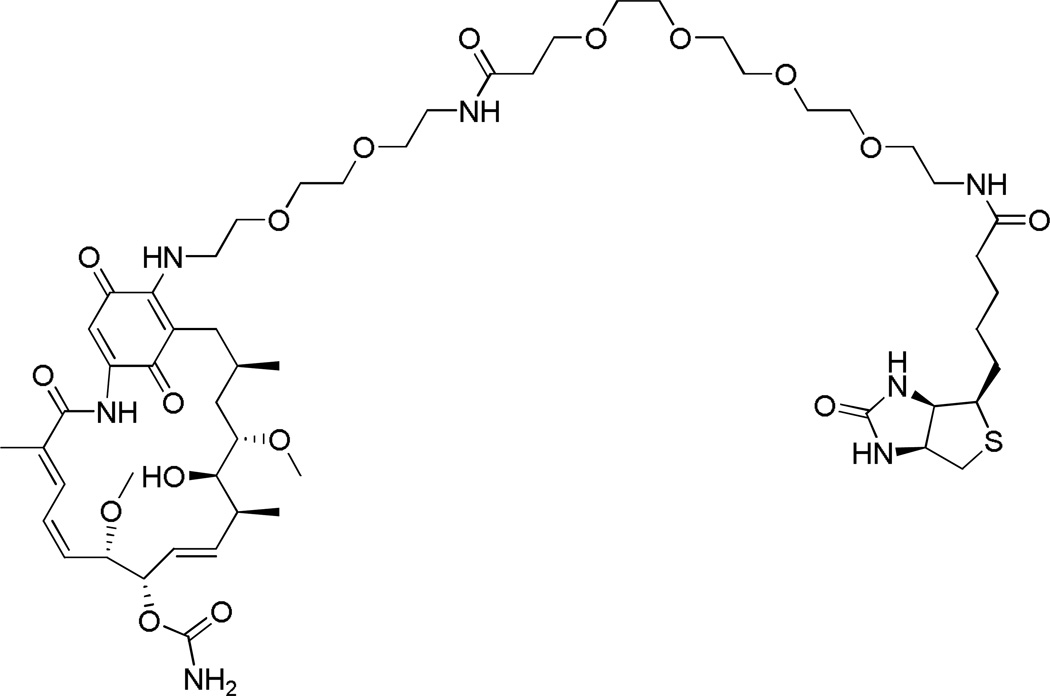
Mapping and quantitative comparison of site-specific adduction reactions on individual proteins still remains an analytical challenge. To identify sites of HNE modification of Hsp90 protein, a biotin-tagged analog of the Hsp90 inhibitor geldanamycin was used to capture endogenous protein from cellular lysates of treated and untreated cells. This compound, geldanamycin-biotin (Figure 1) (9), binds tightly to all isoforms of Hsp90 and displaces proteins bound to Hsp90 as client proteins and co-chaperones by inducing conformational changes around the ATP-binding site (8). To generate adducts on the Hsp90 protein, we treat RKO cells with exogenous HNE in culture, and we use LC-MS/MS to identify and characterize adduction sites on protein isolated from treated cells. Following capture with Neutravidin-agarose, the biotinylated affinity-tagged proteins are eluted and resolved by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), digested with trypsin and the resulting peptides are analyzed by LC-MS/MS. The subsequent immunoblot analysis allows for a rapid screening of the Hsp90 protein-selective affinity capture and determination of whether these proteins have been adducted. Reaction rates for HNE adduction at several sites on both isoforms of Hsp90 in intact cells were characterized by combining geldanamycin-biotin capture with targeted, label-free LC-MS/MS quantification. Our results demonstrate the utility of protein-selective affinity capture for targeted analysis of electrophile adducts.
Fig. 1.
Chemical structure of the Hsp90-capture reagent geldanamycin-biotin. Reprinted with permission from (Connor, R. E., et al. (2011) Chemical Res. Toxicol. 24: 1275–1282). Copyright (2011) American Chemical Society.
3.1. Isolation of Hsp90 from cellular lysates using affinity-tagged inhibitor Geldanamycin
3.1.1. RKO cell culture and preparation of cellular lysate
Grow RKO human colorectal carcinoma cells to 80% confluence in McCoy’s 5A medium supplemented with 10% fetal bovine serum, at 37°C in an atmosphere of 95% air/5% CO2.
