SUMMARY
A novel experience induces Arc/Arg3.1 gene as well as plasticity of CA1 neural networks. To understand how these are linked, we briefly exposed GFP reporter mice of Arc transcription to a novel environment. Excitatory synaptic function of CA1 neurons with recent in vivo Arc-induction (ArcGFP+) was similar to neighboring non-induced neurons (ArcGFP–). However, in response to group 1 mGluR activation, ArcGFP+ neurons preferentially displayed long-term synaptic depression (mGluR-LTD) and robust increases in dendritic Arc protein. mGluR-LTD in ArcGFP+ neurons required rapid protein synthesis and Arc suggesting that dendritic translation of Arc underlies the priming of mGluR-LTD. In support of this idea, novelty exposure increased Arc mRNA in CA1 dendrites and promoted mGluR-induced translation of Arc in hippocampal synaptoneurosomes. Repeated experience suppressed synaptic transmission onto ArcGFP+ neurons and occluded mGluR-LTD ex vivo. mGluR-LTD priming in neurons with similar Arc activation history may contribute to encoding a novel environment.
INTRODUCTION
Salient experience activates rapid induction of immediate early genes (IEGs) in select neuronal populations and brain regions (Miyashita et al., 2008). IEGs are required for learning and the synaptic plasticity paradigms of long-term potentiation (LTP) and depression (LTD) (Shepherd and Bear, 2011). From this work, it is hypothesized that experience-induced IEGs lead to plasticity of synaptic inputs selectively within an activated neural network and this mediates encoding of memories or learning in response to that experience (Miyashita et al., 2008; Shepherd and Bear, 2011). Although numerous studies over the past 2 decades have visualized IEG induction as a means to identify neural networks activated by experience (Miyashita et al., 2008), little is known of how in vivo induction of IEGs with experience affects synaptic function or interacts with synaptic plasticity mechanisms.
An IEG whose induction is strongly correlated with learning and salient experience is Activity-regulated cytoskeletal-associated protein, Arc or Arg3.1. Arc is induced in a select population of hippocampal neurons in response to exploration of a novel environment (Guzowski et al., 1999; Miyashita et al., 2008) and hippocampal inhibition of Arc translation impairs consolidation of long-term memory (Guzowski et al., 2000). Arc protein weakens synaptic transmission by stimulating endocytosis of AMPA subtype glutamate receptors (reviewed in (Shepherd and Bear, 2011)). Interestingly, upon induction, Arc mRNA is rapidly transported to dendrites (Steward et al., 1998) and in CA1 neurons, Arc is translated in dendrites with activation of Group 1 metabotropic glutamate receptors (mGluRs) and leads to long-term synaptic depression (mGluR-LTD) (Park et al., 2008; Waung et al., 2008). These results suggest that mGluR-LTD may be selectively induced in neuronal populations with experience-induced Arc. Importantly, abnormal mGluR-LTD in CA1 is associated with cognitive disorders such as Fragile X Syndrome (FXS), autism, Alzheimer’s disease and age-related memory loss (Auerbach et al., 2011; Bozdagi et al., 2012; Huber et al., 2002; Lee et al., 2005; Li et al., 2009). As a result, mGluR dysfunction is causally linked with disease phenotypes in animal models and patients with FXS and autism (Krueger and Bear, 2010). Despite the strong association of mGluR-LTD with cognitive disorders, the role of hippocampal mGluR-LTD in cognition or experience-dependent hippocampal plasticity is unknown.
Here we utilized transgenic mice harboring a fluorescent reporter of Arc transcription, briefly exposed them to novelty and examined the consequences of in vivo, experience-dependent induction of Arc in CA1 on synaptic function and plasticity. Our results indicate that experience-dependent, in vivo induction of Arc primes individual CA1 neurons for mGluR-LTD. Furthermore, we present evidence that the mechanism of LTD priming involves mGluR-induced, rapid translation of dendritic Arc mRNA. This work reveals a novel metaplasticity of synaptic function (Abraham, 2008) that may contribute to the formation of a CA1 neural network representation of a salient experience.
RESULTS
To identify neurons with recent Arc induction, we used transgenic mice expressing a bacterial artificial chromosome (BAC) with the Arc promoter driving destabilized (4 hr ½ life) GFP (ArcGFP-BAC) (Grinevich et al., 2009). ArcGFP faithfully reports endogenous Arc induction in response to experience and neuronal activity (Grinevich et al., 2009). As observed with in situ hybridization for Arc mRNA (Guzowski et al., 1999; Miyashita et al., 2008), exposure of ArcGFP-BAC mice to a brief (5-min) novel experience increased the number of ArcGFP+ CA1 neurons and intensity of GFP in individual cell soma (Fig.S1A–D). In novelty-exposed mice, somatic levels of Arc protein were elevated in ArcGFP+ neurons as compared to neighboring ArcGFP– neurons (Fig.1A), and highly correlated with soma GFP levels (p<0.0001, Fig.S1E), confirming the reliability of the ArcGFP reporter. To examine the effects of in vivo Arc induction on excitatory synaptic function we performed simultaneous whole-cell recordings of miniature (m) and evoked EPSCs from neighboring ArcGFP+ and ArcGFP– CA1 pyramidal neurons in acute hippocampal brain slices from novelty-exposed ArcGFP-BAC mice (Fig.1B). Based on the known function of Arc (Shepherd and Bear, 2011), we expected to observe depressed synaptic transmission in ArcGFP+ neurons, in comparison to their ArcGFP– neighbors. Contrary to our hypothesis, mEPSC frequency and amplitude and evoked EPSC amplitude were similar between ArcGFP+ and ArcGFP– neurons (Fig.1B). EPSC kinetics, passive membrane properties, action potential threshold and firing frequency in response to depolarizing current injections were also similar between ArcGFP+ and ArcGFP– neurons (Fig. 1C; S1F; Table-S1).
