Abstract
Background: Findings from previous studies on the effects of air pollution exposure on lung function during childhood have been inconsistent. A common limitation has been the quality of exposure data used, and few studies have modeled exposure longitudinally throughout early life.
Objectives: We sought to study the long-term effects of exposure to particulate matter with an aerodynamic diameter ≤ 10 μm (PM10) and to nitrogen dioxide (NO2) on specific airway resistance (sRaw) and forced expiratory volume in 1 sec (FEV1) before and after bronchodilator treatment. Subjects were from the Manchester Asthma and Allergy Study (MAAS) birth cohort (n = 1,185).
Methods: Spirometry was performed during clinic visits at ages 3, 5, 8, and 11 years. Individual-level PM10 and NO2 exposures were estimated from birth to 11 years of age through a microenvironmental exposure model. Longitudinal and cross-sectional associations were estimated using generalized estimating equations and multivariable linear regression models.
Results: Lifetime exposure to PM10 and NO2 was associated with significantly less growth in FEV1 (percent predicted) over time, both before (–1.37%; 95% CI: –2.52, –0.23 for a 1-unit increase in PM10 and –0.83%; 95% CI: –1.39, –0.28 for a 1-unit increase in NO2) and after bronchodilator treatment (–3.59%; 95% CI: –5.36, –1.83 and –1.20%; 95% CI: –1.97, –0.43, respectively). We found no association between lifetime exposure and sRaw over time. Cross-sectional analyses of detailed exposure estimates for the summer and winter before 11 years of age and lung function at 11 years indicated no significant associations.
Conclusions: Long-term PM10 and NO2 exposures were associated with small but statistically significant reductions in lung volume growth in children of elementary-school age.
Citation: Mölter A, Agius RM, de Vocht F, Lindley S, Gerrard W, Lowe L, Belgrave D, Custovic A, Simpson A. 2013. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect 121:1232–1238. http://dx.doi.org/10.1289/ehp.1205961
Introduction
Lung function is an important indicator of respiratory health and long-term survival (Hole et al. 1996). Unlike information collected through questionnaires, measured lung function is an objective health outcome that is not affected by recall or reporting bias. The respiratory tract is at risk from air pollution, because gaseous pollutants and small particles in the air are inhaled through the nose and mouth. Two air pollutants frequently studied are nitrogen dioxide (NO2) and particulate matter (PM). Both are derived from traffic related sources, but are also generated within the home—for example, by gas cookers and cigarette smoke. Both of these pollutants have been associated with respiratory and cardiovascular morbidity and mortality (Brunekreef and Holgate 2002). Several cross-sectional and longitudinal studies have been carried out on the association between NO2 and PM exposure and lung function in children. However, results of these studies have been disparate and conclusions inconsistent. Whereas some studies reported associations with lung volume only (Raizenne et al. 1996; Rojas-Martinez et al. 2007; Sugiri et al. 2006), others reported associations with expiratory flow only (Avol et al. 2001; Oftedal et al. 2008). Some studies reported associations with both lung volume and flow (Gauderman et al. 2000; Horak et al. 2002; Schwartz 1989), whereas others reported no associations at all (Dockery et al. 1989; Hirsch et al. 1999; Neas et al. 1991; Nicolai et al. 2003). In a recent review of studies on air pollution and lung function, Götschi et al. (2008) concluded that it was not possible to perform formal quantitative comparisons of findings because of the heterogeneity of study designs.
One limitation common to many previous studies lies in the assessment of exposure to air pollution. Most studies of the effects of air pollution on lung development in children have estimated associations with more recent air pollution exposure—the average concentration over the previous 12 months, rather than lifetime exposure or early-life exposure (Oftedal et al. 2008), and have estimated exposures based on measurements from central monitoring stations located near the child’s residence, without accounting for geographical factors (Hirsch et al. 1999; Nicolai et al. 2003; Oftedal et al. 2008), indoor as well as outdoor exposures, or time–activity patterns.
We have developed a novel microenvironmental exposure model (MEEM) (Mölter et al. 2012), which allows for spatial (indoor and outdoor microenvironments) and temporal variability in pollutant concentrations (Mölter et al. 2010a, 2010b) and incorporates children’s time–activity patterns to predict personal exposure. The performance of MEEM (for NO2) was evaluated previously through a personal monitoring study of 46 12- to 13-year-old schoolchildren in Manchester, United Kingdom (Mölter et al. 2012); we found good agreement between modeled and measured NO2 concentration (e.g., mean predictor error = –0.75; normalized mean bias factor = 0.04; normalized mean average error factor = 0.27; Spearman’s rank correlation = 0.31, p < 0.05) This performance evaluation also demonstrated that MEEM provided better estimates of exposure than central monitors or an outdoor air pollution model, which tended to overestimate personal exposure levels (Mölter et al. 2012).
The aim of the present study was to estimate the associations of modeled PM10 (particulate matter with an aerodynamic diameter ≤ 10 μm) and NO2 exposure with lung function in elementary-school children enrolled in a population-based birth cohort—the Manchester Asthma and Allergy Study (MAAS). Exposures and lung function were evaluated longitudinally throughout childhood. In addition, we applied a more detailed exposure model in a cross-sectional analysis of lung function measured at 11 years of age.
Methods
Study population. The children studied were participants of MAAS, is an ongoing prospective birth cohort, which initially comprised 1,185 children of mothers who were recruited during pregnancy at two local hospitals between 1995 and 1997 (Simpson et al. 2001). Children attended review clinics at ages 3, 5, 8, and 11 years; the clinics included pulmonary function tests and skin prick tests for common inhalant and food allergens. In addition, parentally completed questionnaires were collected at each review (Custovic et al. 2002, 2004). MAAS received ethical approval by the Local Research Ethics Committee (SOU/00/258; SOU/00/259), and written informed consent was provided by the parents.
Definition of outcomes: lung function. All pulmonary function tests were performed by trained technicians at Wythenshawe Hospital, Manchester. The most informative test to measure lung function was selected for each age group (Beydon et al. 2007; Bisgaard and Klug 1995; Dab and Alexander 1976).