Wash the confluent cells plated in 150-mm culture dishes with 5 ml of cold 1× phosphate-buffered saline. Use a disposable cell scraper to harvest the cells directly in 5 ml of fresh 1× phosphate-buffered saline, and centrifuge at 100×g for 5 min. The phosphate-buffered saline, pH 7.2, is slowly aspirated off the cell pellet. Cell pellet can be stored at −80°C until further use.
Lyse the cell pellets from each 150-mm culture plate on ice in 2 mL of cold NETN buffer supplemented with protease inhibitor and phosphatase inhibitor cocktails. Sonicate the lysate and incubate on ice for 30 min.
Clear the lysate by centrifugation at 10,000×g for 10 min to remove cellular debris. Determine the total protein concentration of the supernatant using the BCA protein assay.
Adjust the protein concentration to 1 mg/mL for each sample using lysis buffer and use it fresh each time.
A 50-µl aliquot from each sample is transferred into a new pre-labeled 1.5-ml Eppendorf tube. To these sample aliquots are added 3.5 µL DTT (1M stock solution to a final concentration of 50 mM) and 16.5 µL of NuPage LDS Sample buffer (4×), and the samples are heated for 10min at 95°C, and then stored at −20°C. This sample is referred to as the Input (Figure 2).
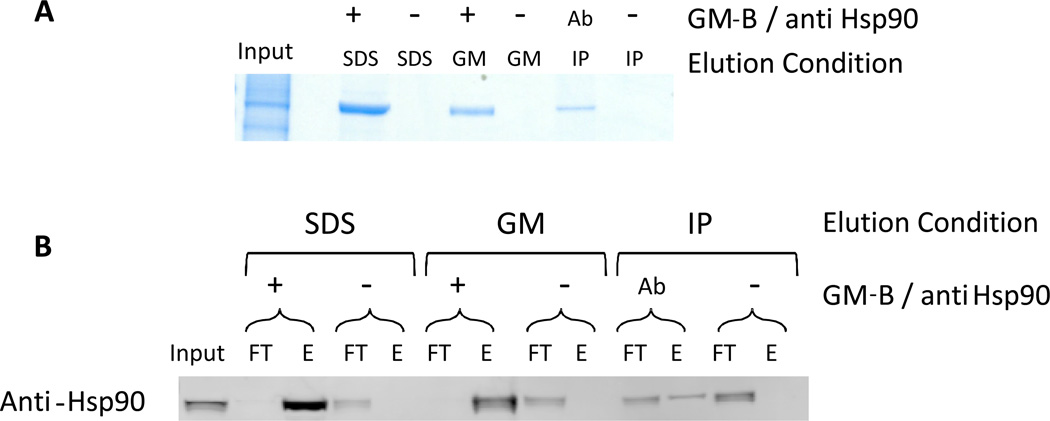
Fig. 2.
Geldanamycin-biotin efficiently captures Hsp90 from cell lysates. A) SDS-PAGE analysis with Safe Stain Blue staining of Hsp90 captured with geldanamycin-biotin (GM-B) or Hsp90 antibody. The input lane contains 5 µg of cell lysate. Lanes are labeled as negative control (−), with GM-B (+), or with anti-Hsp90 (Ab). GM-B-captured protein was eluted either by boiling in LDS sample buffer or with 2 mM geldanamycin (GM). Immunoprecipitated Hsp90 was eluted by boiling in LDS sample buffer. B) Western blot analysis with anti-Hsp90 antibody of the flow-through and elution from GM-B capture and immunoprecipitation of Hsp90. Lanes are labeled as flow-through (FT) or eluant (E), with elution and capture conditions noted as in part A. Flow-through lanes contain 2.5 µg of total protein. Reprinted with permission from (Connor, R. E., et al. (2011) Chemical Res. Toxicol. 24: 1275–1282). Copyright (2011) American Chemical Society.
3.1.2. Geldanamycin-Biotin Protein Capture
Incubate a 250-µL aliquot of diluted cell lysate (1 mg/mL) with geldanamycin-biotin (40 µg in DMSO, 20 µg/µL stock) for at least 3h at 4°C with gentle rotation (Figure 1). Before application to the resin, dilute the lysate further, to a final volume of 1 ml, to give a protein concentration of 0.25 mg/mL.
