Abstract
In this communication, a translational clinical brain-machine interface (BMI) roadmap for an EEG-based BMI to a robotic exoskeleton (NeuroRex) is presented. This multi-faceted project addresses important engineering and clinical challenges: It addresses the validation of an intelligent, self-balancing, robotic lower-body and trunk exoskeleton (Rex) augmented with EEG-based BMI capabilities to interpret user intent to assist a mobility-impaired person to walk independently. The goal is to improve the quality of life and health status of wheelchair-bounded persons by enabling standing and sitting, walking and backing, turning, ascending and descending stairs/curbs, and navigating sloping surfaces in a variety of conditions without the need for additional support or crutches.
I. INTRODUCTION
In 2008, approximately 1.9% of the U.S. population reported some form of paralysis resulting in difficulty or inability to move their arms or legs [1]. Of those, 23% reported being paralyzed due to a spinal cord injury (SCI). There are approximately 12,000 new SCI cases each year [2]. According to The University of Alabama National Spinal Cord Injury Statistical Center and the Centers for Disease Control and Prevention (CDC), the costs of living with SCI can be considerable, and vary greatly due to the severity of injury. One recent estimate indicates that SCI alone costs roughly $40.5 billion annually [1-2]. According to the CDC, it is estimated that by developing novel therapies and preventing new injuries, the United States would save as much as $400 billion on future direct and indirect lifetime costs, thereby reducing the socio-economic burden of disability in the US.
In the last decade, advances in robotic technologies, actuators & sensors, new materials, control algorithms, and miniaturization of computers have lead to the development of wearable lower-body exoskeleton robotic orthoses that augment strength, endurance, and/or mobility of humans. The Cyberdyne’s robot suit HAL [3] is a cyborg-type robot suit that can expand and improve physical capabilities. A hybrid control system combines a voluntary control system, which detects very weak bio-signals from surface sensors on the patient’ skin, with a robotic autonomous control system that provides human-like movements by generating torque that produces limb movements that assist the user in performing intended movements. HAL is internet-enabled, requires upper body function and it is only available in Japan, where is rented to hospitals and rehabilitation clinics. ReWalk, the first commercially available upright walking technology in the US, enables wheelchair users with lower-limb disabilities to stand, walk, and even climb stairs [4]. Currently, it is only suitable for lower-limb mobility impaired adults who have functioning hands, arms and shoulders (as it requires upper arm function to support the body with crutches), as well as the ability to stand (it requires a healthy skeleton and cardio-vascular system). RexBionics’ Robotic Exoskeleton (Rex) is a self-supporting, independently controlled robotic walking device that enables a person with mobility impairment to stand up and walk [5]. It can perform basic functions such as stand-up, sit-down, walk, turn, stair-up and stair-down without the need for crutches or walkers. EksoBionics’ exoskeleton (Ekso) uses remote control (normally operated by a physical therapist) to signal the left or right leg to step forward, while the user’s job, using instrumented crutches, is to balance his/her upper body, shifting the body weight during walking [6]. University of Delaware’s active leg exoskeleton (ALEX) exoskeleton has been designed for gait rehabilitation [7]. It uses a force-field controller, which can apply suitable forces on the leg to help it move on along a desired trajectory. The interaction forces between the subject and the orthosis are designed to be ‘assist-as-needed’ for safe and effective gait training. ALEX however is limited to treadmill-based rehabilitation. NASA’s X1 lower-limb exoskeleton [8], initially designed to help astronauts stay healthier in space, may have the added benefit of assisting paraplegics in walking. The 57-pound device is a robot that a human could wear over his or her body either to assist or inhibit movement in leg joints. More recently, Parker Hannifin has licensed the Vanderbilt’s powered skeleton and renamed Indego [9]. At 27lbs, Indego is modular, small and allows users to stand and walk by using sensors that determine if the patient is standing upright, sitting or leaning and perform accordingly. Thus, if the standing patient leans forward, the skeleton will bend its knee, swivel its hip joint and take a step; when the patient stops leaning forward, the device stops walking. Indego is the only wearable device that incorporates functional electrical stimulation (FES).