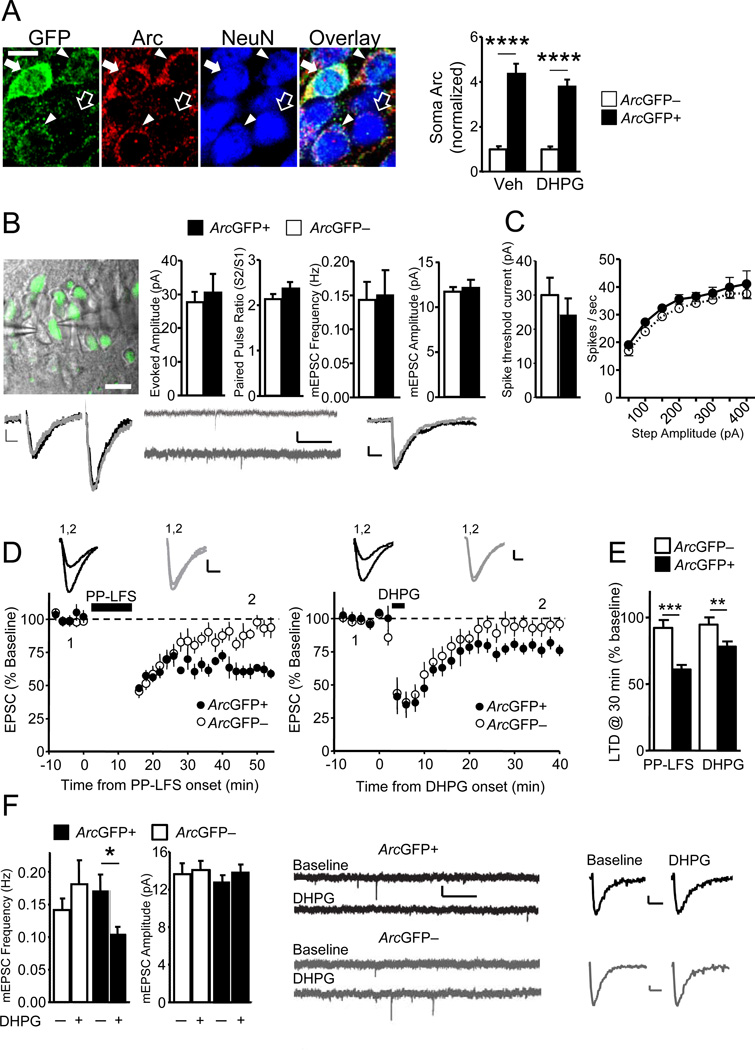
Figure 1. Group 1 mGluR activation induces LTD selectively in CA1 neurons with recent in vivo Arc induction.
A). Left: Immunohistochemistry for NeuN, GFP and Arc in hippocampal CA1 from an ArcGFP-BAC mouse exposed to novelty. Empty and filled arrows denote ArcGFP– and ArcGFP+ neurons, respectively. GFP and Arc levels in neuron soma are correlated (filled arrowheads and arrow). Scale-bar=5 µm. Right: Group averages (±SEM) of soma Arc protein in ArcGFP+ and ArcGFP– neurons (normalized to average soma Arc value in ArcGFP– neurons in the same image). The relative difference in soma Arc levels between ArcGFP+ (n=108) and ArcGFP– neurons (n=32) is similar in ACSF and DHPG treated slices (ArcGFP+;n=97, ArcGFP–;n=28). B) Upper Left: Overlay of confocal fluorescence and infrared DIC images from an acute hippocampal slice during simultaneous recordings from ArcGFP+ and ArcGFP– CA1 neurons. Upper Right: Group averages (±SEM) of evoked EPSC (n=10 cell pairs) amplitudes, paired-pulse ratios, mEPSC frequency and amplitudes (n=11 cell pairs) from ArcGFP+ and neighboring ArcGFP– neurons. Lower: Example evoked EPSCs (average of 4), raw and averaged mEPSCs (Scale bars: 10 ms/5 pA, 1 s/10 pA and 10 ms/2 pA respectively). C) Left: The minimal current required to elicit action potentials is similar ArcGFP+ (n=15) and ArcGFP– (n=10) neurons. Right: Firing frequency versus current injection (F-I curve) is similar between ArcGFP+ (n=19) and ArcGFP– (n=15) neurons. D) Time course of average evoked EPSC amplitude (Avg±SEM) normalized to pre-PP-LFS (left panel) or pre-DHPG (right panel) baseline. mGluR activation by PP-LFS (15-min; black bar; n=6 per group) or 100 µM DHPG (5-min; black bar; n=9 per group) induces LTD in ArcGFP+ but not ArcGFP– neurons. Representative EPSCs taken at times indicated are given on the graph. Scale-bar=10 ms/25 pA. E) LTD of EPSC amplitude at 30 min after cessation of PP-LFS or DHPG in ArcGFP+ and ArcGFP– neurons. F) Left: DHPG application induced a persistent (30-min) decrease in mEPSC frequency in ArcGFP+(n=13) but not in ArcGFP– CA1 neurons (n=10). Right: Raw and averaged mEPSCs (Scale bars: 1 s/10 pA and 10 ms/2 pA) before and 30 min after DHPG (50 µM; 5-min) for ArcGFP+ (black) and ArcGFP– (grey) neurons. *p< 0.05; **p< 0.01; ***p< 0.001; ****p < 0.0001. See also Figure S1.
