Abstract
Background
Atopic dermatitis (AD) is common in children; however, persistence of AD with or without asthma, is less common. Longitudinal studies remain limited in their ability to characterize how IgE antibody responses evolve in AD, and their relationship to asthma.
Objective
To use a cross-sectional study design of children with active AD to analyze age-related differences in IgE antibodies and relation to wheeze.
Methods
IgE antibodies to food and inhalant allergens were measured in children with active AD (5 months to 15 years of age, n=66), with and without history of wheeze.
Results
Whereas IgE antibodies to foods persisted at a similar prevalence and titer throughout childhood, IgE antibodies to all aeroallergens rose sharply into adolescence. From birth, the chance of sensitization for any aeroallergen increased for each 12-month increment in age (OR≥1.21, p≤0.01), with the largest effect observed for dust mite (OR=1.56, p<0.001). A steeper age-related rise in IgE antibody titer to dust mite, but no other allergen, was associated with more severe disease. Despite this, sensitization to cat was more strongly associated with wheeze (OR=4.5, p<0.01), and linked to Fel d 1 and Fel d 4, but not Fel d 2. Comparison of cat allergic children with AD to those without, revealed higher titers to Fel d 2 and Fel d 4 (p<0.05), but not Fel d 1.
Conclusions and Clinical Relevance
Differences in sensitization to cat and dust mite among young children with AD may aid in identifying those at increased risk for disease progression and development of asthma. Early sensitization to cat and risk for wheeze among children with AD may be linked to an increased risk for sensitization to a broader spectrum of allergen components from early life. Collectively, our findings argue for early intervention strategies designed to mitigate skin inflammation in children with AD.
Keywords: Atopic dermatitis, asthma, wheeze, IgE antibodies, age, food allergy, aeroallergens, cat, dust mite
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition that is common in early childhood. It is widely held that the development of AD among a subset of patients, reflects onset of the “atopic march”, which is characterized by progression to respiratory allergies, including asthma and allergic rhinitis [1-3]. This theory is supported by longitudinal studies that cite AD in early life as a predictor of asthma in later childhood [4-6]. Beyond this, patient characteristics that can be evaluated by health care providers, and which might help predict those children with AD at increased risk for asthma, are poorly understood. This is largely owing to the wide variability in risk for development of asthma among AD patients enrolled in longitudinal studies (~10-25%) [7-12].
Sensitization to multiple allergens along with high titer serum IgE antibodies (ab) are each a feature of severe AD in childhood. Regardless of the presence of AD, the prevalence of sensitization to food allergens predominates in infancy, while IgE ab to inhalant allergens appears later [13-17]. In AD, approximately one half of children with moderate to severe disease will have clinically relevant IgE antibodies to food allergens [18-20]. As children grow older, the majority of allergen-specific IgE ab is directed against inhalant sources [21, 22].
Surprisingly little is known regarding age-related differences in IgE ab responses in highly atopic children with AD and the link to asthma phenotype. This is, in part, attributable to the limitations of longitudinal studies which necessitate the enrollment of large cohorts of children with AD in order to track age-specific trends in IgE. Moreover, those large studies typically determine the presence or absence of AD by questionnaire. The present cross-sectional study was designed to evaluate the emergence and evolution of IgE ab directed against diverse allergens among a cohort of children with physician-diagnosed active AD using a rigorous statistical approach, and to examine the relation to wheeze. Our findings yield new insight into the patterns of specific IgE ab responses in childhood disease, and identify sensitization to multiple components of cat allergen as risk factors for wheeze in cat allergic children with AD.
Methods
Subjects
Children with AD were enrolled sequentially through the University of Virginia Pediatric Allergy Clinic. Inclusion criteria were physician-diagnosed eczema with visible itchy rash and at least a topical steroid requirement. Children without AD were recruited through the Allergy Clinic. Exclusion criteria for all subjects included treatment with oral corticosteroids within the last 30 days; systemic immunosuppressive therapy (including azathioprine or cyclosporine A) during the last 6 months; treatment with systemic steroids for any chronic inflammatory disorder other than asthma or eczema; and presence or development of malignancy or any serious medical condition involving major organ systems. Disease severity was assessed by SCORAD (0-25: mild; >25-50: moderate; and >50: severe) [23, 24]. Data related to eczema, including the age of onset, family history of atopy, and foods avoided because they were thought to worsen eczema, were determined by parental questionnaire. Patients were categorized as having a history of wheeze based on physician-documented wheeze occurring within 12 months of study enrollment. Informed consent and subject assent, when indicated, were obtained. The study was approved by the University of Virginia Institutional Review Board (protocols #12309 and #15662).