Equilibrate Neutravidin-agarose resin by washing a 50:50 (w/v) bead slurry three times with lysis buffer. Use 375 µL of bead slurry per sample. Each time the beads are centrifuged at 10,000×g for two min and the supernatant is carefully discarded.
Incubate diluted lysates containing the affinity capture reagent geldanamycin-biotin with Neutravidin-agarose resin previously equilibrated with lysis buffer, and rotate samples at 4°C overnight.
At the end of the incubation period, centrifuge samples at 10,000×g for two min and transfer 50µL of the supernatant into a new pre-labeled 1.5-mL Eppendorf tube. DTT (50mM) and NuPage LDS Sample buffer (4×) are added to each sample. Heat samples for 10 min at 95°C and store at −20°C. This sample is referred to as the Flow Through (Figure 2). Transfer the remainder of the supernatant into another 1.5-mL Eppendorf tube and store at −20°C.
Wash the bound proteins four times with 1 mL of lysis buffer. After each wash step, centrifuge the beads at 10,000×g for 1 min and discard the supernatant.
Elute proteins from the beads in 150µL of either NuPage LDS Sample buffer (4×) with DTT (50mM) or 2 mM geldamycin-biotin in lysis buffer. Heat samples for 10min at 95°C. These samples are referred to as the Eluates (Figure 2).
Subject samples of the protein input, flow through and eluates to gel separation by SDS-PAGE (Figure 2A) for further LC-MS/MS analyses or immunoblot analyses with antibodies directed against Hsp90 proteins (Figure 2B).
3.1.3. Immunoprecipitation of Hsp90
Incubate a 1-mL aliquot of diluted cell lysate (1 mg/mL) with anti-Hsp90 rabbit polyclonal antibody (20 µg) for at least 3h at 4°C with gentle rotation.
Equilibrate protein G-agarose resin by washing a 50:50 (w/v) bead slurry three times with lysis buffer. Use 50 µl of bead slurry per sample. Each time the beads are centrifuged at 10,000×g for two min and the supernatant is carefully discarded.
Incubate diluted lysates containing the anti-Hsp90 rabbit polyclonal antibody bound to Hsp90 proteins with protein G-agarose resin previously equilibrated with lysis buffer, and rotate samples at 4°C overnight.
At the end of the incubation period, centrifuge samples at 10,000×g for two min and transfer 50µL of the supernatant into a new pre-labeled 1.5-ml Eppendorf tube. Add DTT (50mM) and NuPage LDS Sample buffer (4×) to each sample. Heat samples for 10 min at 95°C and store at −20°C. This sample is referred to as the Flow Through (Figure 2). Transfer the remainder of the supernatant into another 1.5-ml Eppendorf tube and store at −20 °C.
Wash the bound proteins four times with 1 mL of lysis buffer. After each wash step, centrifuge the beads at 10,000×g for 1 min and discard the supernatant.
Elute proteins from the beads in 100 µL of NuPage LDS Sample buffer (4×) with DTT (50mM). Heat samples for 10min at 95°C. These samples are referred to as the Eluates (Figure 2).
3.1.4. Western Blotting
Subject samples of the protein input, flow through and eluates (10 µL) from each experimental condition to immunoblot analysis with rabbit polyclonal antibody directed against Hsp90 proteins.
Heat the input, flow through and eluate samples from each experimental condition for 10min at 95°C.
Set up a NuPage Bis-Tris 10% SDS-PAGE gel and follow the manufacturer’s directions to run the gel. One lane of the gel is reserved for the protein standard by adding 5µl of the Precision Plus Protein Standard Kaleidoscope Molecular Weight Marker.
Load 10µl of the samples into the gel in the following order: input, flow through and eluate. This will allow for a more efficient comparison of bands during analysis.
Transfer proteins electrophoretically onto a PVDF membrane, by following the directions of the manufacturer of the transfer apparatus. Block non-specific primary-antibody binding by placing the membrane into 5 mL of Blocking buffer (1:1 1× TBS-Tween: Blocking Buffer) for 1 h at room temperature on a rocking platform (See Note 2).