In vivo Arc induction does not detectably affect synaptic function. Therefore, we next examined if ArcGFP+ and ArcGFP– neurons were differentially susceptible to induction of synaptic plasticity, such as mGluR-LTD (Park et al., 2008; Waung et al., 2008). mGluR-LTD can be induced with synaptic activation of mGluRs, using paired-pulses of low frequency (1 Hz) synaptic stimulation (PP-LFS), or chemically, using the group 1 mGluR selective agonist, (RS)-3,5-Dihydroxyphenylglycine (DHPG). LTD induced by either protocol requires mGluRs, rapid protein synthesis and Arc (Huber et al., 2000; Park et al., 2008; Waung et al., 2008). Delivery of PP-LFS to Schaffer collateral axons acutely depressed evoked EPSCs onto both ArcGFP+ and ArcGFP– neurons (Fig. 1D). However, a persistent LTD was observed in ArcGFP+ neurons (61±3% of baseline 30-min after PP-LFS; n = 6 cells; p<0.0001) but not in ArcGFP– neurons (92±5%; n = 6; Fig. 1D,E). As observed with PP-LFS, brief DHPG application (100µM; 5-min) caused an acute depression in both ArcGFP+ and ArcGFP– neurons but a persistent LTD only occurred in ArcGFP+ neurons (78±4% of baseline 30-min after DHPG; n = 9; p<0.01; ArcGFP–; 95±6%; n = 9; Fig.1D,E). DHPG-induced LTD is associated with decreases in mEPSC frequency, but not amplitude, which relies on postsynaptic AMPAR endocytosis and Arc (Moult et al., 2006; Waung et al., 2008). Consistent with these reports, DHPG (50µM;5-min) induced a persistent depression of mEPSC frequency in ArcGFP+ neurons (69±7% of baseline; n = 13; p<0.05), but not in ArcGFP– neurons (124±15 % of baseline; n = 10; Fig.1F). LTD magnitude of evoked EPSCs and mEPSCs was positively correlated with the GFP intensity of the soma of recorded CA1 neurons (Fig. S1G). DHPG equally affected input resistance and holding current of ArcGFP+ and ArcGFP– neurons (Fig.S1H–I), indicating that ArcGFP– neurons possess functional mGluRs, but are specifically deficient in LTD.
Evidence indicates that mGluR-LTD relies on dendritic translation of Arc mRNA (Huber et al., 2000; Park et al., 2008; Waung et al., 2008). Therefore, we hypothesized that mGluR activation stimulated translation of dendritic Arc mRNA in neurons that have recently undergone Arc transcription. Fluorescent in situ hybridization (FISH) for Arc mRNA in conjunction with immunohistochemistry for GFP and β3-tubulin (a neuron and dendritic marker) in novelty-exposed ArcGFP-BAC mice indicated that ArcGFP+ CA1 pyramidal neurons contained greater dendritic Arc mRNA than neighboring ArcGFP– neurons (304±47% of ArcGFP–;p<0.01 Fig. 2A,B; S2A). Sections incubated with a sense riboprobe for Arc displayed little or no signal (Fig. S2B). To determine if mGluR-stimulation differentially regulated Arc protein levels in the soma or dendrites of ArcGFP+ and ArcGFP– neurons we performed immunohistochemistry for Arc, GFP, and NeuN (neuronal cell bodies;Fig.1A) or β3-tubulin (neuronal cell body and dendrites; Fig.2C,D) in hippocampal slices of novelty-exposed ArcGFP-BAC mice treated with vehicle or DHPG. Arc protein levels were elevated in ArcGFP+ (NeuN+) soma as compared to ArcGFP– (NeuN+) neighbors (437±45% of ArcGFP–;p<0.0001). Surprisingly, this ratio was unaffected by DHPG treatment (380±31% of ArcGFP–;Fig.1A). Similar results were obtained if we measured a ratio of Arc-to-β3 tubulin intensity in the soma (Fig. 2D). Arc/tubulin protein levels in the dendrites of vehicle-treated ArcGFP+ neurons were slightly, but not significantly, elevated in comparison to neighboring ArcGFP– neurons (177±12% of ArcGFP–;p = 0.09; Fig.2D). However, after DHPG treatment Arc/tubulin levels in the dendrites of ArcGFP+ neurons were enhanced in comparison to dendrites from ArcGFP– neurons (292± 29% of ArcGFP–;p< 0.0001). Importantly, DHPG caused an increase in Arc levels in the dendrites of ArcGFP+ (p<0.0001), but not ArcGFP– neurons (Fig.2C,D) suggesting a mechanism for priming of mGluR-LTD in ArcGFP+ neurons.