Analysis of Serum IgE Antibodies
Venous blood samples were collected from all children at the time of enrollment. Levels of serum total IgE and IgE ab to 7 allergens (dust mite, cat dander, egg white, cow's milk, peanut, ryegrass, and ragweed) were measured by Pharmacia CAP assay (Uppsala, Sweden). In addition, a subset of serum samples from children (n=30) were tested for IgE ab to microbial antigens (Candida albicans, Staphylococcal enterotoxin A (SEA), and Staphylococcal enterotoxin B (SEB)). Data for IgE ab to these allergens was available for comparison from 47 adults with physician-diagnosed AD [22], and data for 5 additional allergens (dog epithelium, German cockroach, timothy grass, common silver birch, wheat) was available for 38 of these adults. Sera from children with (n=24) and without (n=17) AD who were cat allergic were assayed for IgE ab to cat components, Fel d 1, Fel d 2, and Fel d 4.
Statistical Methods
Multiple linear regression was used to model serum IgE ab levels in relation to age. A power analysis showed that with a sample size of 66 children with AD, we would have at least 0.80 statistical power to reject the a priori null hypothesis of no age-related change in IgE ab titer if the true correlation between age and IgE ab titer was greater than 0.36. An ordinary least-squares regression model was developed for each allergen, in which the sole predictor variable was the age of the child. Potential linear and nonlinear associations were examined by incorporating linear and nonlinear restricted cubic functions of age into the model. For each allergen, the lower limit of assay detection (0.35 IU/ml) was assigned to those children who had no detectable IgE ab. For cat allergen component testing, the lower limit of assay detection was assigned to 0.3 IU/ml. The Cochran-Armitage test was used to analyze age-related trends in the prevalence of allergen sensitization, independent of IgE antibody magnitude, across four age groups (age <2 years; 2 to < 5 years; 5 to 10 years, and 10 to 15 years). Age-related chance of allergen sensitization was analyzed by logistic regression and generalized estimating equation regression models. The Jonckheere Terpstra test was used to analyze age-related trends in the contribution of specific IgE to total IgE across age groups. Quantitative correlations between IgE ab titers were analyzed by Spearman's test. Among wheezing and non-wheezing children, the between-group prevalence for dichotomous variables was compared by Fisher exact test, and continuous variables analyzed by the Wilcoxon rank sum test.
Results
Patient Characteristics
The study population comprised 66 children with AD (5 months to 15 years of age) who were categorized as follows: (1) 18 subjects under 2 years of age; (2) 23 subjects that were pre-school age (2 to <5 years): (3) 12 pre-adolescent subjects (5-10 years); and (4) 13 adolescents (10-15 years old) (Table 1). Among subjects enrolled after their second birthday, 41/48 (85%) reported onset of AD before age 2. Disease severity was mild in 29% (SCORAD ≤25), moderate in 40% (SCORAD: >25-50) and severe in 31% (SCORAD >50) of subjects. Total IgE ab titers were elevated for age in 55% (10/18) of children under age 2 (total IgE >75 IU/ml), and in 85%(41/48) of older children (total IgE >150 IU/ml). Total serum IgE values were correlated with disease severity (r = 0.46, p=0.001). The prevalence of sensitization to one, two, three or ≥ four of seven key allergen sources (egg, cow's milk, peanut, dust mite, cat, ragweed, and ryegrass) was 90%, 75%, 69% and 54% respectively. These allergens included food and aeroallergens relevant to the pathogenesis of AD and symptom flares [19, 25-28].
Table 1.
Characteristics of Children with Atopic Dermatitis.
| Age group | <2 years | 2-5 years | 5-10 years | 10-15 years |
|---|---|---|---|---|
| N | 18 | 23 | 12 | 13 |
| Enrollment Age* | 1 [0.7, 1.3] yr | 3 [2.7, 3.5] yr | 6.5 [5.7, 7.5] yr | 12 [11, 13] yr |