Prepare dilutions of the primary antibody to proteins of interest, according to the manufacturer’s directions.
Incubate the membrane with primary antibody overnight at 4°C while shaking on an orbital shaker.
Using multiple changes of 1× TBS-Tween, wash membranes for a total of 30min before adding the secondary antibody.
Prepare appropriate dilutions of AlexaFluor®680-labeled secondary antibodies and incubate with the membrane for 1hr at room temperature while shaking. Wash the membrane using multiple changes of 1× TBS-Tween for a total of 30min, before scanning.
Immunoreactive proteins are visualized using the Odyssey™ System and software as described by the manufacturer.
3.1.5. In-gel trypsin digestion and MS analysis
Resolve the protein, purified either by Neutravidin capture, using the geldanamycin-biotin affinity probe, or by immunoprecipitation, using anti-Hsp90 polyclonal antibody as described above (eluate), by 10% SDS-PAGE using NuPAGE Bis-Tris gels and stain with Safe Stain Blue for 1h, followed by detaining in water overnight.
Desired bands corresponding to the correct molecular weight (~ 90 kDa) are excised from the gel and subjected to in-gel digestion with trypsin. Carefully chop each excised band into 1-mm cubes, place in a 1.5 mL Eppendorf tube containing 100 µL of 100 mM ammonium bicarbonate, pH 8.0, and incubate at room temperature for 15 min.
Reduce samples with 10 µL of 45 mM DTT for 20 min at 55°C and alkylate with 10 µL of 100 mM IAM for 20 min at room temperature in the dark.
Discard the liquid and add 100 µL of acetonitrile:50 mM ammonium bicarbonate (50:50, v/v) to distain the samples. Incubate at room temperature for 15 min, and then discard the liquid. Repeat this step twice.
Dehydrate the gel pieces by incubation for 15 min at room temperature in 100 µL of acetonitrile, and discard the supernatant.
Digest the rehydrated gel pieces with trypsin (50 µL of Trypsin Gold (0.01 mg/mL in 25 mM ammonium bicarbonate)) overnight at 37°C.
Extract the peptides twice with 100 µL of 60% acetonitrile, 0.1% TFA, each for 15 min at room temperature; combine the extracts. Evaporate the liquid under vacuum and resuspend peptides in 10 – 20 µL of H2O (0.1% formic acid) for LC-MS/MS analysis.
Perform LC-MS/MS analysis using an LTQ ion-trap mass spectrometer (Thermo Electron, San Jose, CA) equipped with an Eksigent nanoLC (Dublin, CA) and Thermo Surveyor HPLC pump, Nanospray source and Xcalibur 1.4 instrument control.
Carry out the liquid chromatography at ambient temperature at a flow rate of 0.6 µL/min using a gradient mixture of 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B). Acquire centroid MS/MS scans using an isolation width of 2 m/z, an activation time of 30 ms, an activation Q of 0.250 and 30% normalized collision energy, using 1 microscan with a max ion time of 100 ms for each MS/MS scan.
Before analysis, tune the mass spectrometer using the synthetic peptide TpepK (AVAGKAGAR). Some parameters may vary slightly from experiment to experiment, but typically the tune parameters are as follows: spray voltage of 2 KV, a capillary temperature of 150°C, a capillary voltage of 50 V and tube lens of 120 V.
Resolve the peptides on 100 µm × 11 cm fused-silica capillary column (Polymicro Technologies, LLC Phoenix, AZ) packed with 5 µm, 300Å Jupiter C18 (Phenomenex, Torrance, CA). Introduce the peptides eluting from the capillary tip into the LTQ source with a capillary voltage of approximately 2 kV. The heated capillary is operated at 150 °C and 40 V. Acquire the MS/MS spectra in the data-dependent scanning mode, consisting of a full scan obtained for eluting peptides in the range of 350–2000 m/z, followed by four data-dependent MS/MS scans. Record MS/MS spectra using dynamic exclusion of previously analyzed precursors for 30 s with a repeat duration of 2 min.
