Abstract
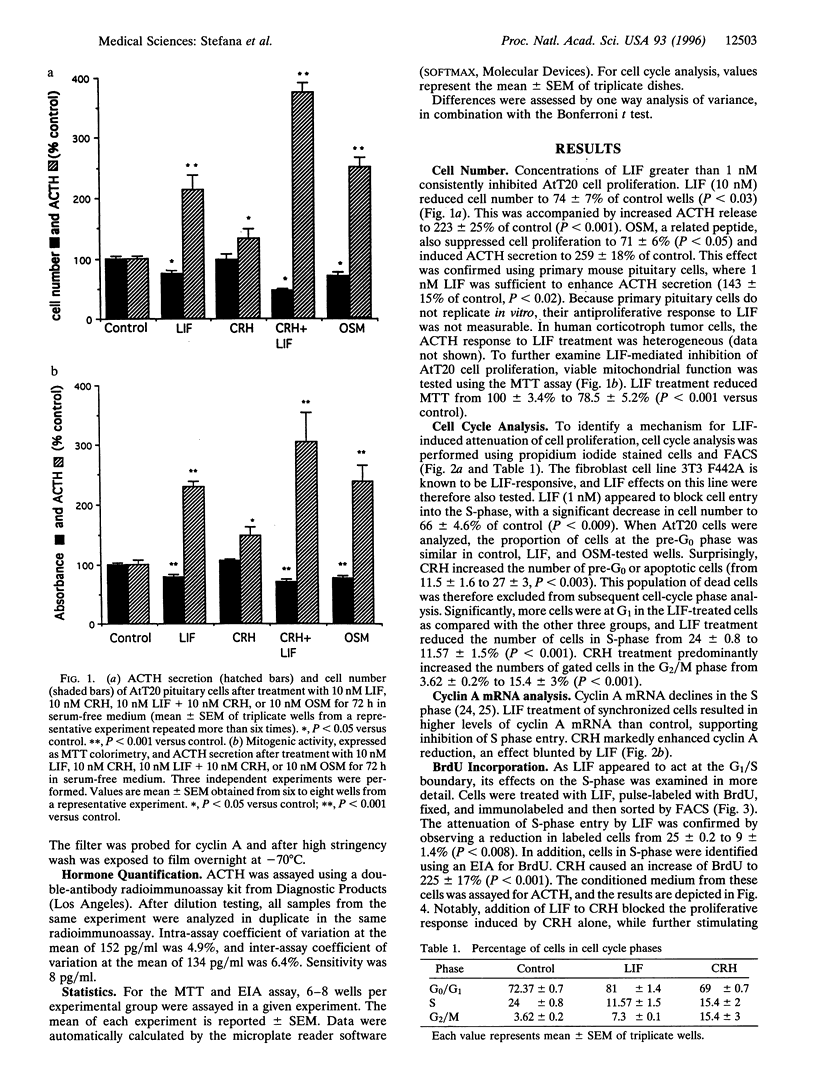
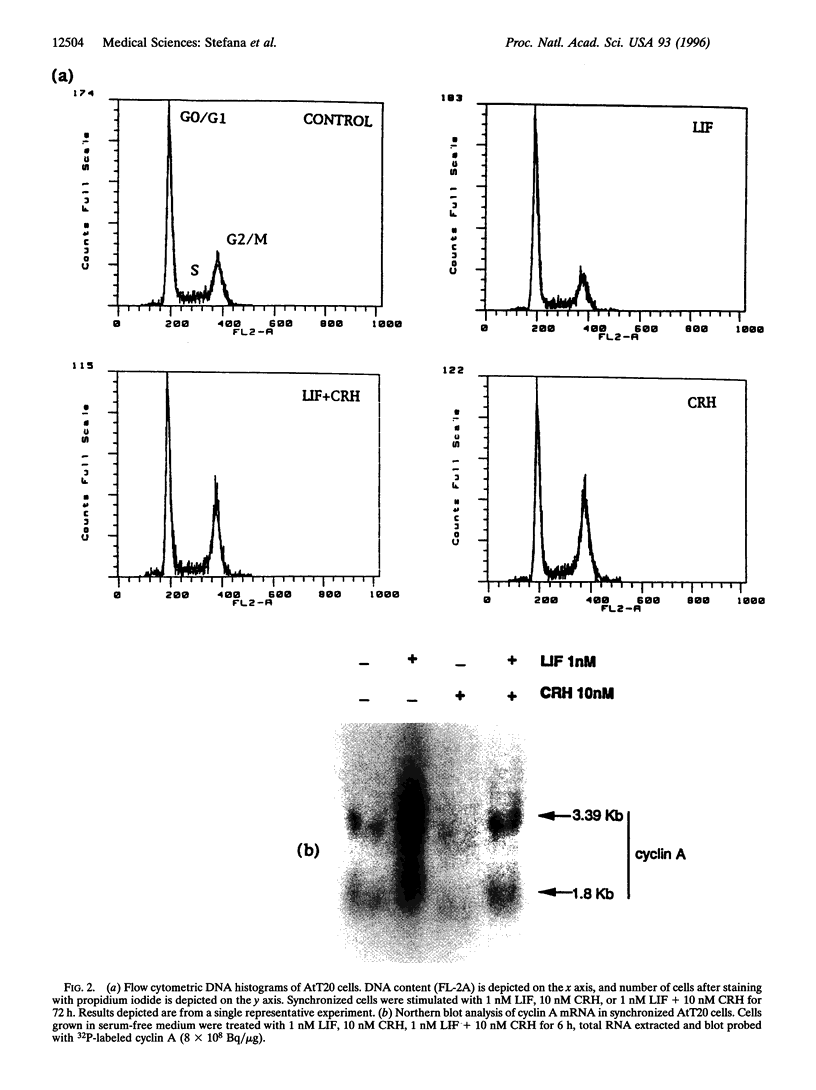
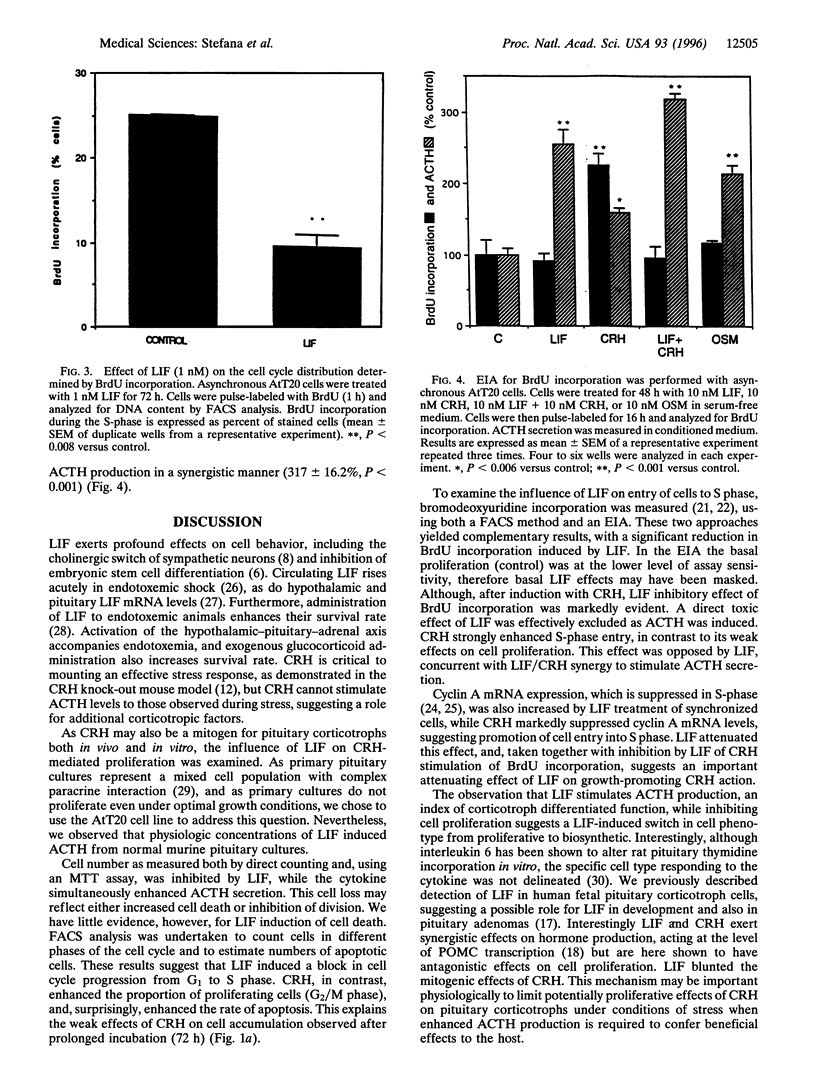
Leukemia inhibitory factor (LIF) promotes differentiated cell function in several systems. We recently reported LIF and LIF receptor expression in human fetal pituitary corticotrophs in vivo and demonstrated LIF stimulation of adrenocorticotrophin (ACTH) transcription in vitro, suggesting a role for LIF in corticotroph development. We therefore assessed the action of LIF on proliferating murine corticotroph cells (AtT20). LIF impairs proliferation of AtT20 cells (25% reduction versus control, P < 0.03), while simultaneously enhancing ACTH secretion (2-fold, P < 0.001) and augmenting ACTH responsiveness to corticotrophin-releasing hormone (CRH) action (4-fold, P < 0.001). This attenuation of cell growth is due to a block of cell cycle progression from G1 into S phase, as measured by flow cytometric analysis (24 +/- 0.8 versus 11.57 +/- 1.5, P < 0.001). Using bromodeoxyuridine incorporation assays, loss of cells in S phase was confirmed (25 +/- 0.08 to 9.4 +/- 1.4, P < 0.008). In contrast, CRH induced the G2/M phase (3.6 +/- 0.2 to 15.4 +/- 3, P < 0.001). This effect was blunted by LIF (P < 0.001 versus CRH alone). Cyclin A mRNA levels, which decline in S phase, were stimulated 3.5-fold by LIF and markedly suppressed by CRH. These results indicate a LIF-induced cell cycle block occurring at G1/S in corticotroph cells. Thus, LIF reduces proliferation, enhances ACTH secretion, and potentiates