Abstract
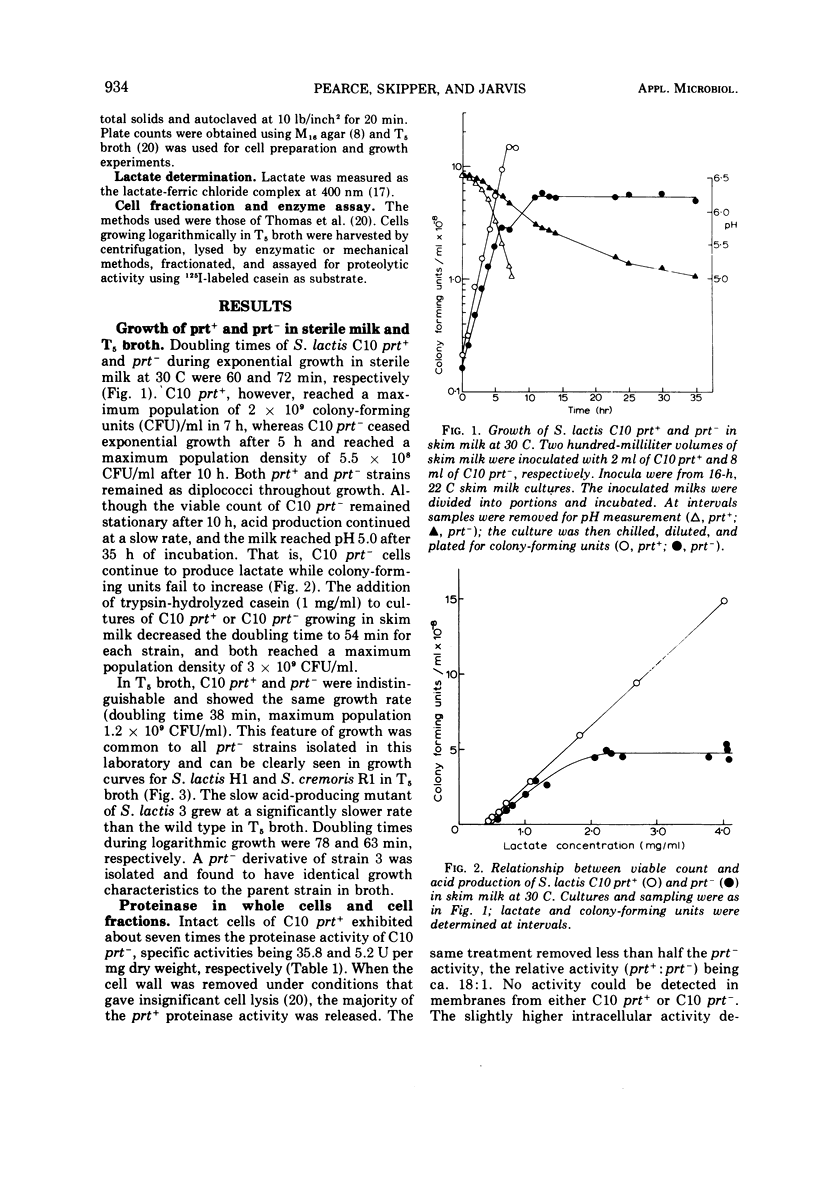
Variants of Streptococcus lactis that produce lactic acid slowly in milk were isolated by inducing plasmid loss in the wild type at 39 to 40 C. Such strains had lost most of their surface-bound proteinase activity and were designated prt-. The specific proteinase activities of S. lactis C10 prt+ whole cells and solubilized cell walls were 7 and 18 times, respectively, those of the prt- strain, but spheroplast lysates of prt+ and prt- strains contained similar proteinase activity. S. lactis H1 showed a similar relative distribution of activity between prt+ and prt- cellular fractions, although the overall level was lower. The limited growth in milk, characteristic of prt- strains, can be explained in terms of their low surface-bound proteinase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CITTI J. E., SANDINE W. E., ELLIKER P. R. COMPARISON OF SLOW AND FAST ACID-PRODUCING STREPTOCOCCUS LACTIS. J Dairy Sci. 1965 Jan;48:14–18. doi: 10.3168/jds.s0022-0302(65)88152-8. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Regulation of the formation of protease in Bacillus megaterium. I. The influence of amino acids on the enzyme formation. Folia Microbiol (Praha) 1966;11(2):82–88. doi: 10.1007/BF02878835. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Porterfield N., Matsumura P. Acid-base titration of streptococci and the physical states of intracellular ions. J Bacteriol. 1973 May;114(2):491–498. doi: 10.1128/jb.114.2.491-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martley F. G., Jayashankar S. R., Lawrence R. C. An improved agar medium for the detection of proteolytic organisms in total bacterial counts. J Appl Bacteriol. 1970 Jun;33(2):363–370. doi: 10.1111/j.1365-2672.1970.tb02208.x. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMARK R., CITRI N. Repression of protease formation in Bacillus cereus. Biochim Biophys Acta. 1962 Jun 4;59:749–751. doi: 10.1016/0006-3002(62)90669-8. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Peptide transport and metabolism in bacteria. Annu Rev Biochem. 1971;40:397–408. doi: 10.1146/annurev.bi.40.070171.002145. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A., Speck M. L. Isolation and partial characterization of a particulate proteinase from a slow acid producing mutant of Streptococcus lactis. J Dairy Sci. 1971 Sep;54(9):1253–1258. doi: 10.3168/jds.S0022-0302(71)86016-2. [DOI] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A. Substrate specificity of the intracellular proteinase from a slow acid producing mutant of Streptococcus lactis. J Dairy Sci. 1971 Sep;54(9):1265–1269. doi: 10.3168/jds.S0022-0302(71)86018-6. [DOI] [PubMed] [Google Scholar]



