Abstract
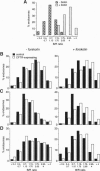
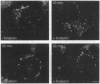
Previous studies have suggested a role for cystic fibrosis transmembrane conductance regulator (CFTR) in the regulation of intracellular vesicular trafficking. A quantitative fluorescence method was used to test the hypothesis that CFTR expression and activation affects endosome-endosome fusion in intact cells. Endosomes from CFTR-expressing and control (vector-transfected) Swiss 3T3 fibroblasts were labeled by internalization with 4,4-difluoro-5,7-dimethyl-4-bora-3a, 4a-diaza-s-indacene (Bodipy)-avidin, a fluid-phase marker whose fluorescence increases approximately 8-fold upon biotin binding. Cells were washed, chased, and then labeled with biotin-albumin or biotin-transferrin. The fraction of Bodipy-avidin-labeled endosomes that fused with biotin-containing endosomes (f(fusion)) was quantified by ratio imaging microfluorimetry. Endosome fusion in unstimulated CFTR-expressing cells was similar to that in control cells. However, in CFTR-expressing cells activated by forskolin, ffusion was increased by 1.30 +/- 0.18- and 2.65 +/- 0.17-fold for a 0 and 10 min chase time between avidin and biotin-albumin pulses; f(fusion) also increased (1.32 +/- 0.11-fold) when biotin-transferrin replaced biotin-albumin. The stimulation of endosome fusion was not due to differences in rates of endocytosis or endosomal acidification. Endosome fusion was not stimulated by forskolin in Cl--depleted CFTR-expressing cells, suggesting that the increase in endosome fusion is due to the CFTR chloride channel activity. These results provide evidence that CFTR is involved in the regulation of endosome fusion and, thus, a possible basis for the cellular defects associated with cystic fibrosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Bhat S. V., Dohadwalla A. N., Bajwa B. S., Dadkar N. K., Dornauer H., de Souza N. J. The antihypertensive and positive inotropic diterpene forskolin: effects of structural modifications on its activity. J Med Chem. 1983 Apr;26(4):486–492. doi: 10.1021/jm00358a006. [DOI] [PubMed] [Google Scholar]
- Biwersi J., Verkman A. S. Functional CFTR in endosomal compartment of CFTR-expressing fibroblasts and T84 cells. Am J Physiol. 1994 Jan;266(1 Pt 1):C149–C156. doi: 10.1152/ajpcell.1994.266.1.C149. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Cohn J. A., Venglarik C. J., Bridges R. J. Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J Biol Chem. 1994 Mar 18;269(11):8296–8302. [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992 Apr 24;256(5056):530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Carson M. R., Welsh M. J. Structural and functional similarities between the nucleotide-binding domains of CFTR and GTP-binding proteins. Biophys J. 1995 Dec;69(6):2443–2448. doi: 10.1016/S0006-3495(95)80113-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dho S., Grinstein S., Foskett J. K. Plasma membrane recycling in CFTR-expressing CHO cells. Biochim Biophys Acta. 1993 Nov 25;1225(1):78–82. doi: 10.1016/0925-4439(93)90125-k. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988 Mar;106(3):617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., Park J., Semrad C. E., Gelman D. L., Shevell T., McGraw T. E. Regulation of endocytic trafficking and acidification are independent of the cystic fibrosis transmembrane regulator. J Biol Chem. 1994 Feb 18;269(7):5336–5345. [PubMed] [Google Scholar]
- Emans N., Biwersi J., Verkman A. S. Imaging of endosome fusion in BHK fibroblasts based on a novel fluorimetric avidin-biotin binding assay. Biophys J. 1995 Aug;69(2):716–728. doi: 10.1016/S0006-3495(95)79947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N., Verkman A. S. Real-time fluorescence measurement of cell-free endosome fusion: regulation by second messengers. Biophys J. 1996 Jul;71(1):487–494. doi: 10.1016/S0006-3495(96)79250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Skach W., Baker O., Calayag M. C., Lingappa V., Verkman A. S. A multifunctional aqueous channel formed by CFTR. Science. 1992 Nov 27;258(5087):1477–1479. doi: 10.1126/science.1279809. [DOI] [PubMed] [Google Scholar]
- Lukacs G. L., Chang X. B., Kartner N., Rotstein O. D., Riordan J. R., Grinstein S. The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem. 1992 Jul 25;267(21):14568–14572. [PubMed] [Google Scholar]
- Lukacs G. L., Mohamed A., Kartner N., Chang X. B., Riordan J. R., Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994 Dec 15;13(24):6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan P., Dearborn D. G., McPherson J. M., Smith A. E. Sequence homologies between nucleotide binding regions of CFTR and G-proteins suggest structural and functional similarities. FEBS Lett. 1995 Jun 12;366(2-3):87–91. doi: 10.1016/0014-5793(95)00463-j. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Sugimoto T., Kurachi Y. Effects of anions on the G protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992 May;99(5):665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Grout M., Zaidi T. S., Olsen J. C., Johnson L. G., Yankaskas J. R., Goldberg J. B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996 Jan 5;271(5245):64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen J. H., Fischer H., Illek B., Machen T. E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince L. S., Workman R. B., Jr, Marchase R. B. Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5192–5196. doi: 10.1073/pnas.91.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisin I. L., Prat A. G., Abraham E. H., Amara J. F., Gregory R. J., Ausiello D. A., Cantiello H. F. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994 Aug 12;269(32):20584–20591. [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Santos G. F., Reenstra W. W. Activation of the cystic fibrosis transmembrane regulator by cyclic AMP is not correlated with inhibition of endocytosis. Biochim Biophys Acta. 1994 Oct 12;1195(1):96–102. doi: 10.1016/0005-2736(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Egan M. E., Hwang T. H., Fulmer S. B., Allen S. S., Cutting G. R., Guggino W. B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995 Jun 30;81(7):1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Gesek F., Ercolani L., Wjasow C., Gruenert D. C., Karlson K., Stanton B. A. Heterotrimeric G proteins, vesicle trafficking, and CFTR Cl- channels. Am J Physiol. 1994 Jul;267(1 Pt 1):C272–C281. doi: 10.1152/ajpcell.1994.267.1.C272. [DOI] [PubMed] [Google Scholar]
- Seksek O., Biwersi J., Verkman A. S. Evidence against defective trans-Golgi acidification in cystic fibrosis. J Biol Chem. 1996 Jun 28;271(26):15542–15548. doi: 10.1074/jbc.271.26.15542. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts M. J., Canessa C. M., Olsen J. C., Hamrick M., Cohn J. A., Rossier B. C., Boucher R. C. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995 Aug 11;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Ward C. L., Kopito R. R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994 Oct 14;269(41):25710–25718. [PubMed] [Google Scholar]
- Webster P., Vanacore L., Nairn A. C., Marino C. R. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol. 1994 Aug;267(2 Pt 1):C340–C348. doi: 10.1152/ajpcell.1994.267.2.C340. [DOI] [PubMed] [Google Scholar]
- Wine J. J. Cystic fibrosis: How do CFTR mutations cause cystic fibrosis? Curr Biol. 1995 Dec 1;5(12):1357–1359. doi: 10.1016/s0960-9822(95)00269-7. [DOI] [PubMed] [Google Scholar]
- Zen K., Biwersi J., Periasamy N., Verkman A. S. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J Cell Biol. 1992 Oct;119(1):99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]