| Age of Onset* | 3 [2, 5] mo | 4 [2, 6] mo | 7 [3, 15] mo | 12 [5, 32] mo |
| Gender (M/F) | 67% / 33% | 61% / 39% | 67% / 33% | 69% / 31% |
| Race - Caucasian Black Other |
61% 22% 17% |
65% 18% 17% |
67% 25% 8% |
69% 16% 15% |
| SCORAD | 34 [24, 47] | 31 [26, 37] | 49 [36, 66] | 34 [21, 55] |
| Total IgE (IU/ml)* | 134 [58, 310] | 445 [204, 971] | 1218 [401, 3,700] | 3338 [816, 13,647] |
| Distribution of Lesions | ||||
| Face | 89% | 61% | 50% | 31% |
| Hands | 56% | 52% | 42% | 46% |
| Feet | 39% | 48% | 58% | 54% |
| Arms | 89% | 70% | 83% | 92% |
| Legs | 94% | 91% | 100% | 92% |
| Torso | 56% | 57% | 42% | 62% |
| Foods Avoided | ||||
| Hen's Egg | 33% | 43% | 25% | 23% |
| Cow's milk | 44% | 35% | 25% | 15% |
| Peanut | 33% | 67% | 33% | 38% |
| Other | 17% | 43% | 17% | 38% |
Geometric mean [95% confidence interval]
Age-Related Profiles of IgE Antibody Titers
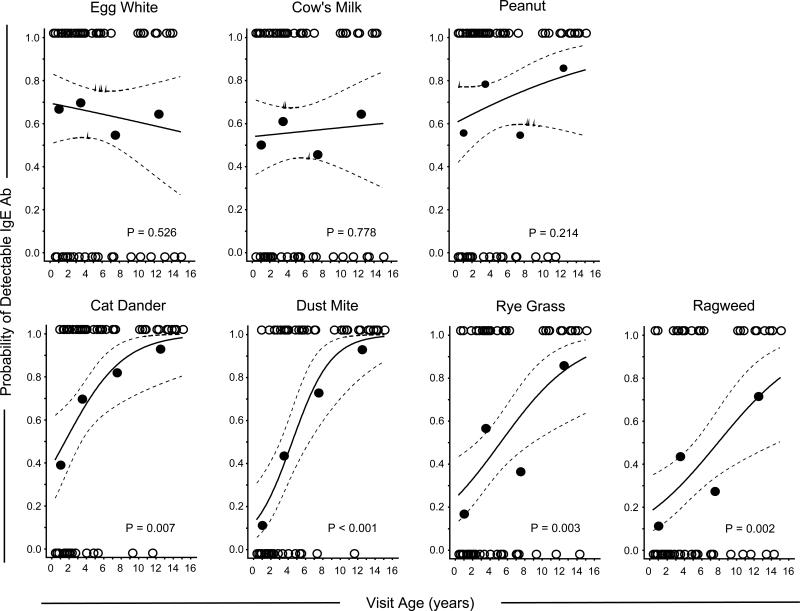
To evaluate the evolution of specific IgE ab directed against diverse sources in AD, we modeled age-related changes in IgE ab titers specific for each of the key allergen sources. Using specific IgE ab as a continuous variable, regression models for food allergens demonstrated early emergence of high titer IgE ab with little increase in IgE ab titers to any food allergen into adolescence. By contrast, age-related increases in IgE ab titer were significant for all aeroallergens (p<0.001)(Figure 1). Furthermore, while age-related increases in IgE ab titer were more pronounced among children with more severe disease (SCORAD > 40), this effect was only significant for dust mite (Figure S1). Interestingly in adolescents with severe AD, while IgE ab for outdoor inhalants increased in a linear fashion, titers of IgE ab to indoor allergens plateaued with age (Figure S1). Measurement of IgE ab to microbial antigens known to colonize AD skin among a subset of children (n=30), confirmed that microbial sensitization was a feature of severe but not mild disease (prevalence = 82% versus 15%, p<0.001), and anti-microbial IgE ab titers did not increase with age (Figure S1).
Figure 1. Regression Modeling of the Relationship between Age and Specific IgE Ab Titers in Children with AD.
Open circles denote IgE ab values for each child (n=66). Solid lines denote prediction curves, and hatched lines denote 95% simultaneous confidence intervals.
Analysis of Multi-sensitization
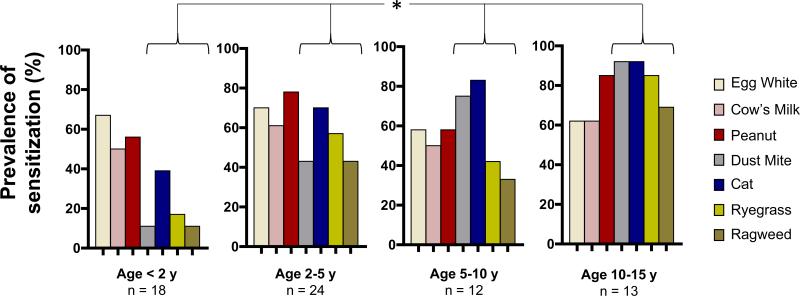
Further analysis of the prevalence of sensitization to each allergen among children in each of the 4 age groups revealed differences in profiles according to age. IgE ab to food allergens appeared early with a prevalence >50% for each allergen among children < 2 years. Interestingly, IgE ab to cat allergen also emerged early (39%); whereas, the prevalence of IgE ab to dust mite was low (11%)(Figure 2). IgE ab responses to ragweed and ryegrass allergens emerged during the pre-school years (prevalence = 43% and 57% respectively at 2-5 years, compared with 11% and 17% at <2 years). There was a trend in increased prevalence of sensitization from infancy into adolescence for dust mite, cat, ryegrass and ragweed (p≤0.004), but not for egg white, cow's milk, or peanut (Figure 2). Whereas less than one quarter of infants and toddlers were sensitized to 4 or more allergens, the prevalence of sensitization to at least 4 allergens among adolescents exceeded 80%. Regression modelling by age group showed that the probability of detecting IgE ab specific for any aeroallergen significantly increased for each 12-month increment in age (OR≥1.21, p≤0.007), with the largest effect observed for dust mite (OR=1.56 [95% CI 1.22, 1.98], p<0.001). The order of onset of sensitization to inhalants was cat before dust mite (p<0.01), and dust mite before ryegrass (p=0.04), with no difference in age of onset between ryegrass or ragweed. No increase in the risk for sensitization with age was observed for any food allergen (Figure 3 & Table 2).
Figure 2. Age-Related Prevalence of Sensitization in AD.
