Abstract
Successful locomotion depends on postural control to establish and maintain appropriate postural orientation of body segments relative to one another and to the environment, and to ensure dynamic stability of the moving body. This paper provides a framework for considering dynamic postural control, highlighting the importance of coordination, consistency, and challenges to postural control posed by various locomotor tasks such as turning and backward walking. The impacts of aging and various movement disorders on postural control are discussed broadly in an effort to provide a general overview of the field and recommendations for assessment of dynamic postural control across different populations in both clinical and research settings. Suggestions for future research on dynamic postural control during locomotion are also provided and include discussion of opportunities afforded by new and developing technologies, the need for long-term monitoring of locomotor performance in everyday activities, gaps in our knowledge of how targeted intervention approaches modify dynamic postural control, and the relative paucity of literature regarding dynamic postural control in movement disorder populations other than Parkinson disease.
Keywords: gait, locomotion, posture, motor control, Parkinson disease
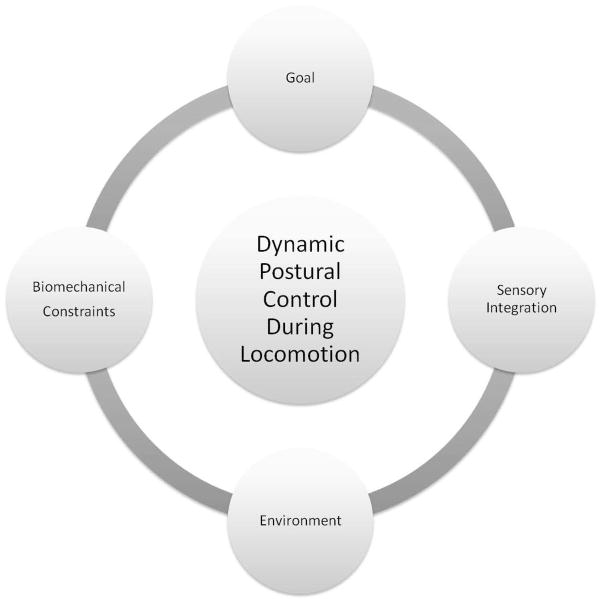
Upright, bipedal locomotion is a hallmark of human mobility, allowing for independent movement through a variety of environments for various purposes. Successful locomotion depends on postural control to establish and maintain appropriate postural orientation of body segments relative to one another and to the environment and to ensure dynamic stability of the moving body. This process critically depends on integration of sensory inputs and must operate within the limits of biomechanical constraints inherent to the individual and the task (Figure 1). Without adequate postural control, locomotion becomes dyscoordinated, inefficient, unstable and potentially hazardous given the risk for falls during walking. Given the importance of postural control during locomotion, the objectives of this paper are to: 1) review recent evidence regarding postural control during gait, highlighting how control changes during challenging locomotor tasks and in the face of aging and various movement disorders, 2) propose a framework for assessment of postural control across locomotor tasks in both clinical and research settings, and 3) recognize gaps in our current knowledge and areas of need for future research regarding postural control during locomotion.
Figure 1.
Model depicting factors that impact dynamic postural control during locomotion.
Coordination and Consistency: The Foundations of Dynamic Postural Control
During ongoing locomotion, the basic stepping patterns for forward walking have for some time been well characterized with regard to expected average joint movement profiles and patterns of muscle activity. These fundamental, spatiotemporal patterns of coordination within and between limbs (Figure 2) provide the foundation for dynamic postural control during gait. Locomotion may be controlled by internal models that determine the difference between actual and desired body locations within the environment, and then transmit this “error” signal to neurons that subsequently work to diminish this difference by sending commands that will result in moving the body closer to the desired, or referent, position.1 In this schema, muscle activation is dependent upon the mismatch between the actual and referent positions, with the referent position being constantly shifted in the desired direction of locomotion as one continues to progress through the environment. Postural control in the fore-aft direction during gait may be maintained through a series of controlled falls that are passively and actively stabilized.2 One example of active stabilization is the braking of the COM during the transition into double support, with older adults adopting reduced step lengths and reduced gait speeds that may assist in maintaining effective COM control.3 The capacity to regulate COM braking is also reduced in those with PD relative to controls and may be related to non-dopaminergic midbrain lesions.4
Figure 2.

Illustration of key aspects of postural control, the foundation being coordination of a fundamental gait pattern, the next level being consistency of pattern production, and the top level being ability to modify control in the face of challenges.