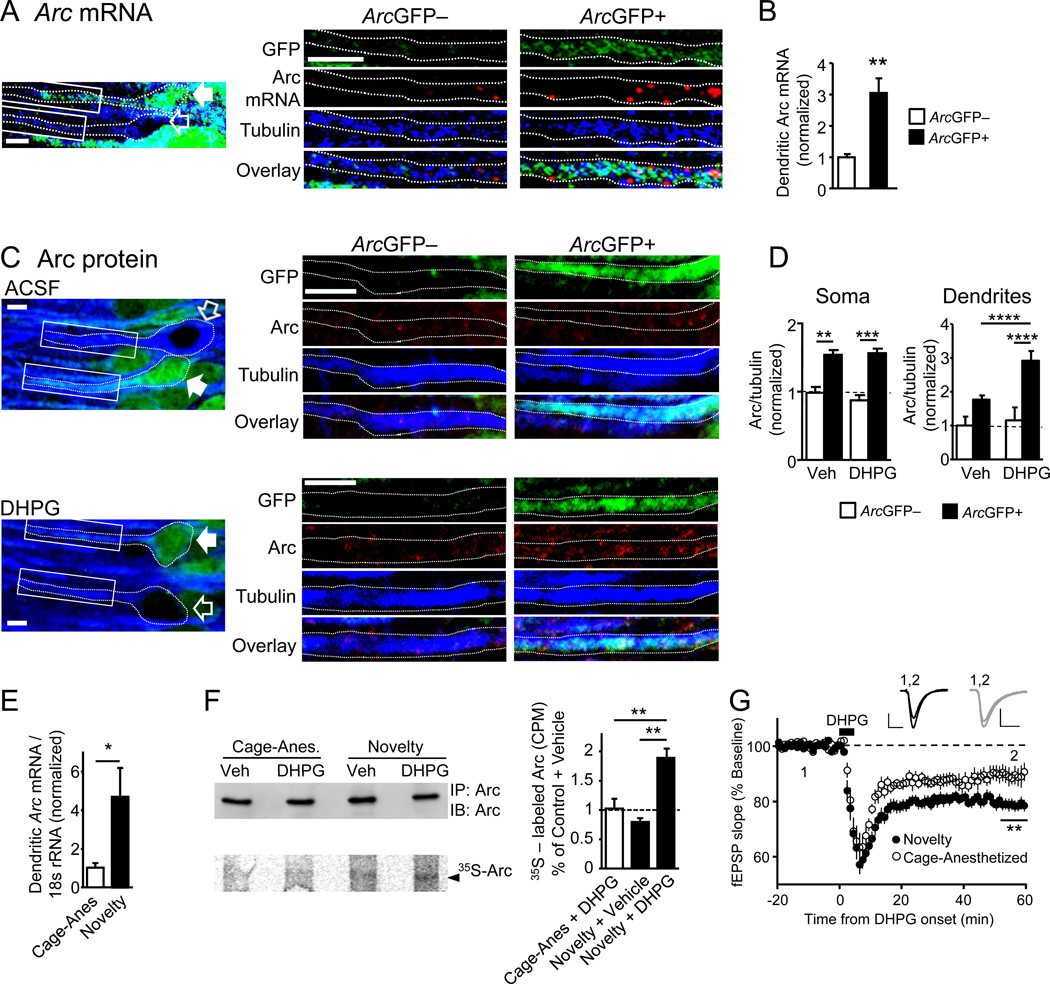
Figure 2. Novelty induction of Arc in vivo enhances group 1 mGluR stimulated dendritic Arc translation.
A) Representative image of CA1 neurons from ArcGFP-BAC mice exposed to brief novelty and processed for FISH of Arc mRNA (red) and immunohistochemistry for GFP (green) and β3-tubulin (blue). Dotted outline of ArcGFP+ neuron (filled white arrow) and ArcGFP– neuron (empty arrow) indicates source of dendrites at right. Scale-bar=5 µm. B) Quantification of dendritic Arc mRNA levels in ArcGFP+ neurons (n=48) normalized to that in neighbor ArcGFP– neurons (n=14). C) Representative images of CA1 neurons from ArcGFP-BAC mice exposed to brief novelty and processed for immunohistochemistry of β3-Tubulin (blue), GFP (green) and Arc protein (red). Dotted outline of neurons in left panel indicates the source of dendrites at right. Scale-bar=10 µm D) Quantified group data of Arc/tubulin immunofluorescence in soma or dendrites of ArcGFP+ (n for soma, dendrites- Vehicle; =155,135; DHPG; n=87,77) and ArcGFP– (Vehicle; n=29,19; DHPG; n=22,19) CA1 neurons from vehicle or DHPG-treated acute slices in novelty exposed mice. E) qPCR of Arc mRNA in microdissected CA1 dendritic regions from mice with brief novelty exposure or cage-anesthetized (n = 6 mice/condition) F) Western blot of total Arc immunoprecipitated (upper) and phosphorImager gel scan (lower) of immunoprecipitated, 35S-Met-labeled Arc from Vehicle (Veh) and DHPG-treated hippocampal synaptoneurosomes from cage-anesthetized or novelty-exposed mice. Right: Quantified group data of 35S Met incorporation into Arc immunoprecipitates expressed as a fraction of total immunoprecipitated Arc and normalized to cage-anesthetized+vehicle condition. G) Group average of DHPG (100 µM; 5 min)-induced LTD of extracellularly recorded, evoked field (f)EPSPs (Avg±SEM) in cage-anesthetized (89±3% of baseline; n=10 slices) and novelty-exposed mice (79±2%; n=20), normalized to pre-DHPG baseline. *p<0.05;**p< 0.01; ***p< 0.001; ****p < 0.0001. See also Figure S2.