Specific airways resistance (sRaw) was measured at ages 3, 5, 8, and 11 years, using a constant volume whole-body plethysmograph (Masterscreen Body 4.3; Erich Jaeger GmbH, Würzburg, Germany) (Lowe et al. 2002; Nicolaou et al. 2008). High values of sRaw indicate poor lung function. Forced expiratory volume in 1 sec (FEV1) was measured at ages 5, 8, and 11 years using a pneumotachograph-based spirometer (Erich Jaeger Gmbh). The protocol for measuring FEV1 was in accordance with American Thoracic Society guidelines (American Thoracic Society 1995). All children were asymptomatic at the time of testing, and β2-agonists were withheld for at least 4 hr before testing. The test was repeated at intervals of 30 sec until three technically acceptable traces were obtained, the highest two of which were within 5% of each other. The percent predicted FEV1 was calculated using reference equations developed by the Asthma UK Collaborative Initiative (Stanojevic et al. 2009). Postbronchodilator FEV1 was measured when the children were 5 and 11 years of age by repeating the FEV1 measurement 15 min after inhalation of 400 μg of albuterol. Results were analyzed as percent predicted FEV1.
Definition of exposures: modeled PM10 and NO2 exposure. The exposure estimates in this study are based on the concept of microenvironments (ME)—a defined space with a homogenous pollutant concentration (Ott 1982). MEs can represent spaces outdoors or indoors, and different methods can be used to estimate concentrations in different types of microenvironments. The microenvironmental models used in this study assumed that children spend the majority of their time in three types of MEs: home, school, and the journey between home and school.
Information on children’s home and school addresses from birth to 11 years of age was collected through a parental questionnaire, completed at the age 11 review. In this questionnaire parents were asked to list the dates and addresses for all homes the child had lived in and each school the child attended, the mode of transport between each home and respective schools. These data were entered into an SQL database (MS SQL2008R2; Microsoft, Redmond, WA, USA) to create a timeline for home and school addresses from birth to 11 years of age for each child. In addition, the shortest driving route between each home and school was estimated using the network analyst extension of ArcGIS9.2 (ESRI, Redlands, CA, USA).
Figure 1 summarizes the methods used to estimate NO2 and PM10 concentration in each ME. Concentrations for outdoor MEs (i.e., home outdoor ME, school outdoor ME, journey outdoor ME) were estimated using land use regression (LUR) models, as described in detail elsewhere (Mölter et al. 2010a, 2010b). In brief, LUR models were developed using estimated annual mean NO2 and PM10 concentrations at 208 locations derived from an air dispersion model. The final LUR models mainly comprised traffic-related predictor variables, such as vehicle counts on major roads, and had determination coefficients (R2) of 0.71. Performance evaluations using a set-aside data set (70 locations), and concentrations measured at automatic monitoring stations showed an acceptable level of agreement (R2 range, 0.33–0.86). To model children’s exposure from 1996 through 2008, the above LUR models were recalibrated to provide 13 annual models for PM10 and NO2, respectively (Mölter et al. 2010b): Data from the air dispersion model and the United Kingdom year adjustment calculator were used to estimate annual PM10 and NO2 concentrations from 1996 through 2008 at the 278 receptor sites described above. These concentrations were entered into regression analyses that included the same predictor variables used in the original LUR models. This resulted in individual models for each year; all models used the same predictor variables but generated different coefficients. A performance evaluation of these models against monitored data showed good agreement [R2 range, 0.35–0.97; root mean square error (RMSE) range, 1.8–8.3] (Mölter et al. 2010b).
Figure 1.
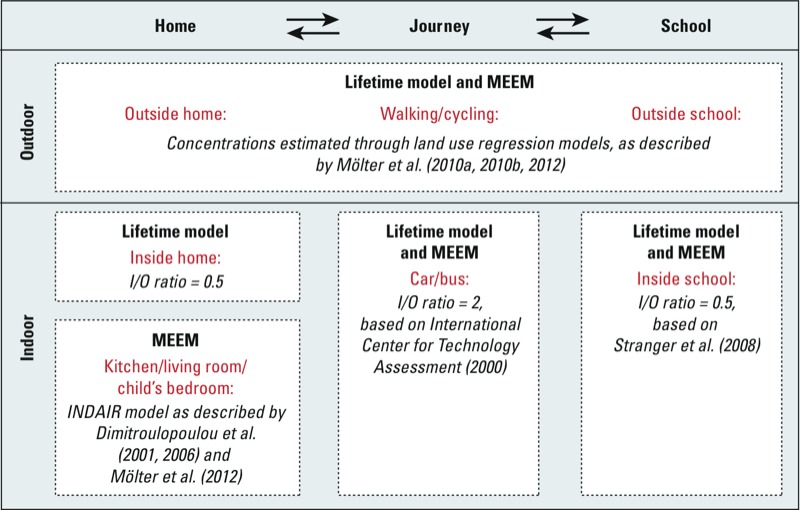
Outline of exposure assessment showing methods used to estimate concentrations in each microenvironment (with relevant references). The same methods were used at all time points except for the year before the age 11 review. A detailed indoor model could be used to estimate concentrations inside the kitchen, living room, and child’s bedroom. Abbreviations: I/O, Indoor to outdoor ratio; MEEM, microenvironmental exposure model.
Concentrations for journey indoor MEs (i.e., inside cars or buses) and school indoor MEs were estimated based on indoor to outdoor (I/O) ratios published in the literature (International Center For Technology Assessment 2000; Stranger et al. 2008). Concentrations in the Home indoor MEs were estimated using I/O ratios or a mass balance model (INDAIR), depending on the time period being modeled (Dimitroulopoulou et al. 2006). This resulted in two slightly different models: the MEEM and the lifetime models (Figure 1).
MEEM was used to estimate each child’s exposures during the summer and winter before the review visit at 11 years of age (Mölter et al. 2012). We modeled winter and summer exposures separately to capture variation in home indoor air concentrations because of seasonal differences in air exchange rates. In MEEM home kitchen ME, home living room ME, and home bedroom ME, concentrations were estimated individually using the INDAIR model, designed specifically to estimate indoor concentrations of NO2 and PM10 concentrations within residential buildings in the United Kingdom (Dimitroulopoulou et al. 2001, 2006).
A parent questionnaire administered at the child’s age 11 review was used to collect input parameters for the INDAIR model, such as room sizes, air exchange rates, and the presence of indoor sources of NO2 and PM10. The indoor sources included in the model were gas cooking and cigarette smoke, which are considered to be the main sources of NO2 and PM10 inside homes in the United Kingdom (Berry et al. 1996; Coward et al. 2001). In addition, the questionnaire collected time–activity data used to estimate the timing and duration of time in each ME. Therefore, MEEM provided spatially resolved time-weighted exposure estimates for each child.
