Abstract
EMBO J (2013) 32: 2672–2684 ; DOI: 10.1038/emboj.2013.188; published online August 23 2013
Long noncoding RNAs (lncRNAs) are a novel class of regulators that play crucial roles in development and disease. Here we highlight the findings by Huang and colleagues that a regulatory lncRNA (treRNA) acts as a scaffold for a new ribonucleic protein complex that inhibits translation of E-cadherin and promotes cell invasion. This work underscores the potential importance of lncRNAs in cancer metastasis.
Genome-wide transcriptome sequencing efforts have led to the discovery of thousands of long noncoding RNAs (lncRNAs). LncRNAs are polyadenylated transcripts, typically more than 200 base pairs in length, that show polymerase II transcription initiation and elongation marks (Ulitsky and Bartel, 2013). Little is known about the biological roles of lncRNAs, but several potential mechanisms for the action of nuclear and cytoplasmic lncRNAs have been proposed. Nuclear lncRNAs mainly regulate the transcription of target genes by recruiting chromatin-modifying complexes to specific regions (Rinn and Chang, 2012), while cytoplasmic lncRNAs may interact with other RNAs, serve as molecular decoys for microRNAs and RNA-binding proteins, or function as cytoplasmic scaffolds of RNA–protein complexes. Interestingly, cytoplasmic lncRNAs may also interact with target mRNAs and protein complexes to regulate mRNA stability and translation (Carrieri et al, 2012; Yoon et al, 2012; Kretz et al, 2013). Recently, lncRNAs have been found to play important roles as drivers of tumour progression and as oncogenic suppressors in various cancer types. A report by Gumireddy et al (2013) in this issue now adds another lncRNA to this list by identifying a role for translational regulatory lncRNA (treRNA) in cellular invasion and metastasis.
Gumireddy et al (2013) examined the expression of treRNA in matched primary and lymph node metastatic tumours and found higher treRNA levels in all metastatic tumours, suggesting that increased treRNA expression could be associated with metastasis. To investigate treRNA function, the authors ectopically expressed the lncRNA in the non-invasive breast cancer cell line MCF7 and observed increased cell migration and invasion capacity. Conversely, knockdown of treRNA in A549 cells (a metastatic cell line with high endogenous expression of treRNA) strongly suppressed cell migration and invasion. Using an in vivo bioluminescence system, the authors furthermore found that treRNA-expressing MCF7 cells promoted lung metastasis, while depletion of treRNA in A459 cells reduced the formation of lung tumours relative to controls, substantiating a potential role for treRNA in metastasis.
Loss of E-cadherin is known to allow epithelial cells to undergo changes in cell morphology and motility in order to adopt mesenchymal characteristics, a process known as epithelial-to-mesenchymal transition (EMT; Valastyan and Weinberg, 2011). Close examination of MCF7 cells with overexpression of treRNA revealed loss of the epithelial protein markers E-cadherin, zonula occludens (Z0-1), and β-catenin, while the mesenchymal markers fibronectin and vimentin were upregulated, indicating that the cells underwent partial EMT. The mRNA levels for the epithelial markers remained constant, indicating that regulation by treRNA occurs at the protein synthesis level. Interestingly, treRNA expression was predominant in the cytoplasm, and polysome analysis confirmed that treRNA affects the translational efficiency of E-cadherin mRNA.
But how exactly does treRNA affect E-cadherin protein expression? Gumireddy et al (2013) further show that treRNA associates with the RNA-binding proteins hnRNP K, FXR1, FXR2 and PUF60, as well as with SF3B3, a subunit of splicing factor 3b. Among those proteins, hnRNP K, FXR1, FXR2, and PUF60 were necessary for treRNA-mediated suppression of E-cadherin translation. Moreover, hnRNP K, FXR1, and FXR2 were also required for treRNA-mediated metastasis formation in vivo. RNAi-mediated depletion of the three RNA-binding proteins diminished the number of lung metastases, and combinations of double and triple knockdowns completely abolished the ability of treRNA to induce metastases in the lung.