effects of CRH on ACTH secretion while blocking effects of CRH on the cell cycle. Responses of these three markers of differentiated corticotroph function indicate LIF to be a differentiation factor for pituitary corticotroph cells by preferential phenotypic switching from proliferative to synthetic.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita S., Webster J., Ren S. G., Takino H., Said J., Zand O., Melmed S. Human and murine pituitary expression of leukemia inhibitory factor. Novel intrapituitary regulation of adrenocorticotropin hormone synthesis and secretion. J Clin Invest. 1995 Mar;95(3):1288–1298. doi: 10.1172/JCI117779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzt E., Buric R., Stelzer G., Stalla J., Sauer J., Renner U., Stalla G. K. Interleukin involvement in anterior pituitary cell growth regulation: effects of IL-2 and IL-6. Endocrinology. 1993 Jan;132(1):459–467. doi: 10.1210/endo.132.1.8419142. [DOI] [PubMed] [Google Scholar]
- Bamber B. A., Masters B. A., Hoyle G. W., Brinster R. L., Palmiter R. D. Leukemia inhibitory factor induces neurotransmitter switching in transgenic mice. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7839–7843. doi: 10.1073/pnas.91.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A. Immune-neuroendocrine circuits: integrative role of cytokines. Front Neuroendocrinol. 1992 Jan;13(1):61–94. [PubMed] [Google Scholar]