We evaluated the performance of MEEM using a personal monitoring study of schoolchildren (12–13 years of age) attending a local secondary school in Manchester (Mölter et al. 2012). MEEM performed well when compared with NO2 concentrations measured with personal monitors (Ogawa passive samplers; Ogawa & Co. USA, Inc., Pompano Beach, FL, USA), with a mean prediction error of –0.75 μg/m3. A paired analysis of measured and predicted concentrations showed no significant difference between measured concentrations and MEEM estimates (Wilcoxon’s signed rank test: z = –0.05, p = 0.96).
Input parameters for the INDAIR model were available for the current (at 11 years of age) home of each child, but most children had changed residence at least once since birth. Therefore, we used a simplified lifetime model to estimate the average PM10 and NO2 exposure of each child for each month from birth to 11 years. In contrast with MEEM, the lifetime model used an I/O ratio to calculate exposure inside the home, instead of using the INDAIR model, and it assumed that all children were in the school indoor ME from 0900 to 1500 hours. However, as for MEEM, outdoor ME exposures (i.e., home outdoor ME, school outdoor ME, journey outdoor ME) were estimated using LUR models, and journey indoor MEs (i.e., inside cars or buses) and school indoor MEs were estimated based on I/O ratios.
Definition of potential confounders. Potential confounding variables and covariates were identified based on previous research within MAAS and previous publications (Lowe et al. 2002, 2004; Nicolaou et al. 2008; Oftedal et al. 2008) and included sex, age, ethnicity, older siblings, sensitization, asthma or current wheeze, family history of asthma, parental smoking, parental atopy, child care attendance during the first 2 years of life, hospitalization during the first 2 years of life, presence of a gas cooker in the home, presence of a dog or cat in the home, visible signs of dampness or mold in the home, body height, body weight, body mass index, maternal age at birth, gestational age, duration of breastfeeding, Tanner stage (age 11 years only), and socioeconomic status (paternal income). In addition, average PM10 and NO2 concentrations over 3 days before the child’s review visit were collected from four (for PM10) or five (for NO2) urban background monitoring stations across the Greater Manchester area (Oftedal et al. 2008).
We classified children as having current wheeze based on a positive response to the question “Has your child had wheezing or whistling in the chest in the last 12 months?” and classified them as having asthma based on positive answers to at least two of the following three variables: doctor diagnosis of asthma ever; current wheeze; asthma medication during the previous 12 months, consistent with the GA2LEN (Global Allergy and Asthma European Network) definition of asthma (Carlsen et al. 2006; Håland et al. 2006). At each review, potential allergic sensitization to common inhalant and food allergens was determined through skin prick tests for inhalant allergens (mites, cat, dog, mold, grass pollen, and tree pollen) and food allergens (milk, egg, and peanut). All allergens were tested at each review except for tree pollen and peanut allergens, which were tested at the age 8 and age 11 reviews only. Children were classified as having atopy, if they had at least one positive skin prick test (defined as a mean wheal diameter 3 mm greater than the negative control). Parental atopy was also established through skin prick tests, which were carried out during the recruitment stage.
Statistical analysis. All analyses were carried out with SPSS 16.0 (IBM SPSS, Chicago, IL, USA). Before all analyses, sRaw was ln-transformed because it follows a log-normal distribution. FEV1 and postbronchodilator FEV1 were not transformed because these variables were normally distributed. Multivariable linear regression was used to cross-sectionally estimate associations of PM10 and NO2 exposure during the summer and winter before children were 11 years of age (estimated by MEEM), with sRaw and FEV1 at 11 years. All potential confounders were entered individually into bivariate models with the exposure and outcome variables, and potential confounders that were significant predictors of the outcome (p < 0.05) were evaluated using multivariate stepwise analyses that retained only covariates that significantly predicted the outcome, or that were retained a priori (age and sex in all sRaw models, Tanner stage for all models of outcomes at age 11). Models of FEV1 outcomes were not adjusted for age, sex, and body height, because these factors were used to calculate the percent predicted values. Models of MEEM exposures at 11 years of age were not adjusted for cigarette smoking because information on smoking was already included in the INDAIR model.
We analyzed the association between lifetime exposure and the development of lung function using generalized estimating equations to account for the within-subject correlation of repeated measures, with the same covariates included in the cross-sectional models. Monthly exposures were averaged into the following time windows: for sRaw, 0–3, 3–5, 5–8, and 5–11 years of age; for FEV1, 0–5, 5–8, and 8–11 years of age; for FEV1 after bronchodilator treatment, 0–5 and 5–11 years of age. For completeness, exposure estimates from the lifetime exposure model were also analyzed cross-sectionally against lung function at 3, 5, 8, and 11 years of age. For these analyses the monthly exposure estimates were averaged into the following time windows: first year of life (0–1), birth to review ages (0–3, 0–5, 0–8, 0–11 years), 1 calendar year before reviews (2–3, 4–5, 7–8, 10–11 years). The level for statistical significance was set at p < 0.05.
Results
Participants and descriptive data. Participant flow with numbers of individuals at each stage of the study, the number of lung function measurements collected and the number of exposure estimates available is shown in Figure 2. Descriptive statistics of the study population and the covariates included in the final models are presented in Table 1; descriptive statistics of potential confounders not included in the final models are shown in Supplemental Material, Table S1. As expected, the prevalence of atopy increased from 3 to 11 years of age, whereas the prevalence of asthma or current wheeze remained fairly constant during this time period. A complete data set of FEV1, pollutant exposures, and covariates at two or more reviews was available for 342 children (Table 1). Children included in the longitudinal analysis of the effect of PM10 and NO2 exposure on the change in FEV1 were more likely to be female and were less likely to have asthma or wheeze in early life. By 8 years of age, there were no differences in asthma/wheeze between children with full sets of longitudinal data and those without. Table 2 summarizes the lung function measurements at each age. The mean FEV1 increased from 1.05 L at 5 years to 2.30 L at 11 years, resembling typical values for Caucasian children of these ages (Stanojevic et al. 2009).
Figure 2.
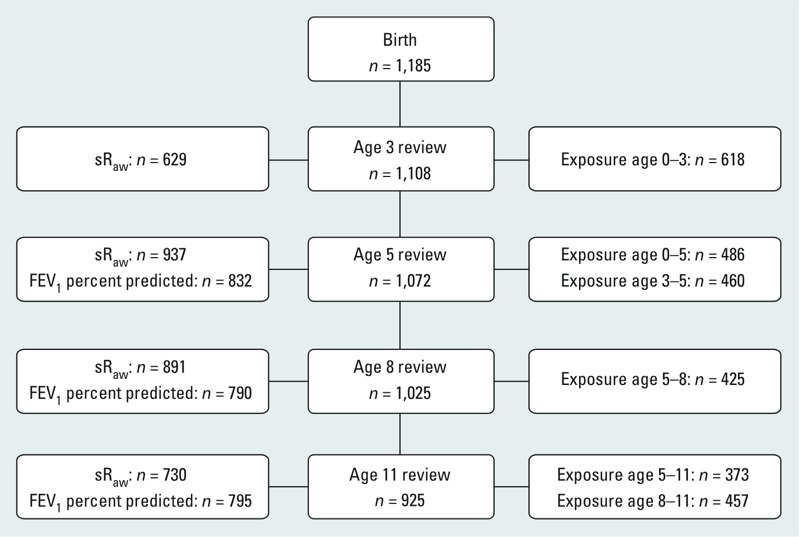
Flow diagram of MAAS cohort showing participation rates at each review, the number of lung function measurements collected, and the number of exposure estimates available.