While much insight can be gained about postural control through assessment of fundamental coordination of movement, there is increasing recognition that consistency of step to step performance, once thought to reflect noise in the control system, provides an important additional level of information about locomotor control (Figure 2). In fact, variability has been identified as an important and unique domain of gait that is impacted by aging and disease, related to fall risk and predictive of future mobility decline in older adults5–9. For example, stride time variability is increased in those with high level gait disorders10 and movement disorders such as Huntington disease (HD) and PD11,12. In fact, pre-manifest mutations carriers for HD can be distinguished from healthy controls based upon stride to stride variability.13 In addition, carriers of the LRRK2-G2019S mutation demonstrate higher gait variability than non-carriers in fast and dual task walking conditions14. Furthermore, among those with PD, fallers and freezers exhibit higher stride time variability than non-fallers and non-freezers.12,15 Step length and step timing are also more variable during gait initiation in PD.16
Both coordination and consistency can be incorporated into single measures of gait performance. One example of such a measure is phase coordination index (PCI), which examines temporal coordination of interlimb phasing and variability of this phasing across strides.17 PCI reveals poorer or less consistent interlimb phasing is also associated with aging, PD, and freezing of gait17–21. Performance of more challenging gait tasks such as backward walking and turning, as well as forced manipulations of step length and cadence away from preferred baseline values during forward walking, are associated with reduced coordination as measured by PCI, with old being more affected than young, those with PD being more affected than age-matched controls, and those with PD and history of freezing being more affected than those with PD but no history of freezing.18,22
Recent work suggests that upper extremity coordination during bimanual tasks requiring anti-phase movements is affected by forced manipulations of amplitude and cadence in a manner very similar to the effects on actual locomotion as outlined in the preceding paragraph.23 Other studies also suggest clear links between locomotor control and upper extremity control in health and disease. For example, studies showing freezing of upper extremity movements and their correlation with freezing of gait suggest the possibility of common control mechanisms for coordination of bilateral upper extremity and bilateral lower extremity tasks.23–25 Moreover, arm swing is clearly coordinated with lower extremity movement during typical gait, suggesting persistence of the basic quadrupedal limb coordination pattern during bipedal gait.26 However, this coordination is task-dependent, as the arms can be uncoupled from the lower limbs for use in voluntary activities such as carrying objects. Changing movement of the arms impacts locomotor coordination, with prevention of arm swing resulting in a switch from anti-phase coordination between the pelvic and scapular girdles to an in-phase pattern.27 Reduced arm swing is common in PD and correlates with rigidity and bradykinesia28. Arm swing amplitude and phasing relative to the lower extremities improve with both levodopa and deep brain stimulation.29 Given the mounting evidence for the importance of upper extremity control in gait, comprehensive clinical assessments and future research studies should consider both lower extremity and upper extremity coordination and consistency to obtain a complete picture of dynamic postural control during gait.
Beyond Coordination and Consistency: Postural Control in Challenging Gait Tasks
While coordination and consistency form the foundations of postural control, ability to regulate posture in the face of challenging gait tasks is equally important to successful locomotion (Figure 2). The ability to produce a coordinated and consistent forward walking pattern is not sufficient, and as such it is important to consider how postural control changes in the face of different environments, goals, biomechanical constraints and sensory conditions (Figure 1). A thorough evaluation of dynamic postural control, whether done for clinical or research purposes, should assess performance in a variety of conditions. Suggested conditions include walking at different speeds, in different directions, with eyes closed and with head turns, with biomechanical constraints such as obstacles and narrow base of support (i.e. tandem walking), and in dual task paradigms where additional non-locomotor demands are placed on the system (Table 1). The following paragraphs highlight some of these areas, while others are covered in more detail elsewhere in this special issue.
Table 1.
Challenging gait conditions for assessment of dynamic locomotor control.
| Challenge | Task | Goal | Environment | Biomechanical Constraints | Sensory Integration |
|---|---|---|---|---|---|
| Speed | Preferred | Baseline assessment | Everyday tasks with no time constraints | Baseline | Visual, vestibular and somatosensory integration |
| Fast | Progress as quickly as possible without running | Crossing a busy street, late for a meeting | Normal | Vestibular information less important | |
| Slow | Progress as slowly as possible while maintaining stability | Walking in crowd moving slowly | Normal | Vestibular information more important | |
| Direction | Backward | Walk backward at preferred pace | Backing out of closet, stepping back from curb, serving as a tour guide | Reverse of forward kinematics | Visual input less effective – not looking in direction of progression |
| Turning | Change direction | 50% of everyday steps are turn steps | Task is inherently asymmetric | Vision/gaze key for leading trajectory change, vestibular upweighting prior to turn | |
| Sensory Inputs | Eyes Closed | Walk without vision | Walking at night, poor lighting | Normal | Vision eliminated, vestibular and somatosensory inputs upweighted |
| Head Turns | Walk while turning head side to side | Reading a sign while walking | APAs required in advance of head turns | Vestibular stimulation via head turns | |
| Biomechanical Requirements | Obstacles | Step over or around obstacle in path | Uneven sidewalk, person stops in front of you, puddle | Step height, length and/or direction modification to avoid obstacle, upper extremity contribution | Visual guidance of limb/body trajectory |
| Tandem | Walk with one foot directly in front of the other along a line | Walking on a curb, crossing a stream by walking on a log | Narrow base of support necessitates tighter mediolateral COM control | Normal | |
| Non-Locomotor Demands | Cognitive Task | Walk and perform a mental task simultaneously | Walking while having a conversation | Normal | Normal but divided attention may impact utilization |
| Secondary Motor Task | Walk and do another motor task simultaneously | Walking and carrying packages | Increased load, upper extremities may be constrained | Normal but divided attention may impact utilization |
Postural control is influenced by the integration of visual, vestibular, and somatosensory inputs. With increasing age, a more conscious strategy for locomotor and postural control may be utilized as evidenced by increases in cortical BOLD signals in vestibular, somatosensory and visual areas of the cerebral cortex in older compared to younger adults during imagined walking.30 Simple means of exploring the role of sensory inputs include walking with eyes closed and with head turns (Table 1). Walking at different speeds also probes sensory inputs, as vestibular influences on gait are reduced during faster walking and running relative to slower walking31,32. More complex experimental methods use perturbations presented during gait initiation and walking to probe the role of sensory inputs. For example, Rogers et al.33 introduced a sudden drop or elevation of the support surface to assess the contribution of somatosensory information during gait initiation, demonstrating that controls and people with PD can rapidly adapt to this type of perturbation. This suggests a feedforward neural control of gait initiation in which sensory information regarding limb load and/or foot pressure can modulate temporal and spatial components of step initiation.