Percentage of children with IgE ab to egg white, cow's milk, peanut, dust mite, cat dander, ryegrass, and ragweed, by age group. Asterisk denotes allergens showing a significant increase in prevalence with age (dust mite: p<0.001; cat: p=0.002; ryegrass: p=0.002; and ragweed: p=0.004).
Figure 3. Probability of Allergen-specific Sensitization as a Function of Age.
Open symbols at 0 and 1 on the y-axis denote subjects without and with specific IgE ab respectively. Closed circles denote the proportion of subjects with detectable IgE in age groups 0-2, 2-5, 5-10, and 10-15 years.
Table 2.
Odds Ratio for Detectable IgE as a Function of Age.
| Specific IgE ab | Estimated Odds Ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Egg white | 0.96 | 0.86 | 1.08 | 0.526 |
| Cow's milk | 1.02 | 0.91 | 1.14 | 0.778 |
| Peanut | 1.09 | 0.95 | 1.25 | 0.214 |
| Dust mite | 1.56 | 1.22 | 1.98 | <0.001 |
| Cat | 1.34 | 1.08 | 1.67 | 0.007 |
| Ryegrass | 1.25 | 1.08 | 1.44 | 0.003 |
| Ragweed | 1.21 | 1.07 | 1.39 | 0.002 |
1 Ratio = age x + 1: age x
Age-Related Changes in the Relationship Between Specific IgE Antibodies and Total IgE
The relationship between specific and total IgE was assessed in relation to age. To extend this analysis to older subjects with AD, serum samples from our current cohort were compared with 47 serum samples from a cohort of previously characterized atopic adults with AD ( geometric mean (gm) total IgE = 1,262 IU/ml [95% CI: 826, 1,929 IU/ml]; and age of onset of AD = 2.2 years [95% CI: 1.5, 3.3 years]) [22]. During infancy, IgE ab directed against food allergens constituted the major contributor to total IgE, while inhalant allergens were the major contributor in adults (Figure 4). The contribution of food-specific IgE ab to total IgE significantly declined from infancy into adulthood (p=0.001)(Figure 4).
Figure 4. Contribution of Specific IgE Antibodies to Total IgE in AD.
The sum of specific IgE ab for food allergens (egg, cow's milk and peanut); indoor allergens (dust mite and cat); and outdoor allergens (ragweed and ryegrass)) expressed as a percentage of total IgE for each age group. Boxes denote median and interquartile range values. Whiskers denote 90% confidence interval. *p=0.001.
Despite a strong correlation between total IgE and specific IgE ab in children with AD, only a small fraction of total IgE was accounted for by the 7 major allergen sources tested, regardless of age (median = 11% [interquartile range (IQR) 9-14%]) (Figures 4 and S2A). Furthermore, IgE ab specific for microbial antigens constituted only a nominal proportion of total IgE (median = 0.02% [IQR 0-0.2] n=30). Among both children and adults, the percentage of total IgE that could be accounted for by specific IgE ab declined in relation to the highest levels of total IgE, and this observation was most pronounced among adults (Figure S2B). Expanding the panel of IgE ab tested from 7 to 14 allergen sources among adults with AD bolstered this inverse relationship (Figure S2B), and increased the median contribution of specific IgE ab to total IgE from 8% [IQR 3-18%] to 12% [IQR 5-28%] (p< 0.001). Collectively, these observations suggest that total IgE continues to increase beyond adolescence owing to production of non-cognate IgE, or IgE to multiple undefined specificities.
Sensitization to Fel d 1 and Fel d 4 Predict Wheeze in Children with AD
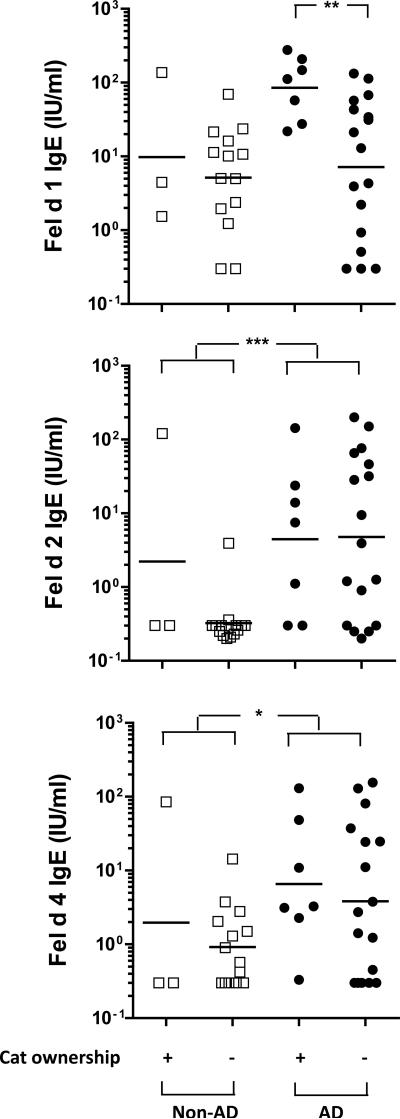