Our results suggest that experience-induced Arc mRNA is transported to CA1 dendrites and translated upon mGluR stimulation. To test this idea more quantitatively, we used quantitative (q) real-time (RT) PCR to measure Arc mRNA in microdissected CA1 dendritic regions (str. radiatum) of acute slices prepared from home cage-anesthetized mice or mice exposed to brief (5 min) novelty. Because Arc mRNA is not expressed in glia or inhibitory neurons in CA1 (Vazdarjanova et al., 2006), the primary source of Arc mRNA in these microdissected regions should be from CA1 pyramidal cell dendrites. Three hours after brief novelty, qRT-PCR revealed a 4-fold increase in Arc mRNA in CA1 dendritic regions (Fig. 2E). To determine if novelty exposure promoted dendritic translation of Arc, we prepared synaptoneurosomes from CA3-CA1 hippocampal regions from cage-anesthetized or novelty-exposed mice and 35S Met incorporation into Arc was measured after immunoprecipitation (Waung et al., 2008). The specificity of 35S Met incorporation into Arc was confirmed using mice with genetic deletion of Arc (ArcGFP-KI−/−; described below; Fig. S2C) (Wang et al., 2006). Although basal Arc translation was not affected by novelty exposure (novelty+vehicle), DHPG stimulated Arc translation in synaptoneurosomes from novelty-exposed, but not cage-anesthetized mice (Fig. 2F). The enhanced mGluR-stimulated Arc translation at synapses of novelty exposed mice would be expected to lead to enhanced mGluR-LTD, which is exactly what we observed in population field (f) EPSP measurements in CA1 of acute slices from novelty-exposed mice in comparison to cage anesthetized mice (Fig. 2G; S2I). Together these results indicate that novelty experience increases Arc mRNA in CA1 dendrites, which in turn, promotes mGluR-stimulated dendritic Arc translation and LTD.
We hypothesized that enhanced DHPG-induced translation of Arc in dendrites underlies the priming of DHPG-induced LTD in ArcGFP+ neurons. In support of this hypothesis, the protein synthesis inhibitor anisomycin (25 µM) blocked DHPG-induced LTD of mEPSC frequency in ArcGFP+ neurons (Fig.3A, S3C). To test if Arc itself is required, we employed another Arc reporter mouse with GFP knocked in to the endogenous Arc locus (ArcGFP-KI) and the homozygous mice (ArcGFP-KI−/−) report Arc promoter activation with GFP, but do not produce Arc protein(Wang et al., 2006). Because novelty induces IEGs in addition to Arc, such as Homer1a, in similar CA1 neuronal populations (Vazdarjanova et al., 2002), these experiments will determine whether the Arc gene itself is required for the priming of LTD in ArcGFP+ neurons. We first confirmed that ArcGFP+ neurons in novelty-exposed ArcGFP-KI mice were primed for mGluR-LTD. For these experiments we used heterozygous ArcGFP-KI+/−mice which display normal DHPG-induced LTD as measured by extracellular recordings of population EPSPs in CA1 (Fig.S3A). LTD is normal in ArcGFP-KI+/−(het) mice likely because Arc protein levels are ∼80% of wildtype (Wang et al., 2006). As observed in ArcGFP-BAC mice, mEPSC frequency, amplitude (Fig.S3B), kinetics and passive membrane properties (Table S1) were similar between ArcGFP+ and ArcGFP– neurons in slices from novelty-exposed ArcGFP-KI+/−mice. Furthermore, DHPG application to ArcGFP+ neurons induced LTD of mEPSC frequency and had no effect on mEPSCs of ArcGFP– neurons (Fig.3B), thus confirming the priming effect of in vivo Arc induction on LTD in an independent mouse line. In ArcGFP-KI−/−(Arc KO) mice, ArcGFP+ neurons responded acutely to DHPG application, as measured by input resistance and holding current (Fig. S3D), but did not display LTD of mEPSC frequency (Fig. 3C). Consistent with previous reports (Plath et al., 2006), no differences were detected in baseline mEPSC frequency (ArcGFP+ neurons of: ArcGFP-KI+/−=0.17±0.04, ArcGFP-KI−/−=0.12 ± 0.04,p=0.13;n=8,11 cells) or kinetics or passive membrane properties (Table S1) between heterozygous ArcGFP-KI+/−and homozygous ArcGFP-KI−/−(ArcKO) mice.