Other perturbation paradigms introduce unexpected movements of the support surface during ongoing walking. Perturbation studies support the concept of modular or synergistic control of gait, hypothesizing that changes in the basic gait pattern in response to perturbations or even to increased loads can be accomplished through variations in temporal recruitment from a library of locomotor muscle synergies, with this recruitment being accomplished through different parallel pathways at the spinal, brainstem, and cortical levels.34,35 The specific strategies employed depend upon the particular demands of the perturbation. For example, mediolateral translations of the support surface during gait result in shorter steps with wider step width to allow for stability and adaptability.36,37 Other tasks, such as tandem walking and obstacle negotiation, introduce specific biomechanical constraints (Table 1). Tandem, or heel-to-toe walking along a line, requires tighter mediolateral control of the COM given the narrow base of support. Obstacles require individuals to adjust step length or step height in order to avoid contact with the object. People with PD have particular difficulty increasing step length as compared to step height,38 and adopt a conservative strategy with reduced anterior-posterior and increased mediolateral center of mass motion, as well as a reduced distance between the center of mass and center of pressure compared to controls.39
Obstacle negotiation tasks also highlight the importance of the upper extremities to balance recovery. Upper extremity muscle activity is higher during obstacle crossing; the upper extremities are coupled with the lower extremities and play a role in equilibrium control.40 The coupling between the upper and lower extremities during obstacle crossing is preserved in PD.41 In conditions which mimic unsuccessful obstacle negotiation, such as sudden arrests of the forward movement of one leg or recovery from a trip, upper extremity movements are asymmetric and may assist in balance recovery by impacting orientation of other body segments in order to facilitate braking of the impending fall.42 As walking continues after the perturbation, stability is recovered and interlimb phasing between the upper and lower limbs is restored, with older adults requiring more cycles to recover stability and appropriate interlimb phasing.43
Older adults ability to successfully negotiate obstacles may also be influenced by vision, which provides critical information about body position relative to the environment.44 Several changes in visual processing and sampling in older individuals have been related to changes in locomotor performance. Ability to reweight visual information declines with aging, resulting in larger gait deviations in response to visual perturbations in older compared to younger individuals.45 Older adults also have reduced ability to maintain gaze fixation and this ability is correlated with gait initiation performance, with those less able to maintain fixation requiring more time to initiate a step.46 Gaze behaviors during ongoing locomotion are also related to falls47,48. During performance of walking tasks where one is required to step on particular targets along the walking path, elderly fallers demonstrate premature transfer of gaze to the upcoming target47 and longer latencies between making a saccade to a target and initiating a step to that target.48 Effective gaze control is critical not just for tasks requiring specific foot placements, but also for turning, where transfer of gaze initiates change in locomotor trajectory.
Changes in locomotor trajectory are critical for daily locomotor activities; in fact, turning steps compose up to 50% of everyday tasks.49 Relative to straight walking, local dynamic stability is reduced during turning50, which is accomplished by a top down temporal sequence of body segment rotations. The top down rotation sequence begins with a saccade in the new heading direction, and this anticipatory redirection of gaze is thought to be critical for initiating changes in locomotor trajectory.51 Without vision, axial segments rotate more slowly and more synchronously during turning.52 However, the top down rotation sequence is not affected by walking velocity52 or sharpness of the turn. Tighter curvatures are associated with greater spatial anticipation of the upcoming turn, but are still executed with a top down sequence.53 The anticipation of turning is characterized by an upweighting of vestibular inputs just before a turn54, along with anticipatory postural adjustments evidenced by a posteriolateral lean.55
Control of locomotor direction is thought to be governed at the level of whole body trajectory, with implementation occurring through specific motor strategies.56 In older adults, strategies for changes of body orientation are characterized by a longer latency between gaze reorientation and body segment reorientation.57 In addition, older adults with lower balance confidence are more likely to use multiple steps in order to accomplish changes in direction more gradually than do those with higher balance confidence.58 Changes in turning performance are even more pronounced in movement disorders. Individuals with cerebellar ataxia use more steps and require a longer time to turn, taking shorter steps with a wider step width and adopting a more extended knee position compared to controls.59,60 These changes in turning performance in cerebellar ataxia may relate to deficits in intralimb coordination and/or compensatory strategies to reduce instability during turns.