Table 1.
Description of study population.
| Variable | MAAS cohort at birth | Children with longitudinal FEV1 and longitudinal exposure data | p-Valuec | ||
|---|---|---|---|---|---|
| Na | nb (%) or mean ± SD | Na | nb (%) or mean ± SD | ||
| Female sex | 1,185 | 543 (45.8) | 342 | 173 (50.6) | 0.036 |
| Family history of asthma | 1,185 | 441 (37.2) | 342 | 125 (36.5) | 0.763 |
| Child is atopicd | |||||
| Age 3 | 983 | 225 (22.9) | 306 | 72 (23.5) | 0.748 |
| Age 5 | 963 | 294 (30.5) | 334 | 94 (28.1) | 0.241 |
| Age 8 | 927 | 314 (33.9) | 330 | 100 (30.3) | 0.088 |
| Age 11 | 784 | 281 (35.8) | 332 | 116 (34.9) | 0.652 |
| Child has asthma or current wheeze | |||||
| Age 3 | 1,097 | 296 (27.0) | 330 | 71 (21.5) | 0.007 |
| Age 5 | 1,071 | 297 (27.7) | 341 | 75 (22.0) | 0.004 |
| Age 8 | 1,023 | 217 (21.2) | 341 | 65 (19.1) | 0.234 |
| Age 11 | 925 | 214 (23.1) | 341 | 78 (22.9) | 0.886 |
| Hospitalization during first 2 years of life for lower respiratory tract infection | 1,185 | 109 (9.2) | 342 | 34 (9.9) | 0.573 |
| Gas cooker in the home | |||||
| Age 1 | 1,028 | 801 (77.9) | 341 | 270 (79.2) | 0.492 |
| Age 8 | 1,029 | 819 (79.6) | 342 | 270 (78.9) | 0.717 |
| Age 11 | 930 | 727 (78.2) | 342 | 267 (78.1) | 0.954 |
| Age at follow-up (years) | |||||
| Age 3 | 1,081 | 3.0 ± 0.1 | 326 | 3.0 ± 0.0 | 0.208 |
| Age 5 | 1,044 | 5.0 ± 0.1 | 340 | 5.0 ± 0.1 | 0.008 |
| Age 8 | 976 | 8.0 ± 0.2 | 339 | 8.0 ± 0.1 | 0.084 |
| Age 11 | 813 | 11.4 ± 0.5 | 341 | 11.4 ± 0.5 | 0.876 |
| Body mass index (kg/m²) | |||||
| Age 3 | 1,044 | 16.7 ± 1.4 | 321 | 16.7 ± 1.5 | 0.914 |
| Age 5 | 1,017 | 16.3 ± 1.6 | 339 | 16.4 ± 1.7 | 0.776 |
| Age 8 | 923 | 17.1 ± 2.4 | 333 | 17.1 ± 2.6 | 0.643 |
| Age 11 | 816 | 19.1 ± 3.4 | 341 | 19.2 ± 3.4 | 0.885 |
| Short-term PM10 (μg/m3) 3-day average before review visit | |||||
| Age 3 | 1,081 | 21.6 ± 7.7 | 326 | 21.0 ± 6.9 | 0.186 |
| Age 5 | 1,044 | 21.5 ± 7.2 | 340 | 21.6 ± 7.2 | 0.910 |
| Age 8 | 976 | 20.8 ± 6.2 | 339 | 21.0 ± 6.0 | 0.660 |
| Age 11 | 820 | 19.6 ± 9.2 | 337 | 19.7 ± 9.0 | 0.895 |
| Mean Tanner stage | 763 | 2.1 ± 0.9 | 317 | 2.1 ± 0.9 | 0.648 |
| aTotal number of children. bNumber of positive children. cp-Value of chi-square test or Student’s t-test comparing children with longitudinal FEV1 and exposure data against all children in the MAAS cohort at birth. dDetermined through skin prick test, mean wheal diameter 3 mm greater than negative control for at least 1 of 9 allergens tested. | |||||
Table 2.
Summary of lung function measures at each review (mean ± SD).
| Lung function measure | Age 3 | Age 5 | Age 8 | Age 11 |
|---|---|---|---|---|
| sRaw (kPa/sec)a | 1.10 (1.23) | 1.17 (1.21) | 1.22 (1.23) | 1.26 (1.29) |
| FEV1 (L) | 1.05 ± 0.16 | 1.59 ± 0.25 | 2.30 ± 0.40 | |
| Predicted FEV1 (L) | 1.03 ± 0.27 | 1.60 ± 0.17 | 2.34 ± 0.29 | |
| FEV1 (% predicted) | 96.4 ± 12.7 | 99.0 ± 11.8 | 98.5 ± 11.7 | |
| FEV1 postbronchodilator (% predicted) | 104.9 ± 11.3 | 103.8 ± 11.5 | ||
| aGeometric mean (GSD). | ||||
Exposure to pollutants. Figures S1 and S2 (Supplemental Material) describe the distribution of the exposure estimates by pollutant and exposure time window. MEEM predicted higher PM10 and NO2 exposures during the winter than during the summer (see Supplemental Material, Figures S1 and S2), and it predicted a wider range of exposures than the lifetime model. The lifetime exposure estimates decreased from 0–1 to 10–11 years of age (see Supplemental Material, Figures S1 and S2), which most likely reflects the general decrease of PM10 and NO2 levels in the Greater Manchester area from 1996 to 2008 (Department for Environment, Food and Rural Affairs 2009). PM10 and NO2 exposures were moderately to strongly correlated in all exposure time windows (Pearson’s r = 0.59–0.89).