Sixty percent of children with AD in our study had a history of wheeze. In order to determine whether these children represented a clinically distinct subset, several variables were compared among wheezing and non-wheezing children. These included race, gender, family history of allergic disease, allergic rhinitis, exposure to cat or dog, and allergen sensitization. Children with wheeze were older than those without (median age = 5 [IQR 2 -10] versus 2 [IQR 1-5] years respectively (p<0.01)). The prevalence of a history of allergic rhinitis and a family history of asthma was modestly higher among wheezing children (OR=2.7 [95% CI: 0.9, 8.4], p=0.07 and OR=2.5 [95% CI: 0.9, 6.8], p=0.08 respectively) (Table 3a). Of each of the 7 allergens tested, sensitization to cat was the most significant predictor of wheeze (OR=4.5 [95% CI: 1.5, 13.4], p<0.01), followed by dust mite (OR=3.3 [95% CI: 1.2, 9.5], p=0.02). In order to further analyze IgE ab responses to cat and relation to wheeze, we measured IgE ab to Fel d 1 (secretoglobin), Fel d 2 (albumin) and Fel d 4 (lipocalin). Among cat allergic children with AD, sensitization to Fel d 1 (titers >15 IU/ml) and Fel d 4 (titers >0.3 IU/ml), but not Fel d 2, was associated with wheeze (Table 3b). Although cat ownership was neither associated with early sensitization to cat, or history of wheeze, children with cat ownership (n=7) had higher IgE ab titer to Fel d 1 (gm = 85 IU/ml [95% CI: 34, 212 IU/ml] versus 7 IU/ml [95% CI: 2, 22 IU/ml], p=0.01) (Figure 5). Furthermore, cat allergic children with AD, with and without wheeze, had significantly higher titer IgE ab to Fel d 2 and Fel d 4 as compared to cat allergic children without AD (Figure 5 & Table S1). Co-sensitization to cat and peanut, both of which occurred early, gave the same risk for wheeze in AD subjects as cat alone (OR=4.3 [CI:1.5, 12.4], p<0.01). Moreover, co-sensitization to other combinations of inhalant and food allergens did not increase the likelihood of wheeze (Table 3). Wheeze was not associated with the race or sex of the child, age of onset or severity of AD, or total IgE titer. Collectively, these findings support a role for sensitization to indoor inhalant allergens, and most significantly cat, in the development of allergic respiratory symptoms among children with AD.
Table 3a.
Odds Ratios for Predictors of Wheeze Among Children with AD.
| Dichotomous Variables | Wheeze (n=41) | Non-wheeze (n=25) | Odds Ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Caucasian Race | 64% | 64% | 1.0 | 0.3, 2.7 | 1.0 |
| Male Sex | 68% | 60% | 1.4 | 0.5, 4.0 | 0.5 |
| Allergic rhinitis | 80% | 60% | 2.7 | 0.9, 8.4 | 0.07 |
|
Family History Asthma |
66% | 44% | 2.5 | 0.9, 6.8 | 0.08 |
| AD | 71% | 72% | 0.9 | 0.3, 2.8 | 0.9 |
| Food Allergy | 53% | 52% | 1.1 | 0.4, 2.9 | 0.89 |
| Cat ownership | 20% | 12% | 1.8 | 0.4, 7.4 | 0.43 |
| Dog ownership | 39% | 44% | 0.8 | 0.3, 2.2 | 0.69 |
|
IgE Antibodies Dust Mite |
61% | 32% | 3.3 | 1.2, 9.5 | 0.02 |
| Cat | 80% | 48% | 4.5 | 1.5, 13.4 | <0.01 |
| Cat & dust mite | 59% | 28% | 3.6 | 1.2, 10.6 | 0.02 |
| Egg | 61% | 68% | 0.7 | 0.3, 2.1 | 0.57 |
| Milk | 58% | 52% | 1.3 | 0.5, 3.5 | 0.60 |
| Peanut | 76% | 56% | 2.4 | 0.8, 7.1 | 0.10 |
| All foods | 46% | 40% | 1.3 | 0.5, 3.6 | 0.61 |
| Any food | 88% | 80% | 1.8 | 0.5, 7.0 | 0.39 |
| Cat & peanut | 71% | 36% | 4.3 | 1.5, 12.4 | <0.01 |
| Ryegrass | 56% | 36% | 2.3 | 0.8, 6.3 | 0.12 |
| Ragweed | 41% | 32% | 1.5 | 0.5, 4.3 | 0.44 |
Table 3b.
Odds Ratios for Predictors of Wheeze Among Cat Allergic Children with AD.
| IgE antibodies (IU/ml) | Wheeze (n=19) | Non-wheeze (n=5) | Odds Ratio | 95% CI | P value |
|---|---|---|---|---|---|
|
Cat >0.3 |
100% | 100% | NA | - | - |
| >15 | 78% | 20% | 15 | 1.3, 175 | 0.03 |
|
Fel d 1 >0.3 |
89% | 80% | 2.1 | 0.15, 29.6 | 0.52 |
| >15 | 73% | 20% | 11.2 | 0.99, 125 | <0.05 |
|
Fel d 2 >0.3 |
63% | 80% | .42 | 0.04, 4.6 | 0.63 |
| >15 | 36% | 40% | 0.88 | 0.12, 6.6 | 1.0 |
|
Fel d 4 >0.3 |
89% | 20% | 12.8 | 1.2, 128.9 | 0.04 |
| >15 | 37% | 20% | 2.3 | 0.2, 25 | 0.63 |
Figure 5. Comparison of IgE Antibodies to Cat Components Among Cat Allergic Children With and Without AD.