Turning is also impaired in PD, in keeping with evidence that striatal activation is associated with turns.61 Even those with mild PD and normal straight walking performance often have turning difficulty.62 These early changes in turning performance can be captured using wearable sensors to monitor turning performance63,64, and changes in turn duration may be a useful measure of progression in early PD.65 In those with more advanced PD, turning is often obviously impaired as observed by increased turn duration, greater number of steps to turn66,67, and difficultly switching motor patterns from straight walking to turning.68 Performance of turns, and functional mobility more generally, are related to increased postural tone, particularly in the neck.69 Difficulty turning in PD may also be related to the inherently asymmetric nature of turning, which requires asymmetric step lengths and leg velocities.70 Sharper, and therefore more asymmetric, turns are associated with increased step time variability and more freezing in individuals with PD and a history of freezing of gait.71 However, the interaction between the asymmetric nature of turning and the asymmetric nature of PD requires further study, as current evidence suggests that turning toward the disease-dominant side is associated with higher cadence but not with increased frequency of freezing72.
Recent work on turning in PD highlights the importance of altered oculomotor control, noting that relative to controls individuals with PD make fewer preparatory saccades approaching a turn73 and initiate turns with saccades that are slower and smaller.74 Those with PD also demonstrate slowness of head and trunk reorientation movements which may be compensated by greater contribution of eye movements than of head/trunk movements to achieve gaze shifts associated with turning.75 In fact, the characteristics of the saccade initiating a turn are predictive of ensuing turn performance; turns initiated with larger, faster saccades are executed more quickly than turns initiated with smaller, slower saccades.74 Initial turning saccade amplitude and velocity, and overall turn performance, improve with subthalamic nucleus deep brain stimulation.76 Cueing also can improve speed of turning in PD77 and may reduce freezing of gait associated with turning as long as the cues are present, with minimal carryover to uncued conditions.72
Like turning, backward walking represents another challenging locomotor task that continues to yield key insights into locomotor control. Backward walking is associated with greater stride time variability than forward walking78 and is more impaired in elderly individuals, in fallers, and in individuals with PD.79,80 Among those with PD, both forward and backward walking respond similarly to levodopa and to deep brain stimulation.81,82
The movement patterns during backward walking are remarkably similar to time-reversed profiles of forward walking.83 Similar muscles can be utilized to control the COM during forward as during backward walking, with additional supraspinal elements for propulsion helping to partially reconfigure lower level networks that may be common to both backward and forward walking.84 This is debatable, however, as other studies examining locomotor adaptations suggest the presence of separate spinal networks for forward and backward walking, as the two walking directions can be adapted independently of one another during split-belt treadmill training.85 Split-belt treadmill paradigms, as well as other approaches utilizing moving surfaces such as a moving sled or rotating treadmill, have yielded many important insights about locomotor adaptation that are beyond the scope of this paper (for split-belt review see Torres-Ovideo et al.86).
Current Knowledge Gaps and Future Directions
While our understanding of postural control during locomotion has grown substantially over recent years, there remain many gaps in our knowledge. One factor that has limited our understanding of dynamic postural control is the difficulty inherent in neuroimaging studies of locomotor tasks. For example, techniques such as functional magnetic resonance imaging (fMRI) are only possible if there is minimal head movement, obviating the use of fMRI and other movement-limited techniques in the imaging of actual locomotion. Recent work using imagined locomotor tasks has begun to partially tackle this issue, while emerging techniques such as near infrared spectroscopy78 and high-density electroencephalography recorded during actual ongoing locomotion87 hold additional promise for studies of brain activity during ongoing locomotor activities. These methods could also be used for tasks that pose particular challenges to postural control such as obstacle avoidance or walking on a narrow beam. It should be noted, however, that these methods also have inherent limitations such as inability to assess activity in subcortical areas.
Another major limitation of most published work is the focus on short-term measures of locomotion in laboratory settings. Given the growing appreciation for the importance of gait variability along with the emergence of long-term monitoring technologies such as inertial sensors63,65, the field is ripe for studies of everyday locomotor function across days88,89. These studies could provide important insights into gait stability over time in health, aging and disease. Studies of disease should consider not just PD, the most common movement disorder, but also other conditions such as progressive supranuclear palsy, essential tremor, HD, and other diseases that have been little studied compared to PD. These studies would benefit from the use of the comprehensive battery of gait tasks outlined in Table 1. Assessment of performance in different populations across different gait tasks would provide key information to enable determination of whether or not particular profiles exist for different conditions and whether difficulties in postural control on a select set of tasks might be useful for discrimination among conditions. Finally, beyond comprehensive determination of how aging and different diseases impact coordination and consistency of postural control across tasks, there is also a clear need for studies that examine the effects of targeted interventions on dynamic postural control. Given the growing appreciation for the role of eye movements in dynamic control of gait, future studies could examine the effects of eye movement training and teaching of specific visual sampling strategies in order to address deficits in dynamic locomotor performance across different populations. Intervention studies should also consider incorporating neuroimaging to examine the neural underpinnings of changes in postural control through rehabilitative, pharmacologic, surgical or combinatorial approaches. Finally, studies are needed to guide optimization of training paradigms to enhance postural control and maximize transfer of benefit across locomotor tasks and different environmental contexts.