These robotic rehabilitation systems have the potential to offer individualized therapy, increased efficiency of training at a lower cost, and new sensing capabilities to the physical therapist to quantify patient’s progress. Robotic devices that provide feedback to the user, harness user intent, and provide assist-as-needed functionality (e.g., undesirable gait motion is resisted and assistance is provided toward desired motion) may also enhance motor learning and therefore neurological rehabilitation. The availability of safe and reliable robotic therapy can also facilitate intense practice -at a reasonable cost- as well as continuous challenge during rehabilitation, which is known to accelerate recovery and improve rehabilitation outcomes. However, most exoskeleton devices are currently limited to patients with intact upper body function for aided support via crutches (a notable exception is Rex, which does not require crutches for balancing and stability). This is an important limitation in current exoskeleton systems as stroke patients and quadriplegics lack control on at least one side of their bodies and cannot use a walker or crutches to stabilize their body effectively. Moreover, exoskeleton control depends on residual motor signals at the periphery (HAL, ALEX, ReWalk), fine motor control (Rex), or external control via joystick (Ekso); therefore further limiting the type of patients that can benefit from these devices. Currently, Rex is the only robotic platform that provides independent, unassisted walking capabilities. Indego on the other hand is the only exoskeleton that provides the option of functional electrical stimulation in a small light package. A common set of challenges for these systems include shared control issues, the regulatory path to follow for use at the clinic and home, cost, and reliability.
In this paper we present the clinical and systems engineering roadmap for NeuroRex - the first BMI-capable robotic exoskeleton that can interpret user intent to assist a mobility-impaired person to walk independently without the need for additional support or crutches. We first identify critical scientific, clinical and engineering challenges:
II. CHALLENGES
A. Reliable BMI Systems
There is a critical need for reliable BMIs that interpret user intent directly from brain signals and make context-based decisions from the user’s current internal state, thus allowing direct and voluntary operation of their exoskeletons beyond their diminished physical, cognitive or sensory capabilities. This involves developing 1) reliable discrete (classifiers) and/or continuous (model-based) neural interfaces to predict the user’s intent (at both high and low levels) from EEG; 2) developing BMI-robot systems with long-term prognostic-based reliability and fault-tolerant performance, 3) self-calibration, 4) self-diagnostic capabilities with backward-forward failure attribution analysis and error-correction, and 5) suitable behavioral testing methods for reliability and performance assessments of the system.
B. Shared Control
BMI systems should allow for multitasking, require minimal effort and release attentional resources to other cognitive-motor tasks. This implies a coordinated effort (shared-control) between brain control and autonomous robot control, whereby intelligent robot control algorithms can implement intended user’s goals extracted via the BMI system without demanding continuous supervisory control, but rather ‘assist-as-needed’ control from the neural interface.
C. Safety vs. Benefit
Clinical evaluation of NeuroRex requires systematic safety and tolerability assessment of key cardio-metabolic, musculoskeletal, skin, and biomechanical factors along with assessment of neurological and cognitive-behavioral deficit profiles that define the user profile. Cardiopulmonary safety is paramount as individuals with stroke and SCI may have autonomic instability that can alter blood pressure, and their heart rates may not reflect or respond correctly to increased cardiopulmonary demands, depending on the lesion level and completeness [10-11]. The cardiopulmonary demands of steady state and sustained BMI-Robot usage must be initially assessed and carefully monitored for two further reasons: the mean peak cardiovascular fitness levels after spinal cord injury vary considerably depending on the lesion characteristics, but are generally much lower than normal; and skeletal muscle after SCI (or any CNS injury such as stroke) shifts in a deficit severity dependent manner from slow twitch to a fast twitch molecular phenotype, which predisposes to anaerobic metabolism, reduced insulin sensitivity, and oxidative injury. Patients with abnormal gait biomechanics, anaerobic muscle metabolism, and fitness levels similar to those in heart failure patients must show adequate cardiopulmonary tolerance based on subject perceived exertion scales, and objective monitoring of cardiopulmonary and metabolic profiles. These metabolic measures, along with careful clinical surveillance and blood markers to assess for muscle injury are key to validating cardiopulmonary, metabolic, and muscle safety during exoskeleton use. Rehabilitation clinician-scientists are highly aware that robotics may impose unusual joint kinetics and kinematics that could potentially injure bone or skin, particularly in SCI or stroke populations that characteristically have accelerated osteopenia or osteoporosis, unusual spasticity patterns, abnormal movement synergy patterns, or contractures. Systematic screening for bone health using dual X ray absorptiometry and assessment ahead of time for “hot spots” of abnormal torque or impulses that could predispose to injury is vital to safe utilization. While impedance control and torque cut-offs successfully assure safety in lower extremity robotics, cumulative experience is limited for mobility devices, warranting caution and careful consideration between engineers, clinicians, and individuals with neurological disability to appropriately apply this exciting new technology.