3.1.6. Database Searching
The "ScanSifter" algorithm, an in-house developed software (10), reads MS/MS spectra stored as centroid peak lists from Thermo RAW files and transcodes them to mzData v1.05 files. Spectra that contain fewer than six peaks do not result in mzData files. Only MS/MS scans are written to the mzData files; MS scans are excluded. If 90% of the intensity of a tandem mass spectrum appears at a lower m/z than the precursor ion, a single precursor charge is assumed; otherwise, the spectrum is processed under both double and triple precursor charge assumptions.
Tandem mass spectra are assigned to peptides from the IPI Human database version 3.56 (May 05, 2009; 153,182 proteins) by the MyriMatch algorithm (11). To estimate false discovery rates, each sequence of the database was reversed and concatenated to the database, for a total of 135,674 entries. Candidate peptides are required to feature trypsin cleavages or protein termini at both ends, though any number of missed cleavages is permitted. All cysteines are expected to undergo carboxamidomethylation and are assigned a mass of 160 kDa. All methionines are allowed to be oxidized. Precursor ions are required to fall within 1.25 m/z of the position expected from their average masses, and fragment ions are required to fall within 0.5 m/z of their monoisotopic positions. The database searches produced raw identifications in pepXML format.
Peptide identification, filtering and protein assembly are done with the IDPicker algorithm (12). Initial filtering takes place in multiple stages. First, IDPicker filters raw peptide identification to a target false-discovery rate (FDR) of 5%. The peptide filtering employs reversed-sequence database-match information to determine thresholds that yield an estimated 5% FDR for the identifications of each charge state by the formula (13) FDR = (2R)/(R+F), where R is the number of passing reversed-peptide identifications and F is the number of passing forward (normal orientation)-peptide identifications. The second round of filtering removes proteins supported by less than two distinct peptide identifications in the analyses. Indistinguishable proteins are recognized and grouped. Parsimony rules are applied to generate a minimal list of proteins that explain all of the peptides that pass the entry criteria.
3.2. Analysis of Electrophile-Protein Adducts
3.2.1 Hsp90 modification by HNE in RKO cells
Grow RKO human colorectal carcinoma cells to 80% confluence in McCoy’s 5A medium supplemented with 10% fetal bovine serum, at 37°C in an atmosphere of 95% air / 5% CO2.
Carry out the treatments with either varying concentrations of HNE dissolved in ethanol for 1 h at 37°C, or with 250 µM HNE for varying time intervals between 0 and 60 min. Wash confluent cells plated in 150-mm culture dishes first with 5 mL of cold phosphate-buffered saline, then incubate with 0, 50, 100 or 250 µM HNE delivered in 10 ml fresh McCoy’s 5A medium without fetal bovine serum. The total concentration of ethanol per culture should be ≤0.1% of the total medium volume.
Expose cells to electrophile for 1 h at 37°C in an atmosphere of 95% air/5% CO2, then, using a disposable cell scraper, scrape off cells from the culture dishes directly in the treatment medium, and centrifuge at 100×g for 5 min. Slowly aspirate off the treatment medium (supernatant) and wash the cell pellets twice with cold phosphate-buffered saline, pH 7.4, before freezing.
Lyse cell pellets, as described above, and reduce the Michael adducts of HNE in cell lysates with 2 mM NaBH4, before capture with geldanamycin-biotin.
Visualize the accumulation of HNE-adducted proteins by immunoblot analysis of treated cellular lysates with an anti-HNE rabbit polyclonal antibody directed against reduced Michael adducts of HNE (Figure 3).
For isolation of adducted Hsp90 protein from HNE-treated cellular lysates, reduce the incubation with geldanamycin-biotin to 1h and the incubation with Neutravidin resin to 1.5h at 4°C, followed by four washes with 1 ml of lysis buffer. After the last wash is removed, add 1/5 bed volume of LDS sample buffer to the resin and incubate at 95°C for 10 minutes, to elute Hsp90. This sample is then subjected to gel electrophoresis and the Hsp90 band is excised for digestion trypsin and analysis by LC-MS/MS.