Conclusion
Dynamic postural control during locomotion involves a critical interplay of environment, goals, biomechanical constraints, and sensory integration. At the foundation of postural control is production of a fundamental, coordinated locomotor pattern where appropriate relationships of body segments to one another and to the environment are produced in order to provide progression and stability during walking. The consistency of this coordinated pattern from step to step and across longer periods of time is also a key consideration, as variability of gait is a unique domain that provides additional predictive insight regarding fall risk and future mobility decline. Higher level postural control requires adaptability in the face of challenges introduced through different gait tasks. Assessment of coordination and consistency in the face of challenges is key to the comprehensive assessment and study of dynamic postural control. With the emergence of new models, new tools, and new intervention strategies the field is poised for substantial growth in our understanding and treatment of dynamic postural control across locomotor tasks.
Acknowledgments
This work was supported through grants from the National Institutes of Health (R01 NS077959, UL1 TR000448). General support came from the Greater St. Louis American Parkinson Disease Association (APDA) and the APDA Center for Advance PD Research at Washington University.
Footnotes
Financial disclosure related to research covered in this article: This work was funded by NIH grants R01 NS077959 and UL1 RR000448, the Greater St. Louis American Parkinson Disease Association (APDA) and the APDA Center for Advanced PD Research at Washington University.
Full financial disclosure for the previous 12 months: Gammon Earhart has received funding from the following sources in the last 12 months: NIH (R01 NS077959, UL1 RR000448, R01 HD070855, R01 NS041509), the Greater St. Louis American Parkinson Disease Association, Parkinson’s Disease Foundation, and a grant from the Washington University Department of Neurology funded by HealthSouth Corporation.
Gammon Earhart was responsible for the organization, execution, drafting, review and revision of this manuscript. The concept for the paper and guidelines regarding desired focus of the work were provided by the editors of this special issue.
References
- 1.Feldman AG, Krasovsky T, Banina MC, Lamontagne A, Levin MF. Changes in the referent body location and configuration may underlie human gait, as confirmed by findings of multi-muscle activity minimizations and phase resetting. Exp Brain Res. 2011;210(1):91–115. doi: 10.1007/s00221-011-2608-0. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. 2009;102(3):1411–9. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong RK, Chastan N, Welter ML, Do MC. Age-related changes in the center of mass velocity control during walking. Neurosci Lett. 2009;458(1):23–7. doi: 10.1016/j.neulet.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Chastan N, Do MC, Bonneville F, Torny F, Bloch F, Westby GW, et al. Gait and balance disorders in Parkinson’s disease: impaired active braking of the fall of centre of gravity. Mov Disord. 2009;24(2):188–95. doi: 10.1002/mds.22269. [DOI] [PubMed] [Google Scholar]
- 5.Hamacher D, Singh NB, Van Dieen JH, Heller MO, Taylor WR. Kinematic measures for assessing gait stability in elderly individuals: a systematic review. J R Soc Interface. 2011;8(65):1682–98. doi: 10.1098/rsif.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait Posture. 2010;31(2):175–9. doi: 10.1016/j.gaitpost.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):983–8. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21(2):178–85. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, et al. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol. 1997;82(1):262–9. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187–94. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 13.Delval A, Bleuse S, Simonin C, Delliaux M, Rolland B, Destee A, et al. Are gait initiation parameters early markers of Huntington’s disease in pre-manifest mutation carriers? Gait Posture. 2011;34(2):202–7. doi: 10.1016/j.gaitpost.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–7. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 15.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1–2):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 16.Roemmich RT, Nocera JR, Vallabhajosula S, Amano S, Naugle KM, Stegemoller EL, et al. Spatiotemporal variability during gait initiation in Parkinson’s disease. Gait Posture. 2012;36(3):340–3. doi: 10.1016/j.gaitpost.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res. 2007;181(4):561–70. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- 18.Peterson DS, Plotnik M, Hausdorff JM, Earhart GM. Evidence for a relationship between bilateral coordination during complex gait tasks and freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(9):1022–6. doi: 10.1016/j.parkreldis.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008;27(8):1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- 20.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80(3):347–50. doi: 10.1136/jnnp.2008.157362. [DOI] [PubMed] [Google Scholar]