Mechanistically, pull-down experiments indicated that treRNA expression is necessary for binding of PUF60-SF3B3 to hnRNP K, FXR1, and FXR2 to form a ribonucleic complex (RNP) that associates with the translation initiation factor eIF4G1. Furthermore, the authors found that the 3′UTR of E-cadherin mRNA is required for translation suppression by the treRNA–RNP complex. The E-cadherin 3′UTR contains multiple potential binding sites for hnRNP K, FXR1, and FXR2 and mutating these binding sites in reporter constructs served to rescue the suppressive effect of treRNA, confirming these sites as docking platforms for treRNA–RNP complexes.
Collectively, the findings presented by Huang, Gummireddy and colleagues provide evidence that lncRNAs can function as cytoplasmatic scaffolds for the formation of RNP complexes (Figure 1). Such RNP complexes interact with target mRNAs to control the rate of protein synthesis for key regulators of the EMT process. However, the study of Gumireddy et al (2013) also raises several intriguing questions. First, how does treRNA specifically interact with regulatory RNA-binding proteins? Second, how many targets does treRNA–RNP have? Third, it remains to be determined how the expression of treRNA is generally controlled, and more specifically whether transcription factors that induce EMT (e.g., TWIST, SNAI1, ZEB1, and others) have a role here. Last but not least, the in vivo role of treRNA should be examined using mouse models for EMT and cancer metastasis. Nevertheless, in the past few years several studies have indicated that lncRNAs are associated with and promote metastasis (Ji et al, 2003; Gupta et al, 2010). Although the mechanism of action of these lncRNAs seems to be very different in nature, the intricate interaction between lncRNAs and tumour metastasis may have important implications for cancer diagnosis and therapy.
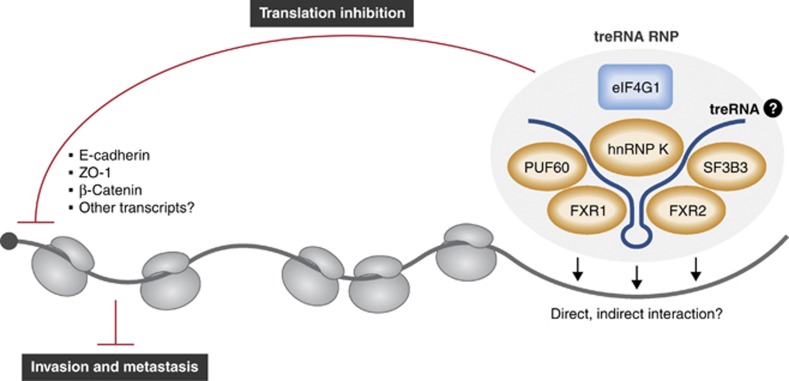
Figure 1.
TreRNA promotes the formation of a protein complex (treRNA–RNP) consisting of hnRNP K, FXR1, FXR2, PUF60, and SF3B3. treRNP interacts with the 3′UTR of E-cadherin and other transcripts, and inhibits translation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C, Forrest ARR, Carninci P, Biffo S, Stupka E, Gustincich S (2012) Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 491: 454–457 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 15: 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumireddy K, Li A, Yan J, Setoyama T, Johannes GJ, Ørom UA, Tchou J, Liu Q, Zhang L, Speicher DW, Calin GA, Huang Q (2013) Identification of a long non-coding RNA-associated RNP complex regulating metastasis at the translational step. EMBO J 32: 2672–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C (2003) MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22: 8031–8041 [DOI] [PubMed] [Google Scholar]
- Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, Lee CS, Flockhart RJ, Groff AF, Chow J, Johnston D, Kim GE, Spitale RC, Flynn RA, Zheng GXY, Aiyer S, Raj A, Rinn JL, Chang HY, Khavari PA (2013) Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81: 145–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154: 26–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147: 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M (2012) LincRNA-p21 suppresses target mrna translation. Mol Cell 4: 648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]