To provide accurate mass characterization of HNE adducts on Hsp90, a Thermo LTQ-Orbitrap instrument with an Eksigent Nano 1D Plus pump and autosampler is used. Liquid chromatography is performed as described above. The data-dependent inclusion list screening is carried out with the following parameters, derived from previously described methods (14). An Orbitrap MS scan from m/z 300–2000 at 60,000 resolution is followed by 10 LTQ ion trap MS/MS scans. If eight or more ions on the inclusion list are present, then the eight most intense ions are selected for tandem MS analysis. If fewer than eight ions on the inclusion list are present, then those ions plus the most intense ions in the initial scan (up to eight) are targeted. Dynamic exclusion is enabled, with a repeat count of 3 and a repeat duration of 10 s. The exclusion list size is 50 and the exclusion duration is 20 s. Threshold intensity for triggering peak detection is set at 100 with collision energy of 28% set for the entire list. The data are analyzed using MonsterMod, an in-house developed algorithm (15), to identify MS/MS spectra corresponding to Hsp90 peptides with mass shifts greater than 1 Da. Mass shifts of 158 Da correspond to the reduced Michael adducts of HNE. Spectra of the adducted precursor and fragment ions are verified manually, with a requirement of less than 10 ppm error for peptide-adduct precursor m/z measurements.
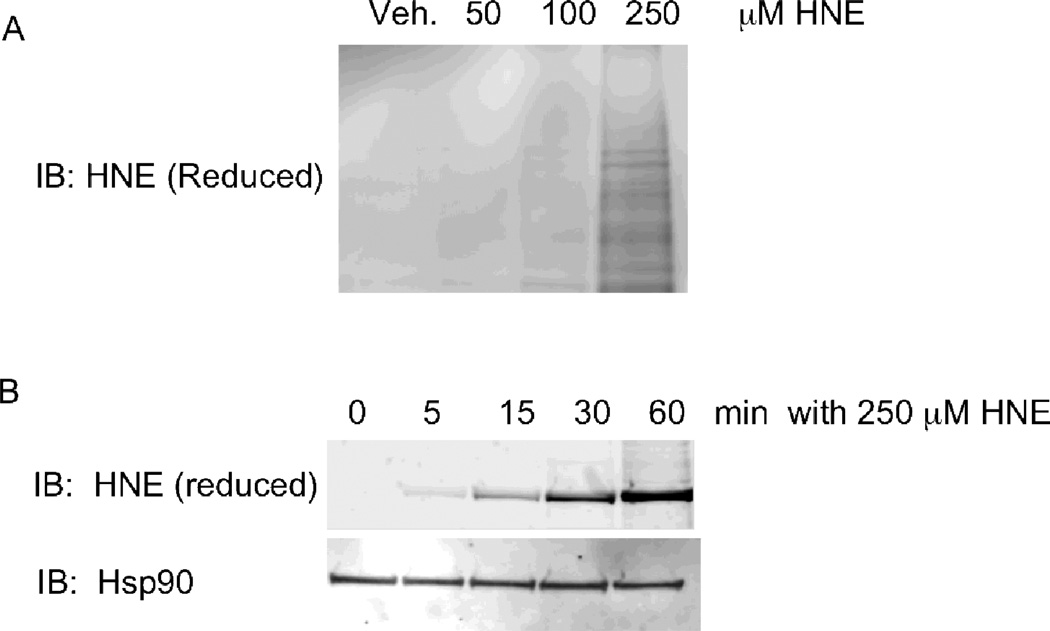
Fig. 3.
Analysis of HNE adduction in RKO cells. A) Immunoblot analysis of lysates from cells treated with EtOH, 50, 100 or 250 µM HNE and detected with an antibody to reduced HNE adducts. B) Immunoblot analysis of Hsp90 isolated from RKO cells treated with 250 µM HNE for 0, 5, 15, 30 or 60 minutes and detected with an antibody to reduced HNE adducts. Loading control for total Hsp90 detected with anti-Hsp90 is shown below. Reprinted with permission from (Connor, R. E., et al. (2011) Chemical Res. Toxicol. 24: 1275–1282). Copyright (2011) American Chemical Society.