- 21.Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23 (Suppl 2):S444–50. doi: 10.1002/mds.21984. [DOI] [PubMed] [Google Scholar]
- 22.Williams AJ, Peterson DS, Earhart GM. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture. 2013 doi: 10.1016/j.gaitpost.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams A, Peterson DS, Ionno M, Pickett KA, Earhart GM. Upper Extremity Freezing and Dyscoordination in Parkinson Disease: Effects of Amplitude and Cadence Manipulations. Parkinson’s Disease. doi: 10.1155/2013/595378. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29(7):1422–30. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- 25.Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Wenderoth N, Swinnen SP, et al. Abnormalities and cue dependence of rhythmical upper-limb movements in Parkinson patients with freezing of gait. Neurorehabil Neural Repair. 2012;26(6):636–45. doi: 10.1177/1545968311431964. [DOI] [PubMed] [Google Scholar]
- 26.Dietz V. Quadrupedal coordination of bipedal gait: implications for movement disorders. J Neurol. 2011;258(8):1406–12. doi: 10.1007/s00415-011-6063-4. [DOI] [PubMed] [Google Scholar]
- 27.Dedieu P, Zanone PG. Effects of gait pattern and arm swing on intergirdle coordination. Hum Mov Sci. 2012;31(3):660–71. doi: 10.1016/j.humov.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Winogrodzka A, Wagenaar RC, Booij J, Wolters EC. Rigidity and bradykinesia reduce interlimb coordination in Parkinsonian gait. Arch Phys Med Rehabil. 2005;86(2):183–9. doi: 10.1016/j.apmr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Carpinella I, Crenna P, Marzegan A, Rabuffetti M, Rizzone M, Lopiano L, et al. Effect of L-dopa and subthalamic nucleus stimulation on arm and leg swing during gait in Parkinson’s Disease. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6665–8. doi: 10.1109/IEMBS.2007.4353888. [DOI] [PubMed] [Google Scholar]
- 30.Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33(6):1073–84. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Brandt T, Strupp M, Benson J, Dieterich M. Vestibulopathic gait. Walking and running Adv Neurol. 2001;87:165–72. [PubMed] [Google Scholar]
- 32.Jahn K, Strupp M, Schneider E, Dieterich M, Brandt T. Differential effects of vestibular stimulation on walking and running. Neuroreport. 2000;11(8):1745–8. doi: 10.1097/00001756-200006050-00029. [DOI] [PubMed] [Google Scholar]
- 33.Rogers MW, Hilliard MJ, Martinez KM, Zhang Y, Simuni T, Mille ML. Perturbations of ground support alter posture and locomotion coupling during step initiation in Parkinson’s disease. Exp Brain Res. 2011;208(4):557–67. doi: 10.1007/s00221-010-2504-z. [DOI] [PubMed] [Google Scholar]
- 34.Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol. 2011;106(2):999–1015. doi: 10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGowan CP, Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: Adaptations to altered mechanical demands. J Biomech. 2010;43(3):412–9. doi: 10.1016/j.jbiomech.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hak L, Houdijk H, Steenbrink F, Mert A, van der Wurff P, Beek PJ, et al. Speeding up or slowing down?: Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture. 2012;36(2):260–4. doi: 10.1016/j.gaitpost.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Hak L, Houdijk H, Steenbrink F, Mert A, van der Wurff P, Beek PJ, et al. Stepping strategies for regulating gait adaptability and stability. J Biomech. 2013 doi: 10.1016/j.jbiomech.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Galna B, Murphy AT, Morris ME. Obstacle crossing in people with Parkinson’s disease: foot clearance and spatiotemporal deficits. Hum Mov Sci. 2010;29(5):84–2. doi: 10.1016/j.humov.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Stegemoller EL, Buckley TA, Pitsikoulis C, Barthelemy E, Roemmich R, Hass CJ. Postural instability and gait impairment during obstacle crossing in Parkinson’s disease. Arch Phys Med Rehabil. 2012;93(4):703–9. doi: 10.1016/j.apmr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Kloter E, Dietz V. Obstacle avoidance locomotor tasks: adaptation, memory and skill transfer. Eur J Neurosci. 2012;35(10):1613–21. doi: 10.1111/j.1460-9568.2012.08066.x. [DOI] [PubMed] [Google Scholar]
- 41.Dietz V, Michel J. Locomotion in Parkinson’s disease: neuronal coupling of upper and lower limbs. Brain. 2008;131(Pt 12):3421–31. doi: 10.1093/brain/awn263. [DOI] [PubMed] [Google Scholar]
- 42.Pijnappels M, Kingma I, Wezenberg D, Reurink G, van Dieen JH. Armed against falls: the contribution of arm movements to balance recovery after tripping. Exp Brain Res. 2010;201(4):689–99. doi: 10.1007/s00221-009-2088-7. [DOI] [PubMed] [Google Scholar]
- 43.Krasovsky T, Lamontagne A, Feldman AG, Levin MF. Reduced gait stability in high-functioning poststroke individuals. J Neurophysiol. 2013;109(1):77–88. doi: 10.1152/jn.00552.2012. [DOI] [PubMed] [Google Scholar]