IgE ab titers to Fel d 1, Fel d 2, and Fel d 4 in cat allergic children with and without AD (n=24 and 17 respectively). Titers were analyzed by group, and by cat ownership. *p<0.05, **p<0.01, ***p<0.001.
Discussion
In the present study, we examined age-related differences in IgE ab profiles among children with AD during the first 15 years of life. By restricting enrollment to children with active disease we selected for a group of highly atopic subjects at risk for development of asthma. Our findings support the view that sensitization to food and inhalant allergens develops sequentially. Additionally, both IgE ab titers and age-related risk of sensitivity for specific allergens follow divergent pathways during the first years of life. Using regression modeling, we demonstrate a marked increase in both age-related IgE ab titers, as well as in the probability of sensitization, for aeroallergens that contrasts with stable rates and levels of food sensitization. Not reported previously, we found that among children with AD, IgE ab to cat developed at a younger age as compared with IgE ab to dust mite. Moreover, IgE ab directed against Fel d 2 (albumin) and Fel d 4 (lipocalin), as well as Fel d 1, were integral to this response. Despite this, among those with more severe disease, dust mite was the only allergen for which a steeper age-related rise was observed as compared with those who had milder disease. Similarly, distinct patterns of age-related increase in IgE titer among aeroallergens were observed among adolescents with severe disease. These findings imply subtle, but important differences in the timing of IgE ab responses to diverse allergens, which are likely based on their ability to induce, or else promote ab production in children with AD. Taken together, our findings provide a model for the kinetics of IgE ab production in children at high risk of developing asthma. Our findings do not prove that the age-related profiles of sensitization reflect those that would occur within individuals with persistent AD. Indeed, the recent rapid rise in rates of sensitization to both aeroallergens and foods is a confounder in this regard. Longitudinal studies provide the only definitive approach to test this view; however, recruitment of a very large birth cohort would be necessary, since ~80% of children with AD will outgrow their disease by age 7 [28].
Although the association between aeroallergen sensitization and AD is well known, little is known about the timing and patterns of sensitization to individual aoeroallergens in relation the development of asthma among these children. This may be explained, at least in part, by the limitations of longitudinal studies in which only a small proportion of children with AD experience disease progression and the development of asthma [12, 29]. An advantage of our cross-sectional study design is that the children with active AD encompassed a broad age range, and more than half had histories of allergic rhinitis or wheeze. Children with AD were most commonly sensitized to cat or peanut allergens, and sensitization to cat was a risk factor for wheeze. The propensity for both cat and peanut allergens to induce IgE ab within the first 2 years of life was surprising, given the low prevalence of cat ownership and infrequent history of oral exposure to peanut among children under 2 years of age. A novel observation was the high degree of association between AD and IgE ab directed to Fel d 2 and Fel d 4. It is temping to speculate that, in contrast to other cat allergic children in whom Fel d 1 acts as the major sensitizer, inflamed skin in children with AD provides a portal for sensitization to additional allergens including cat albumin (Fel d 2) and lipocalin (Fel d 4) (see www.allergen.org). The lack of association between cat sensitization and ownership is not surprising given that public sites, such as schools, can provide a major source of clinically relevant cat allergen exposure in children without cat ownership [30]. Likewise, sensitization to peanut before oral ingestion has been linked to presence of peanut butter in the home and exposure through skin [31]
Sensitization to cat and dust mite allergens are each a risk factor for the development of asthma in children [32-38]. Interestingly, inspection of published data identified a higher probability of wheeze for cat versus mite at a given IgE value among preschool children [39]. In the same study, summing IgE levels for mite, cat, and dog at age 3 strengthened the risk for wheeze at age 5, suggesting that a cumulative response to multiple inhalant allergens within a narrow developmental window could lead to asthma. In our study, multi-sensitization did not increase the risk for asthma; however, our data suggest that low doses of cat allergen that are typical of those present in homes and public places in the absence of cats [30, 40, 41], are sufficient to promote production of high titer IgE ab among children with AD. Moreover, IgE ab to different cat components are integral to these responses. Although cat ownership was uncommon in our cohort, having a cat in the home was associated with significantly higher IgE ab titers to Fel d 1. In contrast to adults, in whom cat ownership has been associated with development of tolerance, our data support the view that inflamed skin during early childhood can interfere with tolerogenic mechanisms related to high dose allergen exposure. Based on this, and the early onset of sensitization to cat observed in our study, we suggest that screening for aeroallergen sensitivity may be warranted in children with AD beginning within the first year of life in order to identify those at most risk for developing asthma. In addition, our findings provide a rationale for targeting children with early childhood AD who are sensitized to cat in intervention studies designed to decrease the risk for developing asthma [41, 42].
