Abstract
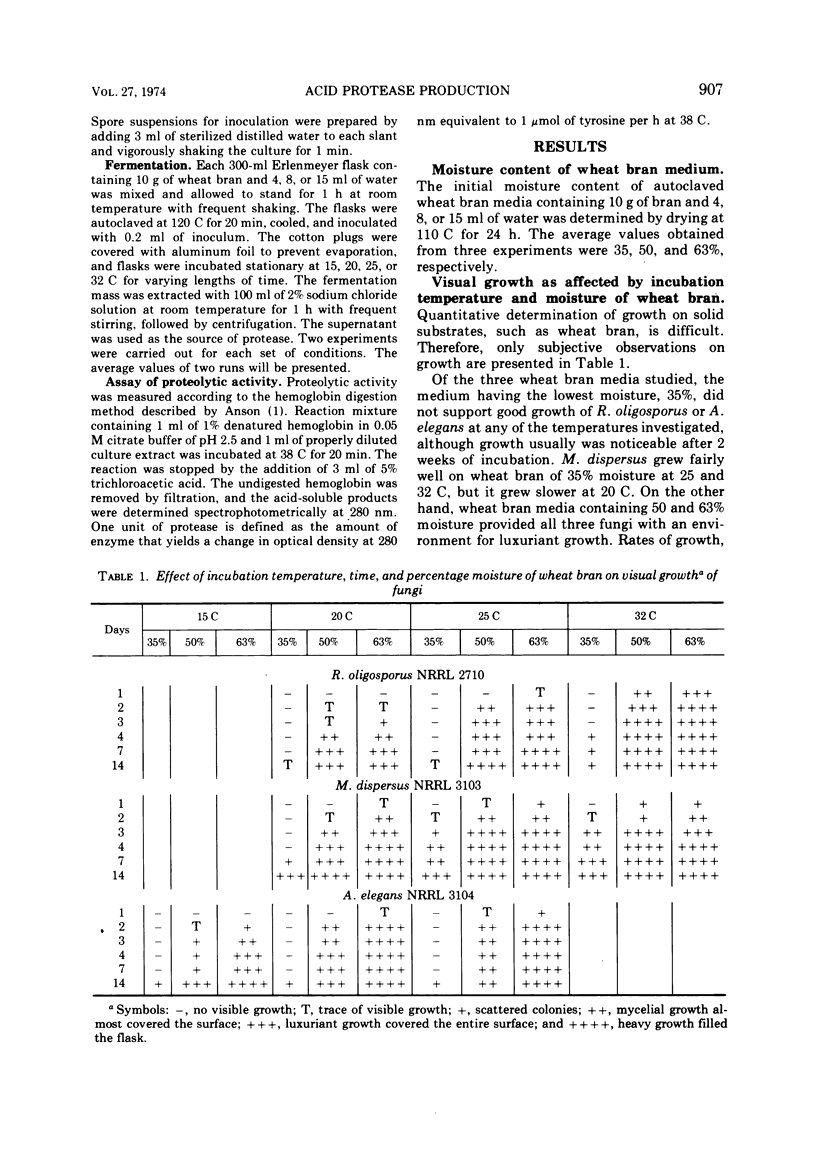
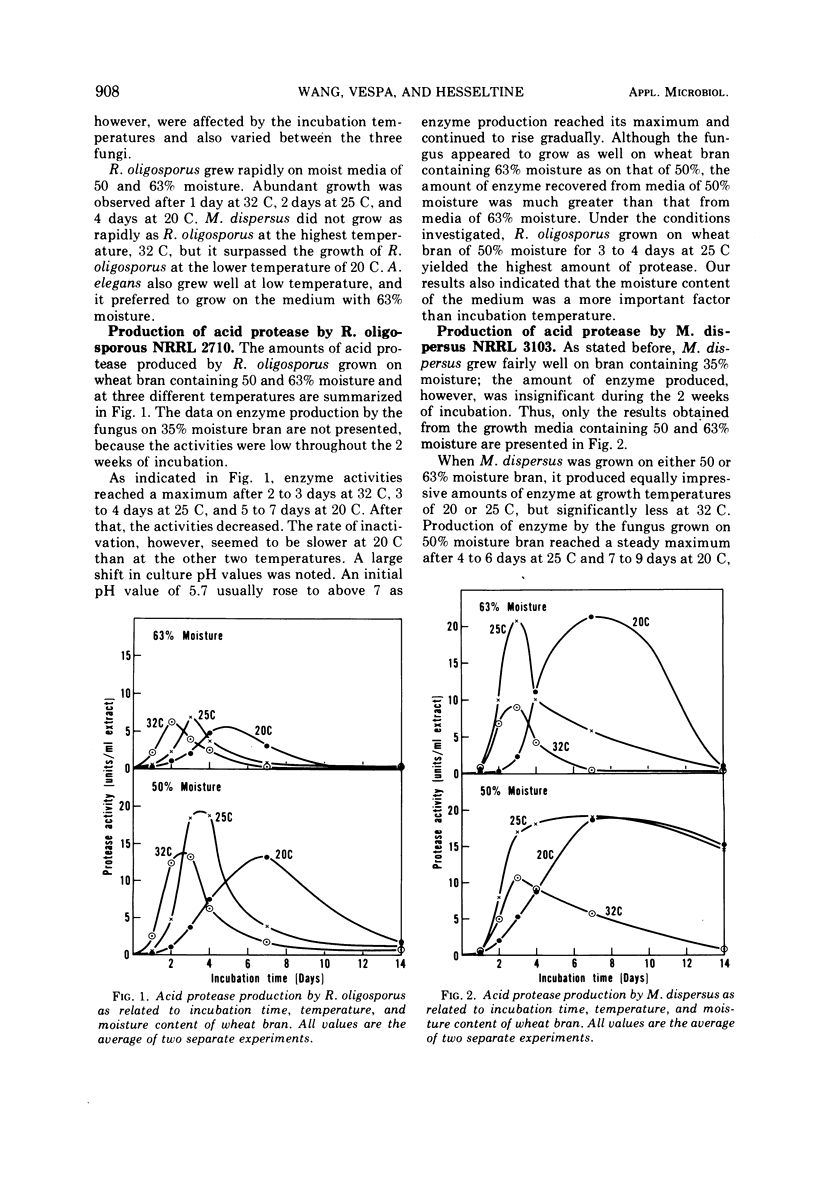
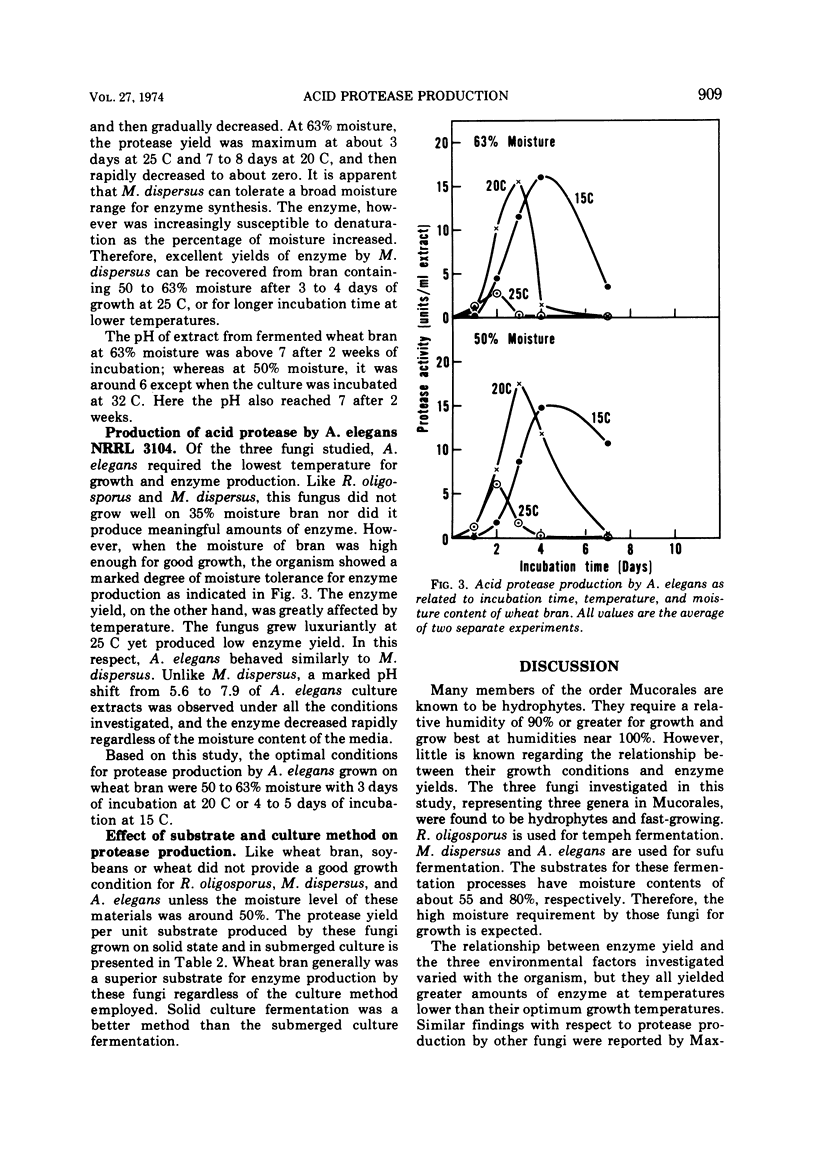
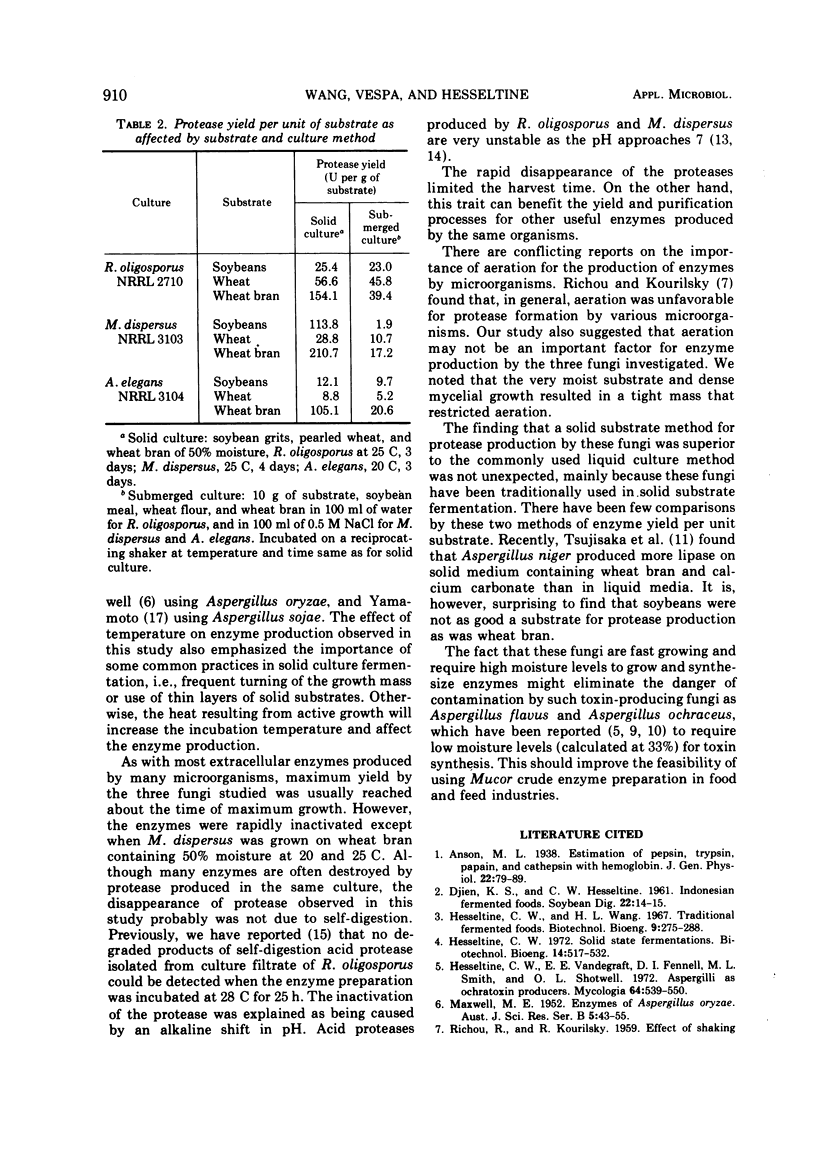
Growth conditions for maximum protease production by Rhizopus oligosporus, Mucor dispersus, and Actinomucor elegans, used in Oriental food fermentations, were investigated. Enzyme yields by all three fungi were higher in solid substrate fermentations than in submerged culture. The level of moisture in solid substrate must be at about 50 to 60%. Very little growth of these fungi was noted when the moisture of substrate was below 35%, whereas many fungi including most storage fungi generally grow well on solid substrate with that level of moisture. Among the three substrates tested—wheat bran, wheat, and soybeans—wheat bran was the most satisfactory one for