D. Reverse Engineering the Brain
A better understanding of the neural representations, at the cortical level, for action and perception of bipedal locomotion is essential for evaluating changes in cortical dynamics during rehabilitation using closed-loop NeuroRex, and assessing how these changes are correlated with gait adaptation induced by BMI-robot therapy.
II. METHODS
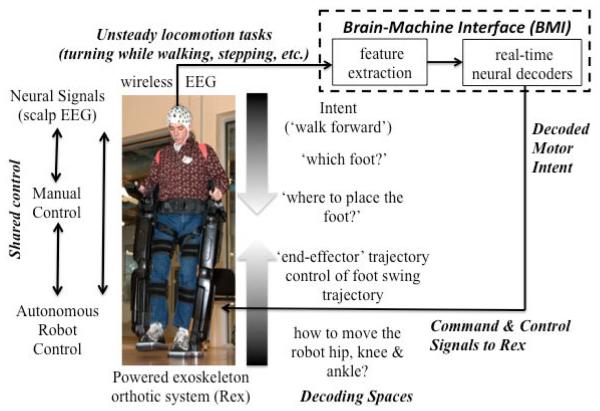
The goal of the this ongoing study is to demonstrate that NeuroRex will be safe and simple to use, have emergency backup systems in the event of failure and have a practical range (Fig. 1). A partnership between the University of Houston and The Methodist Hospital provides the core engineering and clinical setting for validation of NeuroRex.
Figure 1.
Closed-loop EEG-based BMI-to-Rex system. Wireless EEG systems, sophisticated machine learning, system identification methods, and shared control approaches to minimize cognitive effort (and allow multitasking) will be deployed to calibrate the neural interface, control the powered exoskeleton and to reverse-engineer the neural representations for gait production. The BMI will coordinate among brain control (intent), manual control (when available), and the autonomous robot control algorithms. Decoding spaces for BMI control are shown.
A. Human Subjects
The first step is to determine the sensory-motor profile of two classes of individuals, those with paraparesis and those with complete paraplegia whose locomotion can be enhanced by the use of Rex. That is, the severity and neurological segmental levels of motor and sensory deficits that an individual must have to: 1) Benefit from the use of a BMI-Rex (e.g. whether repetitive training and use of Rex leads to gains in mobility, health and quality of life); and 2) be capable of interacting with BMI-Rex to achieve useful mobility. Of particular importance is knowledge of the strength required for maintaining an erect posture in the exoskeleton, the strength required in muscles of the legs, hips, trunk, shoulders, arms, hands and neck.
It is also important to determine if BMI control of Rex provides additional functionality (e.g., multitasking, increased cortical plasticity leading to shorter sensorimotor intervention periods) for individuals who are able to control Rex using hand controls. Thus, we perform comprehensive clinical assessments to ensure safety, assess the extent of cognitive-motor-body adaptations during robot use, and determine whether BMI can replace hand controls and/or cooperate with Rex’s embedded autonomous control schemes to decrease user’s cognitive load.
B. Research Design and Methods
B1. Primary Outcomes
- Maximum degree of motility achieved in:
- Standing from a sitting position. Measure: time to complete action.
- Walking in a straight line. Standardized tests: Measure: 6 minute walk; 10 m walk [12]
- Turning right and left: Measure: modification of the 6 minute and 10 m walks
- Navigating obstacles: Measure: time, number of errors.
- Stand-to-sit and sit-to-stand: Measure: time to complete; errors.
- Climbing, descending stairs. Measure: time to complete, errors.
- fMRI-EEG identification of the neuroanatomical sources of brain signals for BMI control of Rex. Two approaches have been taken with respect to the brain signals used to control the movement of a robot:
- Recording from neurons in the sensory-motor cortex whose firing can be time-linked to the desired movement, and is predictive of gait kinematics [13].
- Recording patterns of the scalp EEG from broad areas of the cortex and correlating them with the desired movement [14-15], which is our approach (Fig. 2). The successful use of this method indicates that information linked to movements is widely distributed throughout the brain. The present study correlates scalp recorded EEG activity with functional BOLD signal magnetic resonance imaging (fMRI) activation and de-activation of cortical and subcortical areas during willed movement. Our protocol will first investigate the motor paradigm of initiating a step with leg flexion. Subsequently stepping movements will be imitated by using a recumbent cycling pedaling apparatus.