- 44.Logan D, Kiemel T, Dominici N, Cappellini G, Ivanenko Y, Lacquaniti F, et al. The many roles of vision during walking. Exp Brain Res. 2010;206(3):337–50. doi: 10.1007/s00221-010-2414-0. [DOI] [PubMed] [Google Scholar]
- 45.Berard J, Fung J, Lamontagne A. Impact of aging on visual reweighting during locomotion. Clin Neurophysiol. 2012;123(7):1422–8. doi: 10.1016/j.clinph.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 46.Diehl MD, Pidcoe PE. The influence of gaze stabilization and fixation on stepping reactions in younger and older adults. J Geriatr Phys Ther. 2010;33(1):19–25. [PubMed] [Google Scholar]
- 47.Chapman GJ, Hollands MA. Evidence for a link between changes to gaze behaviour and risk of falling in older adults during adaptive locomotion. Gait Posture. 2006;24(3):288–94. doi: 10.1016/j.gaitpost.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Greany JF, Di Fabio RP. Saccade to stepping delays in elders at high risk for falling. Aging Clin Exp Res. 2008;20(5):428–33. doi: 10.1007/BF03325148. [DOI] [PubMed] [Google Scholar]
- 49.Glaister BC, Bernatz GC, Klute GK, Orendurff MS. Video task analysis of turning during activities of daily living. Gait Posture. 2007;25(2):289–94. doi: 10.1016/j.gaitpost.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Segal AD, Orendurff MS, Czerniecki JM, Shofer JB, Klute GK. Local dynamic stability in turning and straight-line gait. J Biomech. 2008;41(7):1486–93. doi: 10.1016/j.jbiomech.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Reed-Jones R, Reed-Jones J, Vallis LA, Hollands M. The effects of constraining eye movements on visually evoked steering responses during walking in a virtual environment. Exp Brain Res. 2009;197(4):357–67. doi: 10.1007/s00221-009-1923-1. [DOI] [PubMed] [Google Scholar]
- 52.Akram SB, Frank JS, Fraser J. Coordination of segments reorientation during on-the-spot turns in healthy older adults in eyes-open and eyes-closed conditions. Gait Posture. 2010;32(4):632–6. doi: 10.1016/j.gaitpost.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Bernardin D, Kadone H, Bennequin D, Sugar T, Zaoui M, Berthoz A. Gaze anticipation during human locomotion. Exp Brain Res. 2012;223(1):65–78. doi: 10.1007/s00221-012-3241-2. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy PM, Cressman EK, Carlsen AN, Chua R. Assessing vestibular contributions during changes in gait trajectory. Neuroreport. 2005;16(10):1097–100. doi: 10.1097/00001756-200507130-00013. [DOI] [PubMed] [Google Scholar]
- 55.Xu D, Carlton LG, Rosengren KS. Anticipatory postural adjustments for altering direction during walking. J Mot Behav. 2004;36(3):316–26. doi: 10.3200/JMBR.36.3.316-326. [DOI] [PubMed] [Google Scholar]
- 56.Pham QC, Berthoz A, Hicheur H. Invariance of locomotor trajectories across visual and gait direction conditions. Exp Brain Res. 2011;210(2):207–15. doi: 10.1007/s00221-011-2619-x. [DOI] [PubMed] [Google Scholar]
- 57.Cinelli M, Patla A, Stuart B. Age-related differences during a gaze reorientation task while standing or walking on a treadmill. Exp Brain Res. 2008;185(1):157–64. doi: 10.1007/s00221-007-1266-8. [DOI] [PubMed] [Google Scholar]
- 58.Fuller JR, Adkin AL, Vallis LA. Strategies used by older adults to change travel direction. Gait Posture. 2007;25(3):393–400. doi: 10.1016/j.gaitpost.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Mari S, Serrao M, Casali C, Conte C, Ranavolo A, Padua L, et al. Turning strategies in patients with cerebellar ataxia. Exp Brain Res. 2012;222(1–2):65–75. doi: 10.1007/s00221-012-3197-2. [DOI] [PubMed] [Google Scholar]
- 60.Serrao M, Mari S, Conte C, Ranavolo A, Casali C, Draicchio F, et al. Strategies Adopted by Cerebellar Ataxia Patients to Perform U-Turns. Cerebellum. 2013 doi: 10.1007/s12311-012-0441-z. [DOI] [PubMed] [Google Scholar]
- 61.Wagner J, Stephan T, Kalla R, Bruckmann H, Strupp M, Brandt T, et al. Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Exp Brain Res. 2008;191(2):247–55. doi: 10.1007/s00221-008-1520-8. [DOI] [PubMed] [Google Scholar]
- 62.Crenna P, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R, et al. The association between impaired turning and normal straight walking in Parkinson’s disease. Gait Posture. 2007;26(2):172–8. doi: 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 63.King LA, Mancini M, Priest K, Salarian A, Rodrigues-de-Paula F, Horak F. Do clinical scales of balance reflect turning abnormalities in people with Parkinson’s disease? J Neurol Phys Ther. 2012;36(1):25–31. doi: 10.1097/NPT.0b013e31824620d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81(2):171–6. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salarian A, Zampieri C, Horak FB, Carlson-Kuhta P, Nutt JG, Aminian K. Analyzing 180 degrees turns using an inertial system reveals early signs of progression of Parkinson’s disease. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:224–7. doi: 10.1109/IEMBS.2009.5333970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huxham F, Baker R, Morris ME, Iansek R. Footstep adjustments used to turn during walking in Parkinson’s disease. Mov Disord. 2008;23(6):817–23. doi: 10.1002/mds.21932. [DOI] [PubMed] [Google Scholar]