enzyme production. The optimal conditions for maximum enzyme production of the three fungi grown on wheat bran were: R. oligosporus, 50% moisture at 25 C for 3 to 4 days; M. dispersus, 50 to 63% moisture at 25 C for 3 to 4 days; A. elegans, 50 to 63% moisture at 20 C for 3 days. Because these fungi are fast growing and require high moisture for growth and for enzyme synthesis, the danger of contamination by toxin-producing fungi would be minimal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hesseltine C. W. Biotechnology report. Solid state fermentations. Biotechnol Bioeng. 1972 Jul;14(4):517–532. doi: 10.1002/bit.260140402. [DOI] [PubMed] [Google Scholar]
- Hesseltine C. W., Vandegraft E. E., Fennell D. I., Smith M. L., Shotwell O. L. Aspergilli as ochratoxin producers. Mycologia. 1972 May-Jun;64(3):539–550. [PubMed] [Google Scholar]
- MAXWELL M. E. Enzymes of Aspergillus oryzae. I. The development of a culture medium yielding high protease activity. Aust J Sci Res B. 1952 Feb;5(1):42–55. [PubMed] [Google Scholar]
- RICHOU R., KOURILSKY R. Influence de l'agitation des cultures microbiennes sur la production des enzymes protéolytiques. C R Hebd Seances Acad Sci. 1959 Jul 15;249(2):336–337. [PubMed] [Google Scholar]
- Shotwell O. L., Hesseltine C. W., Stubblefield R. D., Sorenson W. G. Production of aflatoxin on rice. Appl Microbiol. 1966 May;14(3):425–428. doi: 10.1128/am.14.3.425-428.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield R. D., Shotwell O. L., Hesseltine C. W., Smith M. L., Hall H. H. Production of aflatoxin on wheat and oats: measurement with a recording densitometer. Appl Microbiol. 1967 Jan;15(1):186–190. doi: 10.1128/am.15.1.186-190.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L., Hesseltine C. W. Multiple forms of Rhizopus oligosporus protease. Arch Biochem Biophys. 1970 Oct;140(2):459–463. doi: 10.1016/0003-9861(70)90089-5. [DOI] [PubMed] [Google Scholar]
- Wang H. L., Hesseltine C. W. Studies on the extracellular proteolytic enzymes of Rhizopus oligosporus. Can J Microbiol. 1965 Aug;11(4):727–732. doi: 10.1139/m65-096. [DOI] [PubMed] [Google Scholar]
- Wang H. L. Release of proteinase from mycelium of Mucor hiemalis. J Bacteriol. 1967 Jun;93(6):1794–1799. doi: 10.1128/jb.93.6.1794-1799.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L., Ruttle D. I., Hesseltine C. W. Milk-clotting activity of proteinases produced by Rhizopus. Can J Microbiol. 1969 Jan;15(1):99–104. doi: 10.1139/m69-015. [DOI] [PubMed] [Google Scholar]