Time-resolved examination of how cortical networks may adapt to changes in the neural representation of gait due to NeuroRex use. Human locomotor studies involving patients with stroke and SCI suggest that bipedal interlimb coordination requires some level of cerebral control [16-17]. Patients with cerebral damage from stroke show problems in interlimb phasing resulting in asymmetric walking patterns [18-19]. Split-belt treadmill adaptation experiments have shown that right and left legs can be trained individually in healthy subjects [20], for example, by training subject’s legs to walk at different speeds in the same or different directions. This type of manipulation results in early asymmetric walking as interlimb coordination is phase shifted and step lengths become asymmetric not unlike walking with a ‘limp’; however, with practice subjects can improve phasing and reduce gait asymmetries [20]. Analyses of aftereffects showed that locomotor training is both leg- and direction-specific. Bastian et al showed split-belt adaptation partially transfers to overground walking in patients poststroke, and then it could have implications for the restoration of gait function in these patients [21]. We are examining the changes in the cortical contributions to gait, as well as the metabolic, physiological, and biomechanical adaptations induced by the NeuroRex intervention.
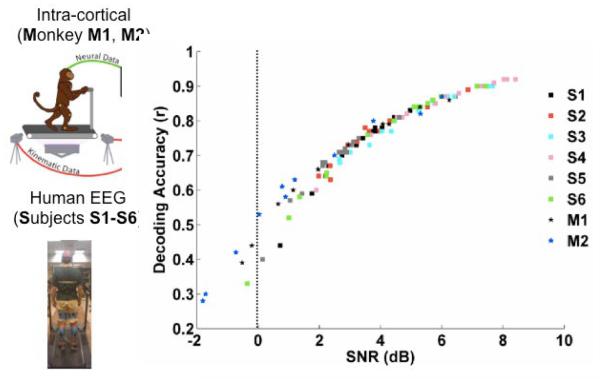
Figure 2.
Decoding of gait kinematics from EEG and intracranial electrodes. Decoding accuracies (Pearson’s r) of non-invasive (EEG, [14-15]) as compared with intra-cortical (spikes-based, [13]) neural decoders for inferring gait parameters demonstrate the feasibility of designing non-invasive BMI systems for command and control of robotic exoskeletons.
B2. Secondary Outcomes
Longitudinal following of improvement or regression in muscle strength, balance and gait function, and in health and quality of life (QOL) measures, including:
Muscle strength – Measure: muscle mass, ASIA motor examination, isokinetic dynamometry [22].
Cardiovascular Function – Measure: blood pressure and orthostatic hypotension [23].
Pulmonary Function – Measure: standardized test of forced vital capacity [24].
Spinal Cord Independence measure (SCIM) – Measure: standardized test: SCIM [25].
Dynamic Postural Stability – Measures: inertial sensors, goniometers, EMG, pressure/load sensors.
Balance & Gait Function (paraparetic group): postural & gait stability, step length/step frequency.
Bone densitometry.
III. DISCUSSION
The use of NeuroRex can be considered to be an augmented form of Locomotor Therapy (LT). LT, implemented by weight-supported treadmill walking facilitated by manually assisted movements of the subjects’ legs by 2 therapists, or by a robotic device such as the Lokomat, has been shown to improve walking measures and balance in AIS C and D subjects in controlled studies [28]. Uncontrolled reports of improvement in orthostasis, vital capacity and bowel and bladder have been given with the use of LT and the use of REX. Thus, one goal of our study is to determine with objective measurements if there are health benefits associated with the use of REX without/with a BMI system. A companion presentation at this conference reviews the initial validation of NeuroRex in a paraplegic patient with spinal cord injury (Kilicarslan et al; see also Bulea et al., this 2013 IEEE EMBS conference).
Footnotes
Research was supported in part by NIH R01NS075889 and Mission Connect - A project of the TIRR Foundation. We gratefully acknowledge Eugene Alford MD, for his advice and support of this project.
Contributor Information
Jose L. Contreras-Vidal, Department of Electrical and Computer Engineering, University of Houston, TX 77004, USA and the Department of Neurosurgery at The Methodist Hospital Research Institute (jlcontreras-vidal@uh.edu).