Association between exposure to pollutants and sRaw. The results of the cross-sectional analyses conducted at 3–11 years of age are shown in Supplemental Material, Table S2. Table S2 indicates a significant negative association between PM10 exposure during early life and sRaw at 3 and 5 years. However, all other analyses showed no statistically significant associations. Furthermore, at 11 years there was no association between PM10 and NO2 exposure (MEEM) during the summer or winter and sRaw (Table 3), and there was no association between lifetime exposure and longitudinal sRaw.
Table 3.
Results of longitudinal analyses (GEE) of longitudinal PM10 and NO2 exposure (based on the lifetime model) and lung function and cross-sectional analyses (multivariable linear regression) of PM10 and NO2 exposure at 10–11 years of age (based on the lifetime model or MEEM) and lung function at 11 years of age.
| Exposure metric/lung function metric | Longitudinal exposure and lung function | Exposure at age 10–11 (lifetime model) and lung function at age 11 | Winter exposure before age 11 review (MEEM) and lung function at age 11 | Summer exposure before age 11 review (MEEM) and lung function at age 11 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βa (95% CI) | p-Value | nb | βa (95% CI) | p-Value | nb | βa (95% CI) | p-Value | nb | βa (95% CI) | p-Value | nb | |
| PM10 (μg/m3) | ||||||||||||
| Ln sRaw (kPa/sec)c | 0.009 (–0.027, 0.010) | 0.37 | 453 | –0.007 (–0.054, 0.040) | 0.77 | 352 | –0.001 (–0.011, 0.008) | 0.78 | 315 | 0.001 (–0.008, 0.009) | 0.90 | 298 |
| FEV1 (% predicted)d | –1.37 (–2.52, –0.23) | 0.019 | 342 | –1.13 (–3.36, 1.09) | 0.32 | 373 | –0.20 (–0.65, 0.26) | 0.39 | 334 | 0.07 (–0.33, 0.47) | 0.73 | 317 |
| FEV1 after bronchodilator treatment (% predicted)d | –3.59 (–5.36, –1.83) | < 0.001 | 176 | –1.71 (–3.94, 0.53) | 0.13 | 366 | –0.14 (–0.61, 0.34) | 0.57 | 327 | 0.15 (–0.27, 0.57) | 0.48 | 310 |
| NO2 (μg/m3) | ||||||||||||
| Ln sRaw (kPa/sec)c | –0.007 (–0.016, 0.003) | 0.16 | 453 | 0.002 (–0.020, 0.023) | 0.88 | 352 | 0.001 (–0.004, 0.007) | 0.64 | 315 | –0.001 (–0.006, 0.004) | 0.57 | 298 |
| FEV1 (% predicted)d | –0.83 (–1.39, –0.28) | 0.003 | 342 | –0.83 (–1.79, 0.14) | 0.093 | 373 | –0.10 (–0.36, 0.17) | 0.47 | 334 | 0.05 (–0.18, 0.29) | 0.66 | 317 |
| FEV1 after bronchodilator treatment (% predicted)d | –1.20 (–1.97, –0.43) | 0.002 | 176 | –1.00 (–1.96, –0.03) | 0.043 | 366 | –0.01 (–0.29, 0.27) | 0.93 | 327 | 0.08 (–0.17, 0.32) | 0.53 | 310 |
| GEE, generalized estimating equation.aβ coefficient per 1-μg/m3 increase in exposure. bNumber of children included in analysis. cAdjusted for age, sex, concurrent body mass index, concurrent atopy, concurrent asthma or wheeze, family history of asthma, hospitalization during first two years of life for lower respiratory tract infection, average 3-day background PM10 concentration prior to sRaw measurement, mean Tanner stage. dAdjusted for age (only in GEE), concurrent atopy, concurrent asthma or wheeze, hospitalization during first two years of life for lower respiratory tract infection, gas cooker in home, mean Tanner stage. | ||||||||||||
Association between exposure to pollutants and FEV1. In the cross-sectional analysis at 11 years of age, there was no association between PM10 and NO2 exposure (MEEM) during the summer or winter and FEV1 percent predicted (Table 3). In contrast, the longitudinal model of lifetime exposure to pollutants and longitudinal measures of FEV1 revealed a significant association between exposure to pollutants and the change in this measure of lung function during childhood. PM10 and NO2 exposures were associated with poorer lung function over time [PM10: β = –1.37 (95% CI: –2.52, –0.23); NO2: β = –0.83 (95% CI: –1.39, –0.28)]. Based on the average predicted FEV1 within MAAS at 5, 8, and 11 years of 1.65 L (Table 2), the model estimated that for each unit increase (1 μg/m3) in PM10 exposure, the growth in FEV1 from 5 to 11 years was 23 mL smaller; and for each unit increase (1 μg/m3) of NO2 exposure, the growth in FEV1 was 14 mL smaller [ΔFEV1 = β / 100 × 1.65 × 1,000]. Results of cross-sectional analyses conducted at other time points are shown in Supplemental Material, Table S3; we observed no statistically significant association between PM10 or NO2 exposure windows and FEV1 in cross-sectional analyses.
Association between exposure to pollutants and postbronchodilator FEV1. At 11 years of age, there was no association between PM10 or NO2 exposure (MEEM) during the summer or winter and postbronchodilator FEV1 percent predicted (Table 3). However, there was a significant negative association between postbronchodilator FEV1 and the annual average NO2 exposure from 10 to 11 years of age estimated by the lifetime model (β = –1.00; 95% CI: –1.96, –0.03, p = 0.043). In the longitudinal models, we observed a significant negative association between postbronchodilator FEV1 and PM10 and NO2 exposure over time [PM10: β = –3.59 (95% CI: –5.36, –1.83); NO2: β = –1.20 (95% CI: –1.97, –0.43)]. Based on the average predicted FEV1 of 1.65 L, these would be equivalent to a growth deficit in post bronchodilator FEV1 of 59 mL from 5 to 11 years of age per unit increase in PM10, and a growth deficit of 20 mL from 5 to 11 years per unit increase in NO2. For completeness results of cross-sectional analyses conducted at other time points are shown in Supplemental Material, Table S4. Table S4 shows significant negative associations between postbronchodilator FEV1 and early-life PM10 (βAge 0–1 = –3.00; 95% CI: –5.29, –0.71; βAge 0–5 = –4.70; 95% CI: –7.85, –1.55) and NO2 exposures (βAge 0–1 = –0.91; 95% CI: –1.77, –0.05).