Similar to previous reports of early sensitization to food allergens in AD [7-9], our findings question whether this phenomenon is a pre-requisite for the development of disease. Peanut allergy, eczema, and asthma have each been linked to loss-of-function mutations in the filaggrin gene that disrupt epithelial barrier function and enhance allergen entry [43-45]. Moreover, evidence from both mouse and human studies supports the view that exposure to allergen through the skin can induce allergic sensitization [46, 47]. In the present study, while co-sensitization to peanut and cat was a risk factor for asthma, the risk was equivalent to that for cat alone. Interestingly, the majority of cat-sensitized subjects (95% of cat-sensitized non-wheezing subjects and 86% of cat-sensitized wheezing subjects) were also sensitized to peanut. These findings raise important questions about how cat and peanut allergen may interact in disease.
Patients with AD often have total IgE levels that far exceed those observed in subjects with allergic respiratory disease [48]. High total IgE titers appeared early in children with AD and were strongly correlated with allergen-specific IgE ab levels. However, allergen-specific IgE ab, including that directed against microbial sources, accounted for only a small proportion of total IgE. Moreover, the contribution of allergen-specific IgE ab to total IgE was lowest among both children and adults with the highest total IgE titers (>10,000 IU/ml). This inverse relationship was more pronounced in adults than in children, suggesting that production of IgE continues into adulthood through preferential induction of non-cognate IgE or IgE ab directed against undefined sources. Such a process might occur through epitope spreading. Collectively, these observations reinforce the notion that targeted strategies aimed at preventing disease progression would need to be implemented early in life in order to be effective.
To summarize, in children with AD, age-related changes in allergen-specific IgE ab exhibit divergent courses for food and aeroallergens. Early sensitization to cat allergen, but more specifically, IgE ab directed towards Fel d 4 in addition to Fel d 1, were strongly associated with wheeze in children with AD. These findings provide a basis for investigating the mechanisms that contribute to the development of high titer IgE ab responses to specific allergen components in children with AD, and their relationship to the development of asthma.
Supplementary Material
Acknowledgements
Dr. Wisniewski was funded by an AAAAI/Food Allergy Initiative Howard Gittis Memorial Fellowship Award. Dr. Heymann receives funding for research from Novartis and NIH (NIAID). Dr. Platts-Mills receives research funding from NIH (NIAID). Dr. Woodfolk receives research funding from NIH (NIAID and NIAMS) and Dupont/Danisco.
This research was supported by NIH/NIAID grants U19 AR070364 (Project 2: J. Woodfolk) and R01 AI052196 (J. Woodfolk), and by an AAAAI/Food Allergy Initiative Howard Gittis Memorial Fellowship Award (J. Wisniewski).
Abbreviations
- Ab
antibody
- AD
atopic dermatitis
- CI
confidence interval
- OR
odds ratio
- gm
geometric mean
- IQR
interquartile range
Footnotes
Conflict of Interest Statement.
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Warner JO. A double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months’ treatment and 18 months’ posttreatment follow-up. J Allergy Clin Immunol. 2001;108:929–37. doi: 10.1067/mai.2001.120015. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–27. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Boulay ME, Boulet LP. The relationships between atopy, rhinitis and asthma: pathophysiological considerations. Curr Opin Allergy Clin Immunol. 2003;3:51–5. doi: 10.1097/00130832-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–9. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55:240–5. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann RL, Edenharter G, Bergmann KE, et al. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin Exp Allergy. 1998;28:965–70. doi: 10.1046/j.1365-2222.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 8.Mrazek DA, Klinnert M, Mrazek PJ, et al. Prediction of early-onset asthma in genetically at-risk children. Pediatr Pulmonol. 1999;27:85–94. doi: 10.1002/(sici)1099-0496(199902)27:2<85::aid-ppul4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–5. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 10.Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108:E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 11.Guilbert TW, Morgan WJ, Krawiec M, et al. The prevention of early asthma in kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Ricci G, Patrizi A, Giannetti A, Dondi A, Bendandi B, Masi M. Does improvement management of atopic dermatitis influence the appearance of respiratory allergic diseases? A follow-up study. Clin Mol Allergy. 2010;8:8. doi: 10.1186/1476-7961-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillet G, Guillet MH. Natural history of sensitizations in atopic dermatitis. A 3-year follow-up in 250 children: food allergy and high risk of respiratory symptoms. Arch Dermatol. 1992;128:187–92. doi: 10.1001/archderm.128.2.187. [DOI] [PubMed] [Google Scholar]
- 14.Hattevig G, Kjellman B, Bjorksten B. Clinical symptoms and IgE responses to common food proteins and inhalants in the first 7 years of life. Clin Allergy. 1987;17:571–8. doi: 10.1111/j.1365-2222.1987.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 15.Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990;117:561–7. doi: 10.1016/s0022-3476(05)80689-4. [DOI] [PubMed] [Google Scholar]