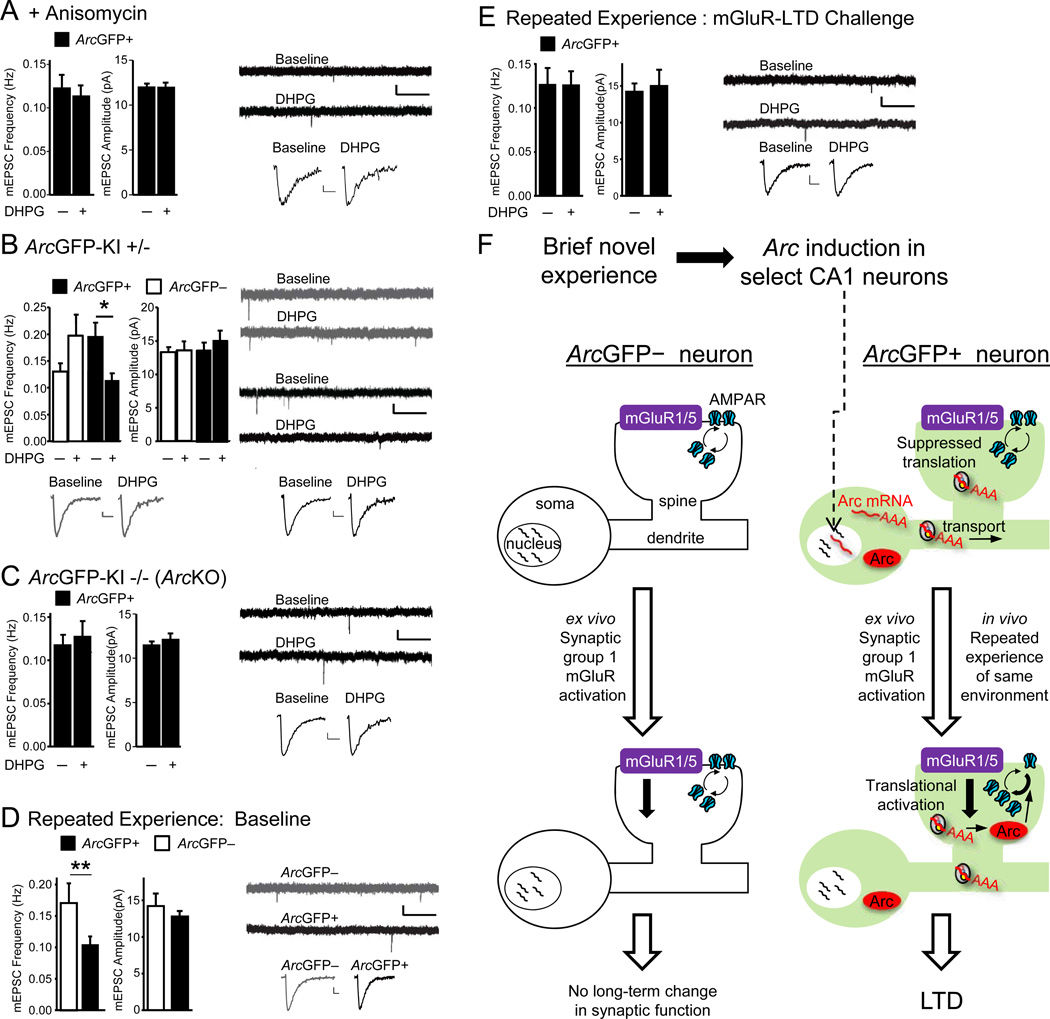
Figure 3. Group 1 mGluR triggered LTD in ArcGFP+ neurons requires de novo protein synthesis and Arc and occurs in vivo with repeated novelty experience.
A) Anisomycin (25 µM) blocks DHPG-induced decreases in mEPSC frequency (Avg±SEM) in ArcGFP+ neurons (n=13) from novelty exposed ArcGFP-BAC mice. B) Novelty-exposed heterozygous ArcGFP-KI+/− mice exhibit DHPG-induced LTD of mEPSC frequency in ArcGFP+, but not ArcGFP– neurons (n=9,12). C) DHPG-induced LTD of mEPSCs is absent in ArcGFP+ neurons of homozygous ArcGFP-KI −/− (ArcKO) mice (n=11). D)ArcGFP+ neurons from ArcGFP-BAC mice exposed to repeated experience of the same environment display reduced mEPSC frequency in comparison to neighboring ArcGFP– neurons (n=10 cell-pairs) E) DHPG has no effect on mEPSCs in ArcGFP+ neurons from ArcGFP-BAC mice with repeated experience (n=9). For Figures 3A–3E: left panels depict group averages (±SEM) of mEPSC frequency and amplitude before and 30 min after DHPG and right panels depict representative raw and average mEPSCs at baseline and 30 min after DHPG for ArcGFP+ (black) and ArcGFP– (grey) neurons. Scale-bars: 1s/10 pA and 10 ms/2 pA. *p< 0.05, **p< 0.01. F) Proposed model of in vivo Arc induction by novelty experience and LTD priming. See text and Figure S3.
Arc induction with brief novelty primes ArcGFP+ neurons for LTD upon subsequent mGluR stimulation. We hypothesized that repeated exposure to the same, initially novel, experience would reactivate synapses onto ArcGFP+ neurons and cause LTD in vivo. In support of our hypothesis, repeated exposure to the same environment activates the same network of CA1 neurons (Guzowski et al., 1999; Thompson and Best, 1990) and mGluR5 is required for synaptic depression triggered by prolonged novelty (Popkirov and Manahan-Vaughan, 2010; Qi et al., 2012). To test our hypothesis, we repeatedly exposed ArcGFP-BAC mice to the same novel environment (5-min exposure, every 20 min for 4 hours), prepared acute slices and examined synaptic function in ArcGFP+ and ArcGFP– CA1 neurons. Since we were unable to selectively probe synapses activated by repeated experience, we assayed all active synapses on an ArcGFP+ neuron by measuring mEPSCs. In contrast to brief in vivo experience (Fig. 1B), multiple repeated experiences resulted in depressed mEPSC frequency in ArcGFP+ compared to neighbor ArcGFP– neurons (Fig 3D). There were no differences in mEPSC amplitudes, kinetics or passive membrane properties after repeated experience between ArcGFP+ cells and a) ArcGFP– cells or b) ArcGFP+ cells in the brief novelty condition (Table S1). If such in vivo synaptic suppression in ArcGFP+ cells occurred via mechanisms common to mGluR-LTD, we would predict that DHPG-induced LTD would be decreased or occluded ex vivo. Consistent with this prediction, DHPG activated mGluRs but had no effect on mEPSC frequency of ArcGFP+ neurons from mice with repeated experience (Fig. 3E, S3E). These results suggest that repeated experience suppresses synaptic function in vivo selectively on neurons with recent Arc induction and this occurs through a mechanism in common with mGluR-LTD.