Discussion
To our knowledge, this is the first study to estimate the effect of modeled individual lifetime exposure to PM10 and NO2, from birth through elementary school, on the development of lung function measured throughout childhood. With both exposure and lung function modeled longitudinally, our results indicated a small but statistically significant impairment in growth of FEV1 with an increase in exposure to air pollutants. We estimated the size of this effect to be a loss of 23 mL in the growth in FEV1 from 5 to 11 years of age per unit increase in PM10 (~ 3.8 mL/year), and 14 mL per unit increase of NO2 exposure (~ 2.3 mL/year). In addition, we observed significant associations of PM10 and NO2 exposures with postbronchodilator FEV1. In cross-sectional analyses, using a detailed assessment of summer and winter pollutant exposure at 11 years, we found no associations between air pollution and contemporaneous measures of lung function.
One of the strengths of this study was the use of the comprehensive validated MEEM model to estimate exposures for cross-sectional analyses of outcomes at 11 years of age. This model provided weighted estimates of exposure based on time–activity patterns and NO2 and PM10 models with a high spatiotemporal resolution. Ideally, we would have used MEEM to estimate lifetime exposure of each child. However, MEEM requires detailed descriptions of the house design that were not available longitudinally for the approximately 50% of children who had moved house from their original home during follow-up. Therefore we used the lifetime model—a slightly simplified version of MEEM that did not require detailed knowledge of the home environment to estimate exposures on a monthly basis from birth to 11 years for longitudinal analyses. The ranges of exposures estimated by MEEM (9.7–28.0 μg/m3 and 6.5–38.1 μg/m3 for PM10 during the previous summer and winter, respectively; and 9.5–43.0 μg/m3 and 10.3–47.2 μg/m3 for NO2, respectively) were greater than the corresponding estimates from the lifetime model at 10–11 years (PM10: 8.8–14.0 μg/m3; NO2: 10.8–23.7 μg/m3). Differences between estimates from each model reflect the different time periods used for averaging (3-month averages during summer and winter for MEEM, 12-month averages at 10–11 years of age for the lifetime model) and the use of the INDAIR model to estimate indoor exposures for MEEM, which captures peaks in exposure due to gas cooking and cigarette smoking, as well as very low exposures due to low air exchange rates. However, the lifetime model also improves over previously used exposure assessment methods by providing retrospective estimates of monthly exposures that can be aggregated into different exposure time windows for longitudinal and cross-sectional analyses. Furthermore, using home and school address histories, we modeled exposure at an individual level, rather than a community level, thereby reducing the potential for exposure misclassification.
Because of the strong correlation between NO2 and PM10 exposures in our study, we used single- rather than two-pollutant models. Many previous cohort studies of air pollution have included cigarette smoking and socioeconomic status as confounders in their analysis (Brunst et al. 2012; Li et al. 2000; Stocks and Dezateux 2003). Although it is likely that parental smoking and socioeconomic status affect lung function in children, we did not include them in our final model because they were not significant predictors of the outcomes, and we therefore assumed that they did not confound associations with air pollution exposures in our study. However, we cannot rule out residual confounding by these or other exposures. In addition, we acknowledge that our estimates of PM10 exposures do not necessarily represent the size fraction of particulate matter that is most damaging and that further studies of associations with fine or ultrafine particles are needed to address this.
Another strength of this study was its setting in the context of a population-based birth cohort with repeated measurements of lung function—an objective outcome that is not affected by recall or reporting bias—at four ages. Assessment of sRaw enabled measurement of lung function from a young age (3 years). Assessing bronchodilator responses is a common diagnostic tool to test for reversible airway obstruction that can also be used to estimate the maximum achievable expiratory volume of a child. The results of our longitudinal analyses suggest an average annual growth deficit of 9.8 mL/year and 3.3 mL/year in the maximum achievable expiratory volume with each unit increase in PM10 and NO2 exposure.
A limitation of this study was the relatively small sample sizes for some of the analyses, mostly due to missing exposure data. Exposure data were missing for children who moved outside the Greater Manchester area and for children with incomplete information on home and school addresses. However, the loss in precision due to sample size limitations may be partly offset by the use of detailed individual-level estimates of longitudinal exposures.
Most published studies have investigated the association between pollutant exposure and FEV1 cross-sectionally—at a single time point only. Some of these studies also reported that PM10 or NO2 exposures were associated with decreases in mean FEV1, but not at a statistically significant level (Avol et al. 2001; Dockery et al. 1989; Oftedal et al. 2008). However, other studies have reported significant negative associations between air pollution exposure and FEV1 (Gauderman et al. 2000, 2004; Horak et al. 2002; Peters et al. 1999; Rojas-Martinez et al. 2007), but often only in subgroups of children [e.g., only in girls (Peters et al. 1999), only in one age group (Gauderman et al. 2004), or only during one season (Horak et al. 2002)].
Few studies have estimated the longitudinal effects of pollutants on the growth in lung function (Table 4). The Children’s Health Study was set in 12 communities of Southern California (USA), with a broad range of pollutant exposures (Gauderman et al. 2000, 2004). After 4 years of follow-up from 10 years of age, increasing community exposure to PM10 was associated with a reduced adjusted mean FEV1 growth rate, with those in the most polluted community having an estimated cumulative reduction in FEV1 of 3.4% over 4 years compared with those in the least polluted communities (Gauderman et al. 2000). After 8 years of follow-up, this association with PM10 was no longer statistically significant, although a much higher proportion of the children who lived in high-PM10 communities had a FEV1 < 80% predicted. By the time children were 18 years of age, the average FEV1 in the community with the highest NO2 exposure was about 100 mL lower than that seen in the community with the lowest exposure (Gauderman et al. 2004). In a population of 975 8-year-old Austrian children who were followed for 3 years, significant negative associations with lung function growth were reported for winter NO2 and summer PM10, even though higher concentrations of PM10 were present during the winter (Horak et al. 2002). A 3-year study of 3,170 children living in Mexico City, which has comparatively high pollution levels, reported statistically significant negative associations of both PM10 and NO2 with growth in FEV1 (Rojas-Martinez et al. 2007). Specifically, the authors estimated that an interquartile range (IQR) increase in PM10 (36.4 μg/m3) was associated with a mean annual deficit in FEV1 of 29 mL in girls and 27 mL in boys. Similarly, they estimated that an IQR increase in NO2 (12.0 ppb) was associated with a mean annual deficit of 32 mL in girls and 26 mL in boys. When estimates are scaled to the same exposure increment and time period (Table 4), it is apparent that past and present longitudinal studies have estimated a very broad range of effect sizes on lung function growth.
Table 4.