- 67.Stack E, Ashburn A. Dysfunctional turning in Parkinson’s disease. Disabil Rehabil. 2008;30(16):1222–9. doi: 10.1080/09638280701829938. [DOI] [PubMed] [Google Scholar]
- 68.Mak MK, Patla A, Hui-Chan C. Sudden turn during walking is impaired in people with Parkinson’s disease. Exp Brain Res. 2008;190(1):43–51. doi: 10.1007/s00221-008-1446-1. [DOI] [PubMed] [Google Scholar]
- 69.Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol. 2009;219(2):430–8. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Courtine G, Schieppati M. Human walking along a curved path. II. Gait features and EMG patterns. Eur J Neurosci. 2003;18(1):191–205. doi: 10.1046/j.1460-9568.2003.02737.x. [DOI] [PubMed] [Google Scholar]
- 71.Bhatt H, Pieruccini-Faria F, Almeida QJ. Dynamics of turning sharpness influences freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(2):181–5. doi: 10.1016/j.parkreldis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Spildooren J, Vercruysse S, Meyns P, Vandenbossche J, Heremans E, Desloovere K, et al. Turning and unilateral cueing in Parkinson’s disease patients with and without freezing of gait. Neuroscience. 2012;207:298–306. doi: 10.1016/j.neuroscience.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 73.Galna B, Lord S, Daud D, Archibald N, Burn D, Rochester L. Visual sampling during walking in people with Parkinson’s disease and the influence of environment and dual-task. Brain Res. 2012;1473:35–43. doi: 10.1016/j.brainres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 74.Lohnes CA, Earhart GM. Saccadic Eye Movements Are Related to Turning Performance in Parkinson Disease. J Parkinsons Dis. 2011;1(1):109–18. doi: 10.3233/JPD-2011-11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anastasopoulos D, Ziavra N, Savvidou E, Bain P, Bronstein AM. Altered eye-to-foot coordination in standing parkinsonian patients during large gaze and whole-body reorientations. Mov Disord. 2011;26(12):2201–11. doi: 10.1002/mds.23798. [DOI] [PubMed] [Google Scholar]
- 76.Lohnes CA, Earhart GM. Effect of subthalamic deep brain stimulation on turning kinematics and related saccadic eye movements in Parkinson disease. Exp Neurol. 2012;236(2):389–94. doi: 10.1016/j.expneurol.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieuwboer A, Baker K, Willems AM, Jones D, Spildooren J, Lim I, et al. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil Neural Repair. 2009;23(8):831–6. doi: 10.1177/1545968309337136. [DOI] [PubMed] [Google Scholar]
- 78.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage. 2012;59(2):1602–7. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 79.Fritz NE, Worstell AM, Kloos AD, Siles AB, White SE, Kegelmeyer DA. Backward walking measures are sensitive to age-related changes in mobility and balance. Gait Posture. 2012 doi: 10.1016/j.gaitpost.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 80.Hackney ME, Earhart GM. Backward walking in Parkinson’s disease. Mov Disord. 2009;24(2):218–23. doi: 10.1002/mds.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bryant MS, Rintala DH, Hou JG, Lai EC, Protas EJ. Effects of levodopa on forward and backward gait patterns in persons with Parkinson’s disease. NeuroRehabilitation. 2011;29(3):247–52. doi: 10.3233/NRE-2011-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McNeely ME, Earhart GM. Medication and subthalamic nucleus deep brain stimulation similarly improve balance and complex gait in Parkinson disease. Parkinsonism Relat Disord. 2013;19(1):86–91. doi: 10.1016/j.parkreldis.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80(4):1868–85. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- 84.Jansen K, De Groote F, Massaad F, Meyns P, Duysens J, Jonkers I. Similar muscles contribute to horizontal and vertical acceleration of center of mass in forward and backward walking: implications for neural control. J Neurophysiol. 2012;107(12):3385–96. doi: 10.1152/jn.01156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10(8):1055–62. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 86.Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res. 2011;191:65–74. doi: 10.1016/B978-0-444-53752-2.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lau TM, Gwin JT, McDowell KG, Ferris DP. Weighted phase lag index stability as an artifact resistant measure to detect cognitive EEG activity during locomotion. J Neuroeng Rehabil. 2012;9:47. doi: 10.1186/1743-0003-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Terashi H, Utsumi H, Ishimura Y, Takazawa T, Okuma Y, Yoneyama M, et al. Deficits in scaling of gait force and cycle in parkinsonian gait identified by long-term monitoring of acceleration with the portable gait rhythmogram. ISRN Neurol. 2012;2012:306816. doi: 10.5402/2012/306816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terashi H, Utsumi H, Ishimura Y, Mitoma H. Independent Regulation of the Cycle and Acceleration in Parkinsonian Gait Analyzed by a Long-Term Daily Monitoring System. Eur Neurol. 2012;69(3):134–41. doi: 10.1159/000345266. [DOI] [PubMed] [Google Scholar]