DISCUSSION
Here we demonstrate that in vivo Arc induction after a brief novel experience primes CA1 neurons for LTD in response to subsequent activation of group 1 mGluR receptors. In vivo Arc induction during brief novelty does not affect baseline synaptic transmission nor excitability, but enhances subsequent induction of synaptic plasticity and therefore may represent a novel form of metaplasticity (Abraham, 2008). We hypothesize that LTD priming by in vivo Arc induction may play a role in targeted suppression of a subset of CA1 neurons previously activated by the same experience and contribute to the formation of a neural network representation of that experience.
Our results suggest a model wherein Arc mRNA is induced by salient experience in select CA1 neurons and is then transported to dendrites, where it remains translationally-suppressed until mGluR activation translates Arc protein and induces LTD(Fig. 3F). Additional support for this model includes: 1) mGluRs stimulate dendritic Arc translation 2) mGluR-LTD relies on translation in dendrites and specifically translation of Arc (Huber et al., 2000; Park et al., 2008; Waung et al., 2008) 3) mGluR-LTD priming requires rapid de novo protein synthesis as well as Arc and 4) brief novelty induces Arc mRNA in select CA1 neurons (Guzowski et al., 1999), increases Arc mRNA levels in CA1 dendrites and enhances mGluR-triggered translation of Arc at synapses and LTD (Popkirov and Manahan-Vaughan, 2010; Qi et al., 2012). Our data suggests that repeated experience of the same environment causes synaptic weakening of CA1 neurons with dendritic Arc mRNA through a mechanism similar to mGluR-LTD (Fig. 3F). Similarly, an mGluR5-dependent synaptic depression occurs in vivo in response to prolonged environment exposure (Popkirov and Manahan-Vaughan, 2010; Qi et al., 2012). Since re-exposure to the same environment is known to prune the network of responsive CA1 neurons (Karlsson and Frank, 2008), one possibility is that re-exposure related mGluR-LTD in Arc+ CA1 neurons contributes to targeted suppression of a subset of neurons activated by the first novel experience, leading to a sparser representation of the original experience.
ArcGFP(+) cells allow single cell assay of recent salient experience-activated network
Arc activation marks specific networks activated by experience (Miyashita et al., 2008). With ArcGFP mice, we cannot be certain that the GFP was specifically induced with novel object exploration. However, cage-anesthetized mice had low ArcGFP+ expression and Arc transcription is not induced by spontaneous firing of cells, or place cell activation without behavioral relevance (Fletcher et al., 2006; Miyashita et al., 2009). These results, combined with the short (2–4 h) half-life of ArcGFP, suggests that ArcGFP+ neurons in our study were at least activated in vivo by recent, salient experience. Studies have observed robust mGluR-LTD in slices from rodents that were not subjected to novel object exploration (Auerbach et al., 2011; Huber et al., 2002; Huber et al., 2000; Moult et al., 2006; Park et al., 2008). How are these studies reconciled with our findings that novelty primes mGluR-LTD? In most slice physiology labs, including our own, mice or rats are not habituated to human handling, and on experiment day animals are removed from their home cage, transported to the lab and anesthetized there. This “standard handling” procedure itself may constitute a brief novel experience since it induces Arc in CA1 neurons (Fig. S2D–G), and enhances mGluR-LTD in comparison to cage-anesthetized mice (Fig. S2H,I).
Translational suppression of dendritic Arc mRNA as a metaplasticity mechanism
We observed that after brief novelty, CA1 as a whole, and Arc-activated neurons in particular, have greater levels of dendritic Arc mRNA, but not Arc protein. Such translational suppression of dendritic Arc mRNA may explain lack of differences in baseline synaptic function between ArcGFP+ and ArcGFP– neurons. Many dendritic mRNAsare translationally suppressed during transportto achieve localized synaptic protein expression (Besse and Ephrussi, 2008). Arc’s 3’UTR harbors an intron and dendritic Arc mRNA is associated with the exon junctional complex (EJC) (Giorgi et al., 2007). Since the EJC is typically removed upon a pioneer round of translation, its association with dendritic Arc mRNA suggests that much of dendritic Arc mRNA has not been translated. The RNA binding protein, Fragile X Mental Retardation Protein (FMRP) may translationally suppress dendritic Arc at steady state (Napoli et al., 2008; Niere et al., 2012). Upon group 1 mGluR-stimulation FMRP is dephosphorylated, altered in its protein interactions, ubiquitinated and degraded which may result in translational de-repression of Arc mRNA and LTD (Napoli et al., 2008; Narayanan et al., 2007; Niere et al., 2012). In addition to FMRP, mGluRs stimulate signaling pathways that lead to translational activation and that are necessary for LTD (Waung and Huber, 2009).