Comparison of average deficit in lung growth with findings from previously published population-based studies.
| Reference, country | Exposure assigned at | Study duration | Range of exposures (μg/m3) | Average deficit in lung growth (mL/year) associated with 1-μg/m3 increase in exposurea | ||
|---|---|---|---|---|---|---|
| PM10 | NO2 | PM10 | NO2 | |||
| Gauderman et al. 2000, 2004, USA | Community level | Age 10–14 | 20–65 | 10–70 | 0.20 | 0.19 |
| Horak et al. 2002, Austria | Community level | Age 8–11 | 9–31 | 2–35 | 8.4 | 9.5 |
| Rojas-Martinez et al. 2007, Mexico | Community level | Age 8–11 | 53–96 | 54–74 | 0.80 (girls), 0.74 (boys) | 1.4 (girls), 1.1 (boys) |
| Present study, United Kingdom | Individual level | Birth–age 11 | 10–16 | 15–28 | 3.8 | 2.3 |
| aCalculated based on published figures, assuming a linear relationship between exposure and lung function. | ||||||
Having found a longitudinal association during childhood, we find it interesting to speculate at which time point exposure to pollutants may be most damaging to lung function. The cross-sectional analysis of the detailed NO2 and PM10 exposure estimates derived from MEEM showed no association between exposure and lung function at 11 years of age. However, for postbronchodilator FEV1 the cross-sectional analyses indicate that early exposures are associated with poorer lung function (see Supplemental Material, Table S4), but this association was not as evident for FEV1 percent predicted (see Supplemental Material, Table S3). Previous research has suggested that lung development during infancy is particularly susceptible to environmental toxins and that exposure can result in irreversible lung damage (Dietert et al. 2000; Plopper and Fanucchi 2000). In the Children’s Health Study, no significant associations of pollutant exposures were reported for older children (recruited at 13 and 15 years of age) who were also followed longitudinally (Gauderman et al. 2000). However, most epidemiological studies on children’s lung function have assessed only present air pollution exposure (Götschi et al. 2008), and very little work has been done on early-life exposure (Oftedal et al. 2008). The results of the present study support the hypothesis that early life exposures may affect lung development in later life.
We found evidence of an impairment in lung function growth at apparently lower exposure levels than those of previous longitudinal studies of air pollution exposure and lung function in children (Avol et al. 2001; Gauderman et al. 2004; Rojas-Martinez et al. 2007). However, exposure estimates in previous studies are not directly comparable with exposure estimates used in our study, because they were based on levels measured at centrally located outdoor pollution monitors. In contrast, our estimates accounted for both indoor and outdoor exposures, because children living in urban areas in industrialized countries spend most of their time indoors (Infante-Rivard 1993). Our previous work on MEEM has shown that a model allowing for indoor and outdoor exposure provides a better estimate of personal exposure than methods based solely on outdoor air pollution, which tended to overestimate personal exposure (Mölter et al. 2012). Therefore, it is possible that exposure levels assigned to children in previous studies based on outdoor monitors overestimated their true personal exposures. Nonetheless, the maximum outdoor concentrations of 70–80 μg/m3 NO2 and 60–90 μg/m3 PM10 found in previous studies in Mexico (Rojas-Martinez et al. 2007) and the United States (Avol et al. 2001; Gauderman et al. 2004) do exceed the current regulatory limits for annual mean concentrations in the United Kingdom (NO2 = 40 μg/m3; PM10 = 40 μg/m3) and are higher than concentrations typically measured at urban background monitoring stations in Manchester (Mölter et al. 2010a, 2010b).
Conclusions
Our findings suggest that lifetime exposure to PM10 and NO2 may be associated with reduced growth in FEV1 in children. Although the observed reductions in FEV1 growth were small, and therefore may have little impact on healthy individuals, they could have implications for individuals with chronic respiratory disease, particularly obstructive lung diseases, or in children who go on to smoke cigarettes. Future follow-up will provide further insight on whether reductions in FEV1 growth associated with air pollution persist into adulthood or disappear during adolescence.
Supplemental Material
Acknowledgments
We thank the families who participate in MAAS (Manchester Asthma and Allergy Study) and all members of the MAAS study team for their tireless effort, particularly J. Nathan and M. Mycock, who carried out the data quality checks. The exposure model used in this study was partly based on the Greater Manchester air dispersion modeling study carried out by the former Atmospheric Research and Information Centre on behalf of the local authorities of Greater Manchester. Therefore, we also thank the Manchester Area Pollution Advisory Council for permitting us to access this data. Furthermore, we are very grateful to M. Ashmore, S. Dimitroulopoulou, and A. Terry for permitting us access to the INDAIR model (Dimitroulopoulou et al. 2006) and for their helpful advice on the use of this model.
Footnotes
MAAS has been supported by Asthma UK, The JP Moulton Charitable Foundation, and The Medical Research Council.
Portions of this work have been published in a PhD thesis submitted to the University of Manchester.
The authors declare they have no actual or potential competing financial interests.