Robert G. Grossman, Chairman of the Department of Neurosurgery and Co-Director, The Neurological Institute, The Methodist Hospital, Houston, TX 77030, USA (Rgrossman@tmhs.org)
REFERENCES
- [1].One Degree of Separation: Paralysis and Spinal Cord Injury in the United States. The Christopher & Dana Reeve Found; 2009. [Google Scholar]
- [2].Spinal Cord Injury Facts and Figures at a Glance. National Spinal Cord Injury Statistical Center; Birmingham, Alabama: 2009. [Google Scholar]
- [3]. http://www.cyberdyne.jp/english/robotsuithal/
- [4]. http://www.argomedtec.com/
- [5]. http://www.rexbionics.com/
- [6]. http://berkeleybionics.com/ekso.
- [7]. http://mechsys4.me.udel.edu/research/medical_robotics/#alo.
- [8]. http://www.nasa.gov/offices/oct/home/feature_exoskeleton.html.
- [9]. http://www.parker.com/portal/site/Market-Tech/menuitem.e9f921bc8ae21676de92b210237ad1ca/?vgnextoid=1914d3ae3339a310VgnVCM100000200c1dacRCRD&vgnextfmt=default.
- [10].Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part 1: Classification and prevalence. Arch Phys Med Rehabil. 1993;74(7):752–60. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]
- [11].Roth EJ. Heart disease in patients with stroke. Part II: Impact and implications for rehabilitation. Arch Phys Med Rehabil. 1994;75(1):94–101. [PubMed] [Google Scholar]
- [12].Van Hedel HJ, Dietz V, Curt A. Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil Neural Repair. 2007;21:295–301. doi: 10.1177/1545968306297861. [DOI] [PubMed] [Google Scholar]
- [13].Fitzsimmons NA, Lebedev MA, Peikon ID, Nicolesis MA. Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity Front. Integr. Neurosci. 2009;3:3. doi: 10.3389/neuro.07.003.2009. doi:10.3389/neuro.07.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Presacco A, Goodman R, Forrester L, Contreras-Vidal JL. Neural decoding of treadmill walking from noninvasive electroencephalographic signals. J Neurophysiol. 2011;106(4):1875–87. doi: 10.1152/jn.00104.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Presacco A, Forrester L, Contreras-Vidal JL. Decoding Intra-Limb and Inter-Limb Kinematics During Treadmill Walking From Scalp Electroencephalographic (EEG) Signals. IEEE Trans Neural Syst Rehabil Eng. 2012;20(2):212–219. doi: 10.1109/TNSRE.2012.2188304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–34. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- [17].Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol. 2005;93:2460–73. doi: 10.1152/jn.00963.2004. [DOI] [PubMed] [Google Scholar]
- [18].Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–93. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- [19].Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–8. doi: 10.1161/01.STR.0000144685.88760.d7. [DOI] [PubMed] [Google Scholar]
- [20].Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–62. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- [21].Reisman DS, Wityk R, Silver K, Bastian AJ. Split-Belt Treadmill Adaptation Transfers to Overground Walking in Persons Poststroke. Neurorehabil Neural Repair. 2009;23(7):735–744. doi: 10.1177/1545968309332880. doi:10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- [23].Krassioukov A, Eng JJ, Warburton DE, Teasell R. Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil. 2009;90:876–85. doi: 10.1016/j.apmr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R. Spirometry testing standards in spinal cord injury. Chest. 2003;123:725–730. doi: 10.1378/chest.123.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, Tonack M, Hitzig SL, Glaser E, Zeilig G, Aito S, Scivoletto G, Mecci M, Chadwick RJ, El Masry WS, Osman A, Glass CA, Silva P, Soni BM, Gardner BP, Savic G, Bergstrom EM, Bluvshtein V, Ronen JA. multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–291. doi: 10.1038/sj.sc.3101960. [DOI] [PubMed] [Google Scholar]
- [26].Jain NB, Sullivan M, Kazis LE, Tun CG, Garshick E. Factors associated with health-related quality of life in chronic spinal cord injury. Am J Phys Med Rehabil. 2007;86:387–96. doi: 10.1097/PHM.0b013e31804a7d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ware JH, Sherbourne C. The MOS 36-Item Short-Form Health Survey (SF-36) Medical Care. 1992;30:473–83. [PubMed] [Google Scholar]
- [28].Harkema SJ, Schmidt-Read M, Lorenz D, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2011;93(9):1508–17. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]