A model for experience-induced Arc and mGluR-LTD priming in the formation of CA1 neural representation of a novel experience
Exploration of novel objects facilitates CA1 LTD in awake rats (Dong et al., 2012; Kemp and Manahan-Vaughan, 2004; Manahan-Vaughan and Braunewell, 1999; Popkirov and Manahan-Vaughan, 2010) suggesting that LTD may contribute to learning or familiarity of a novel experience. In support, inhibition of mGluR5 or LTD mechanisms block novelty-related learning (Dong et al., 2012; Popkirov and Manahan-Vaughan, 2010). Importantly, Arc is implicated in late phase LTP (Bramham et al., 2010; Guzowski et al., 2000; Plath et al., 2006). Exploration of a novel open field (without objects) facilitates LTP induction (Kemp and Manahan-Vaughan, 2004), suggesting that Arc induction in vivo could also prime neurons for LTP. An interesting possibility is that the pattern and frequency of synaptic activation onto a neuron with recent Arc induction determines whether LTP or LTD is primed. Priming of mGluR-LTD by recent salient experience may contribute to the plasticity of active neural networks that encode that experience. Abnormal encoding and habituation of novel experiences occurs in Fragile X syndrome (Hagerman et al., 1985; Restivo et al., 2005), a cognitive disorder associated with altered mGluR-LTD (Huber et al., 2002). Understanding the contribution of mGluR-LTD to normal cognition will help understand how altered mGluR-LTD mechanisms lead to cognitive disorders (Auerbach et al., 2011; Bozdagi et al., 2012; Huber et al., 2002; Lee et al., 2005; Li et al., 2009).
EXPERIMENTAL PROCEDURES
Mice
The two mouse lines that were used, ArcGFP-BAC and ArcGFP-KI have been previously described (Grinevich et al., 2009; Wang et al., 2006) (Supplementary methods).
Novel Experience
Novelty-exposed and cage-anesthetized mice were habituated to human handling (4–5 days). After brief novelty (5 min), mice were left undisturbed in a standard empty cage for 3 hours prior to anesthesia. Cage-anesthesized mice were removed from their home cage and anesthestized within 10 sec with isoflurane. Repeated exposure to the same environment involved 5-min exposures, every 20 min for 4 hours. Mice in the standard-handling group were not habituated to human handling, and on experiment day were removed from their home cage, transported to the lab and anesthetized in the lab. (see Supplementary Methods).
Electrophysiology
Whole cell patch clamp recordings from CA1 pyramidal neurons in acute hippocampal slices was performed as described (Huber et al., 2000) (Supplementary methods).
Immunocytochemistry
Acute hippocampal slices (400 µm) were prepared as for electrophysiology and treated with Vehicle or DHPG. Slices were then fixed in 4% paraformaldehyde and resectioned in 3% agarose to yield 50 µm thick sections. Sections were blocked with 3% goat serum and 0.5% Triton X prior to treatment with primary and secondary antibodies (see Supplementary methods).
Fluorescent in situ hybridization (FISH) with co-immunostaining
ArcGFP-BAC mice were exposed to brief novelty as for electrophysiology experiments but perfused with 4% paraformaldehyde. 20 µm sections were prepared and processed for Arc/Arg3.1-specific FISH using Cy3-coupled tyramide amplification. (see Supplementary Methods)
Synaptoneurosome preparation, 35S-Met/Cys incorporation and immunoprecipitation of Arc was conducted as previously described (Waung et al., 2008) with a minor modification (Supplementary Methods).
Statistical Analysis
Statistical analyses were performed using Prism 5.0 (GraphPad Software). Two-way or one-way ANOVA, independent Student’s t-test, Mann-Whitney U test or Two-sample Kolmogorov-Smirnoff test were applied where appropriate unless specified otherwise. All data are expressed as the mean ± SEM.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the grants from the National Institutes of Health NS045711 (KMH) and HD056370 (JRG), Emory Neuroscience NINDS Core Facilities grant, P30NS055077 (GJB and CG). We would like to thank Dr. John Isaac for discussions and sending us the ArcGFP-BAC mice, Darya Fakhretdinova, Lorea Ormazabal and Nicole Cabalo for technical assistance, and members of the Huber lab for discussions and comments on the manuscript. We thank Drs Darrin Brager and Daniel Johnston for technical training of whole-cell recordings in mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat Rev Mol Cell Biol. 2008;9:971–980. doi: 10.1038/nrm2548. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Dorr N, Pilorge M, Takahashi N, Buxbaum JD. Haploinsufficiency of cyfip1 produces fragile x-like phenotypes in mice. PLoS One. 2012;7:e42422. doi: 10.1371/journal.pone.0042422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J Neurosci. 2012;32:11980–11990. doi: 10.1523/JNEUROSCI.0984-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher BR, Calhoun ME, Rapp PR, Shapiro ML. Fornix lesions decouple the induction of hippocampal arc transcription from behavior but not plasticity. J Neurosci. 2006;26:1507–1515. doi: 10.1523/JNEUROSCI.4441-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Kolleker A, Eliava M, Takada N, Takuma H, Fukazawa Y, Shigemoto R, Kuhl D, Waters J, Seeburg PH, et al. Fluorescent Arc/Arg3.1 indicator mice: a versatile tool to study brain activity changes in vitro and in vivo. J Neurosci Methods. 2009;184:25–36. doi: 10.1016/j.jneumeth.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Kemper M, Hudson M. Learning disabilities and attentional problems in boys with the fragile X syndrome. Archives of Pediatrics and Adolescent Medicine. 1985;139:674. doi: 10.1001/archpedi.1985.02140090036021. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. The Journal of neuroscience. 2008;28:14271. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2010;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. The Journal of Neuroscience. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E–BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, Huber KM. Evidence for a fragile × mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered arc translation and long-term depression. J Neurosci. 2012;32:5924–5936. doi: 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2010;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Hu NW, Rowan MJ. Switching off LTP: mGlu and NMDA Receptor-Dependent Novelty Exploration-Induced Depotentiation in the Rat Hippocampus. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs086. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509:299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. The Journal of neuroscience. 2002;22:10067. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.