References
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- Berry R, Brown V, Coward S, Crump D, Gavin M, Grimes C, et al. London: CRC Ltd; 1996. Indoor Air Quality in Homes. The Building Research Establishment Indoor Environment Study (BRE Reports BR299 and BR300) [Google Scholar]
- Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Klug B. Lung function measurement in awake young children. Eur Respir J. 1995;8:2067–2075. doi: 10.1183/09031936.95.08122067. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Brunst KJ, Ryan PH, Lockey JE, Bernstein DI, McKay RT, Khurana Hershey GK, et al. 2012Unraveling the relationship between aeroallergen sensitization, gender, second-hand smoke exposure, and impaired lung function. Pediatr Allergy Immunol 23479–487.; 10.1111/j.1399-3038.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen KCL, Håland G, Devulapalli CS, Munthe-Kaas M, Pettersen M, Granum B, et al. Asthma in every fifth child in Oslo, Norway: a 10-year follow up of a birth cohort study. Allergy. 2006;61:454–460. doi: 10.1111/j.1398-9995.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- Coward SKD, Llewellyn JW, Raw GJ, Brown VM, Crump DR, Ross DI. London: CRC Ltd; 2001. Indoor Air Quality in Homes in England. [Google Scholar]
- Custovic A, Simpson A, Woodcock A. Manchester cohort. Pediatr Pulmonol Suppl. 2004;26:12–13. doi: 10.1002/ppul.70033. [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol. 2002;13:32–37. doi: 10.1034/j.1399-3038.13.s.15.3.x. [DOI] [PubMed] [Google Scholar]
- Dab I, Alexander F. A simplified approach to the measurement of specific airway resistance. Pediatr Res. 1976;10:996–999. doi: 10.1203/00006450-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Department for Environment, Food and Rural Affairs. Data Selector. 2009. Available: http://uk-air.defra.gov.uk/data/data_selector [accessed 6 September 2013]
- Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, et al. Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect. 2000;108(suppl 3):483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroulopoulou C, Ashmore MR, Byrne MA, Kinnersley RP. Modelling of indoor exposure to nitrogen dioxide in the UK. Atmos Environ. 2001;35:269–279. [Google Scholar]
- Dimitroulopoulou C, Ashmore MR, Hill MTR, Byrne MA, Kinnersley R. INDAIR: a probabilistic model of indoor air pollution in UK homes. Atmos Environ. 2006;40:6362–6379. [Google Scholar]
- Dockery DW, Speizer FE, Stram DO, Ware JH, Spengler JD, Ferris BG., Jr Effects of inhalable particles on respiratory health of children. Am Rev Respir Dis. 1989;139:587–594. doi: 10.1164/ajrccm/139.3.587. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, McConnell R, Gilliland F, London S, Thomas D, Avol E, et al. Association between air pollution and lung function growth in Southern California children. Am J Respir Crit Care Med. 2000;162:1383–1390. doi: 10.1164/ajrccm.162.4.9909096. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Götschi T, Heinrich J, Sunyer J, Künzli N.2008Long-term effects of ambient air pollution on lung function: a review. Epidemiology 195690–701.; 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- Håland G, Carlsen KCL, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- Hirsch T, Weiland SK, von Mutius E, Safeca AF, Grafe H, Csaplovics E, et al. Inner city air pollution and respiratory health and atopy in children. Eur Respir J. 1999;14:669–677. doi: 10.1034/j.1399-3003.1999.14c29.x. [DOI] [PubMed] [Google Scholar]
- Hole DJ, Watt CM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, Studnicka M, Gartner C, Spengler JD, Tauber E, Urbanek R, et al. Particulate matter and lung function growth in children: a 3-yr follow-up study in Austrian schoolchildren. Eur Respir J. 2002;19:838–845. doi: 10.1183/09031936.02.00512001. [DOI] [PubMed] [Google Scholar]
- Infante-Rivard C. Childhood asthma and indoor environmental risk factors. Am J Epidemiol. 1993;137:834–844. doi: 10.1093/oxfordjournals.aje.a116745. [DOI] [PubMed] [Google Scholar]
- International Center For Technology Assessment. In-Car Air Pollution: The Hidden Threat To Automobile Drivers. Report No. 4. Washington, DC:International Center for Technology Assessment. 2000. Available: http://www.andrewkimbrell.org/doc/In-car%20pollution%20report.pdf [accessed 6 September 2013]
- Li YF, Gilliland F, Berhane K, McConnell R, Gauderman WJ, Rappaport E, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet. 2002;359:1904–1908. doi: 10.1016/S0140-6736(02)08781-0. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med. 2004;158:996–1001. doi: 10.1001/archpedi.158.10.996. [DOI] [PubMed] [Google Scholar]
- Mölter A, Lindley S, de Vocht F, Simpson A, Agius R. Modelling air pollution for epidemiologic research–Part I: A novel approach combining land use regression and air dispersion. Sci Total Environ. 2010a;408:5862–5869. doi: 10.1016/j.scitotenv.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Mölter A, Lindley S, de Vocht F, Simpson A, Agius R. Modelling air pollution for epidemiologic research–Part II: Predicting temporal variation through land use regression. Sci Total Environ. 2010b;409:211–217. doi: 10.1016/j.scitotenv.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Mölter A, Lindley S, de Vocht F, Agius R, Kerry G, Johnson K, et al. Performance of a microenviromental model for estimating personal NO2 exposure in children. Atmos Environ. 2012;51:225–233. [Google Scholar]
- Neas LM, Dockery DW, Ware JH, Spengler JD, Speizer FE, Ferris BG., Jr Association of indoor nitrogen dioxide with respiratory symptoms and pulmonary function in children. Am J Epidemiol. 1991;134:204–219. doi: 10.1093/oxfordjournals.aje.a116073. [DOI] [PubMed] [Google Scholar]
- Nicolai T, Carr D, Weiland SK, Duhme H, von Ehrenstein O, Wagner C, et al. Urban traffic and pollutant exposure related to respiratory outcomes and atopy in a large sample of children. Eur Respir J. 2003;21:956–963. doi: 10.1183/09031936.03.00041103a. [DOI] [PubMed] [Google Scholar]
- Nicolaou NC, Simpson A, Lowe LA, Murray CS, Woodcock A, Custovic A.2008Day-care attendance, position in sibship, and early childhood wheezing: a population-based birth cohort study. J Allergy Clin Immunol 122500–506.; 10.1016/j.jaci.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker SE, Nafstad P. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19(1):129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- Ott WR. Concepts of human exposure to air pollution. Environ Int. 1982;7:179–196. [Google Scholar]
- Peters J, Avol E, Gauderman J, Linn W, Navidi WC, London S, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Fanucchi MV. Do urban environmental pollutants exacerbate childhood lung diseases? Environ Health Perspect. 2000;108:A252–A253. doi: 10.1289/ehp.108-a252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizenne M, Neas LM, Damokosh AI, Dockery DW, Spengler JD, Koutrakis P, et al. Health effects of acid aerosols on North American children: pulmonary function. Environ Health Perspect. 1996;104:506–514. doi: 10.1289/ehp.96104506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Lung function and chronic exposure to air pollution: a cross-sectional analysis of NHANES II. Environ Res. 1989;50:309–321. doi: 10.1016/s0013-9351(89)80012-x. [DOI] [PubMed] [Google Scholar]
- Simpson BM, Custovic A, Simpson A, Hallam CL, Walsh D, Marolia H, et al. NAC Manchester Asthma and Allergy Study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy. 2001;31:391–399. doi: 10.1046/j.1365-2222.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- Stanojevic S, Wade A, Cole TJ, Lum S, Custovic A, Silverman M, et al. Spirometry Centile charts for young Caucasian children: the Asthma UK Collaborative Initiative. Am J Respir Crit Care Med. 2009;180:547–552. doi: 10.1164/rccm.200903-0323OC. [DOI] [PubMed] [Google Scholar]
- Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–285. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Stranger M, Potgieter-Vermaak S, Van Grieken R. Characterization of indoor air quality in primary schools in Antwerp, Belgium. Indoor Air. 2008;18:454–463. doi: 10.1111/j.1600-0668.2008.00545.x. [DOI] [PubMed] [Google Scholar]
- Sugiri D, Ranft U, Schikowski T, Kramer U.2006The influence of large-scale airborne particle decline and traffic-related exposure on children’s lung function. Environ Health Perspect 114282–288.; 10.1289/ehp.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