3.2.2. Kinetic Analysis of Hsp90 adduction by HNE in culture
Kinetic analysis of modification sites is performed using a label-free quantification approach we described previously (16,17). This approach measures signals for adducted peptides by LC-MS/MS using a Thermo LTQ instrument. Each peptide adduct is monitored by targeting the m/z of the doubly or triply charged precursor for MS/MS. Two unmodified peptides from each protein are also targeted in the same manner. Specific product ions generated by MS/MS fragmentation of the targeted peptide adducts and reference peptides are extracted with Thermo Xcalibur software and peak areas are integrated. Three product-ion signals are monitored for each peptide or peptide adduct and the peak area for each MS/MS transition is summed, to generate a peak area for each peptide. MS/MS data for both doubly and triply charged precursor ions are acquired in some cases and the product ions yielding the greatest signal are used for subsequent analysis.
The peak area for each peptide adduct is normalized to the average signal of the two unmodified reference peptides at each sample time point. The peak area reflecting HNE adduction after 4 hours treatment is used as the endpoint for reactions with isolated Hsp90, in order to determine an observed rate of reaction.
Values of kobs are calculated from plots of the ratio of normalized peak areas for adducted peptides at each time point to the average normalized peak area for the adducted peptide at the specified endpoint.
Data are fitted to a single exponential association with GraphPad Software (Figure 4).
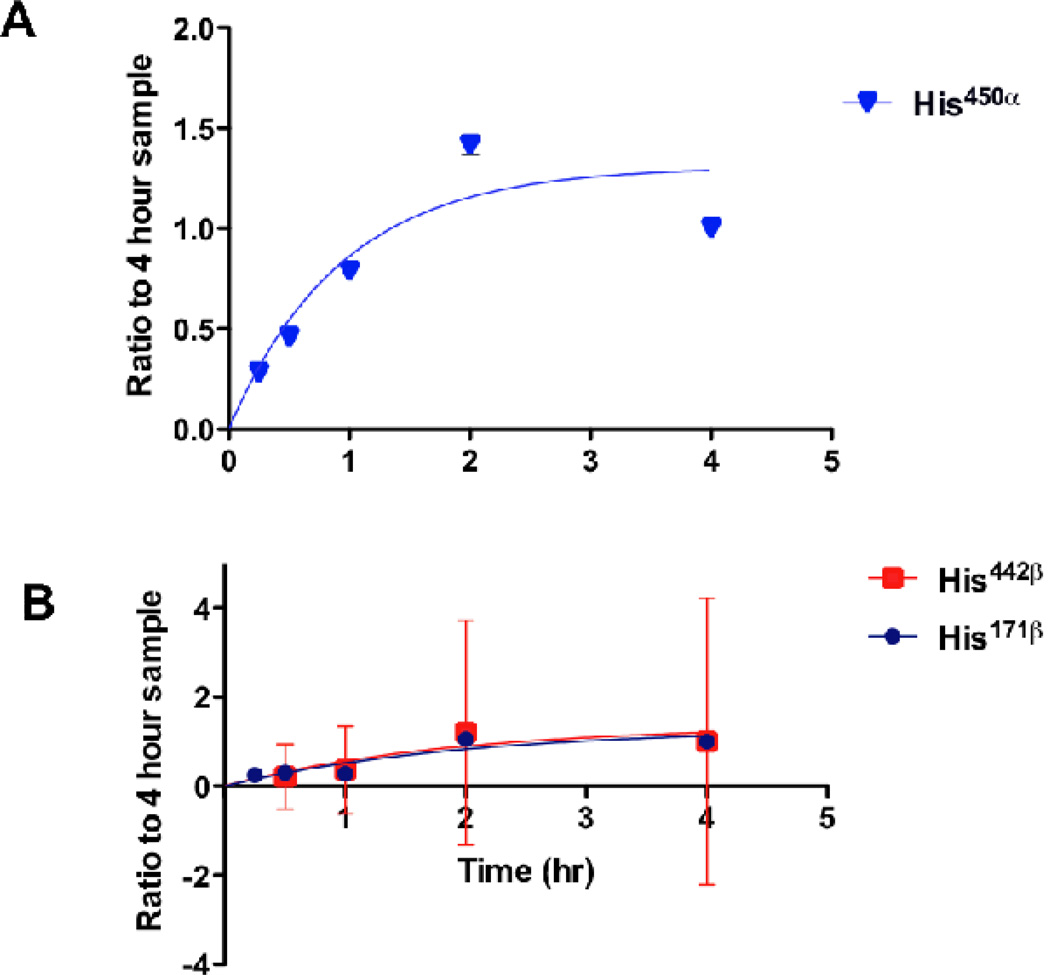
Fig. 4.