- 16.Hattevig G, Kjellman B, Bjorksten B. Appearance of IgE antibodies to ingested and inhaled allergens during the first 12 years of life in atopic and non-atopic children. Pediatr Allergy Immunol. 1993;4:182–6. doi: 10.1111/j.1399-3038.1993.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 17.Sigurs N, Hattevig G, Kjellman B, Kjellman NI, Nilsson L, Bjorksten B. Appearance of atopic disease in relation to serum IgE antibodies in children followed up from birth for 4 to 15 years. Journal of Allergy and Clin Immunol. 1994;94:757–63. doi: 10.1016/0091-6749(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 18.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 19.Eigenmann PA, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatr Allergy Immunol. 2000;11:95–100. doi: 10.1034/j.1399-3038.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia C, El-Qutob D, Martorell A, et al. Sensitization in early age to food allergens in children with atopic dermatitis. Allergol Immunopathol (Madr) 2007;35:15–20. doi: 10.1157/13099090. [DOI] [PubMed] [Google Scholar]
- 21.Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol. 1999;104:1273–9. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 22.Reefer AJ, Satinover SM, Wilson BB, Woodfolk JA. The relevance of microbial allergens to the IgE antibody repertoire in atopic and nonatopic eczema. J Allergy Clin Immunol. 2007;120:156–63. doi: 10.1016/j.jaci.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 24.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–9. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 25.Clark RA, Adinoff AD. The relationship between positive aeroallergen patch test reactions and aeroallergen exacerbations of atopic dermatitis. Clin Immunol Immunopathol. 1989;53:S132. doi: 10.1016/0090-1229(89)90078-0. [DOI] [PubMed] [Google Scholar]
- 26.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985;107:669–75. doi: 10.1016/s0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 27.Sampson HA, Scanlon SM. Natural history of food hypersensitivity in children with atopic dermatitis. J Pediatr. 1989;115:23–7. doi: 10.1016/s0022-3476(89)80323-3. [DOI] [PubMed] [Google Scholar]
- 28.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 29.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–5. e1–3. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Almqvist C, Larsson PH, Egmar AC, Hedren M, Malmberg P, Wickman M. School as a risk environment for children allergic to cats and a site for transfer of cat allergen to homes. J Allergy Clin Immunol. 1999;103:1012–7. doi: 10.1016/s0091-6749(99)70172-7. [DOI] [PubMed] [Google Scholar]
- 31.Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of Parents and Children Study Team Factors associated with the developent of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 32.Custovic A, Soderstrom L, Ahlstedt S, Sly PD, Simpson A, Holt PG. Allergen-specific IgG antibody levels modify the relationship between allergen-specific IgE and wheezing in childhood. J Allergy Clin Immunol. 2011;127:1480–5. doi: 10.1016/j.jaci.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perzanowski MS, Platts-Mills TA. Further confirmation of the relevance of cockroach and dust mite sensitization to inner-city asthma morbidity. Clin Exp Allergy. 2009;39:1291–3. doi: 10.1111/j.1365-2222.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 34.Platts-Mills TA, Erwin EA, Heymann PW, Woodfolk JA. Pro: The evidence for a causal role of dust mites in asthma. Am J Respir Crit Care Med. 2009;180:109–13. doi: 10.1164/rccm.200811-1756PR. [DOI] [PubMed] [Google Scholar]
- 35.Erwin EA, Ronmark E, Wickens K, et al. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. J Allergy Clin Immunol. 2007;119:359–65. doi: 10.1016/j.jaci.2006.12.648. [DOI] [PubMed] [Google Scholar]
- 36.Erwin EA, Wickens K, Custis NJ, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115:74–9. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Kuehr J, Frischer T, Meinert R, et al. Sensitization to mite allergens is a risk factor for early and late onset of asthma and for persistence of asthmatic signs in children. J Allergy Clin Immunol. 1995;95:655–62. doi: 10.1016/s0091-6749(95)70168-0. [DOI] [PubMed] [Google Scholar]
- 38.Sporik R, Platts-Mills TA, Cogswell JJ. Exposure to house dust mite allergen of children admitted to hospital with asthma. Clin Exp Allergy. 1993;23:740–6. doi: 10.1111/j.1365-2222.1993.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 39.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116:744–9. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Bollinger ME, Eggleston PA, Flanagan E, Wood RA. Cat antigen in homes with and without cats may induce allergic symptoms. J Allergy Clin Immunol. 1996;97:907–14. doi: 10.1016/s0091-6749(96)80064-9. [DOI] [PubMed] [Google Scholar]
- 41.Gulbahar O, Sin A, Mete N, Kokuludag A, Kirmaz C, Sebik F. Sensitization to cat allergens in non-cat owner patients with repisratory allergy. Ann Allergy Asthma Immunol. 2003;90:635–9. doi: 10.1016/S1081-1206(10)61868-6. [DOI] [PubMed] [Google Scholar]
- 42.Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy. 1996;51:89–93. [PubMed] [Google Scholar]
- 43.Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119:307–13. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 43.Brown SJ, Asai Y, Cordell HJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–7. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisgaard H, Simpson A, Palmer CN, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5:e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 46.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–23. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39:440–56. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.