Determination of the relative reaction rates of HNE-modified Hsp90 peptides after treatment of RKO cells with 250 µM HNE. A) Reaction kinetics of His450α with HNE in Hsp90α. Replicate analyses of 4–8 samples were used for each time point. Non-linear single-exponential regression was used to fit the curve. B) Reaction kinetics of His442β and His171β with HNE in Hsp90β. Replicate analyses of 4–8 samples were used for each time point. Non-linear single exponential regression was used to fit the curve. Reprinted with permission from (Connor, R. E., et al. (2011) Chemical Res. Toxicol. 24: 1275–1282). Copyright (2011) American Chemical Society.
Acknowledgments
This work was supported by National Institutes of Health Grants ES013125 and the National Foundation for Cancer Research.
Footnotes
All chemical reagents are purchased from commercial sources and are used without further purification. All reagents should be prepared fresh before each use.
Incubation with primary antibody overnight at 4°C gives a much stronger signal for western blotting than does two-hour incubation at room temperature.
References
- 1.Liebler DC. Protein Damage by Reactive Electrophiles: Targets and Consequences. Chem Res Toxicol. 2008;21:117–128. doi: 10.1021/tx700235t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Codreanu SG, Zhang B, Sobecki SM, Billheimer DD, Liebler DC. Global analysis of protein damage by the lipid electrophile 4-hydroxy-2-nonenal. Mol Cell Proteomics. 2009;8:670–680. doi: 10.1074/mcp.M800070-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HY, Tallman KA, Liebler DC, Porter NA. An azido-biotin reagent for use in the isolation of protein adducts of lipid-derived electrophiles by streptavidin catch and photo-release. Mol Cell Proteomics. 2009;8:2080–2089. doi: 10.1074/mcp.M900121-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem Res Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Li Q, Yang X, van Breemen RB, Bolton JL, Thatcher GR. Analysis of protein covalent modification by xenobiotics using a covert oxidatively activated tag: raloxifene proof-of-principle study. Chem Res Toxicol. 2005;18:1485–1496. doi: 10.1021/tx0501738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor RE, Marnett LJ, Liebler DC. Protein-selective capture to analyze electrophile adduction of hsp90 by 4-hydroxynonenal. Chemical Research in Toxicology. 2011;24:1275–1282. doi: 10.1021/tx200157t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J.Pharmacol.Exp.Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 8.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clevenger RC, Raibel JM, Peck AM, Blagg BS. Biotinylated geldanamycin. J Org Chem. 2004;69:4375–4380. doi: 10.1021/jo049848m. [DOI] [PubMed] [Google Scholar]
- 10.Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ, Halvey PJ, Schilling B, Drake PM, Gibson BW, Tabb DL. IDPicker 2.0: Improved protein assembly with high discrimination peptide identification filtering. J Proteome Res. 2009;8:3872–3881. doi: 10.1021/pr900360j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabb DL, Fernando CG, Chambers MC. MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res. 2007;6:654–661. doi: 10.1021/pr0604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Chambers MC, Tabb DL. Proteomic parsimony through bipartite graph analysis improves accuracy and transparency. J Proteome Res. 2007;6:3549–3557. doi: 10.1021/pr070230d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe JD, Keshishian H, Chang B, Addona TA, Gillette MA, Carr SA. Accurate inclusion mass screening: a bridge from unbiased discovery to targeted assay development for biomarker verification. Mol Cell Proteomics. 2008;7:1952–1962. doi: 10.1074/mcp.M800218-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen BT, Davey SW, Ham AJ, Liebler DC. P-Mod: an algorithm and software to map modifications to peptide sequences using tandem MS data. J Proteome Res. 2005;4:358–368. doi: 10.1021/pr0498234. [DOI] [PubMed] [Google Scholar]
- 16.Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem Res Toxicol. 2008;21:705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 17.Connor RE, Marnett LJ, Liebler DC. Protein-selective capture to analyze electrophile adduction of hsp90 by 4-hydroxynonenal. Chem Res Toxicol. 24:1275–1282. doi: 10.1021/tx200157t. [DOI] [PMC free article] [PubMed] [Google Scholar]