- Boutillier A. L., Monnier D., Lorang D., Lundblad J. R., Roberts J. L., Loeffler J. P. Corticotropin-releasing hormone stimulates proopiomelanocortin transcription by cFos-dependent and -independent pathways: characterization of an AP1 site in exon 1. Mol Endocrinol. 1995 Jun;9(6):745–755. doi: 10.1210/mend.9.6.8592520. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995 May 18;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Dolbeare F., Gratzner H., Rice G. C., Gray J. W. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984 Jul;17(4):427–436. doi: 10.1111/j.1365-2184.1984.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Winer J., Henzel W. J. Pituitary follicular cells secrete an inhibitor of aortic endothelial cell growth: identification as leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):698–702. doi: 10.1073/pnas.89.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata J., Usui T., Naitoh Y., Nakai Y., Imura H. Effects of recombinant human interleukin-1 alpha, -1 beta, 2 and 6 on ACTH synthesis and release in the mouse pituitary tumour cell line AtT-20. J Endocrinol. 1989 Jul;122(1):33–39. doi: 10.1677/joe.0.1220033. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S., Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992 Mar 13;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Gertz B. J., Contreras L. N., McComb D. J., Kovacs K., Tyrrell J. B., Dallman M. F. Chronic administration of corticotropin-releasing factor increases pituitary corticotroph number. Endocrinology. 1987 Jan;120(1):381–388. doi: 10.1210/endo-120-1-381. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., Nye S. H., Boulton T. G., Davis S., Taga T., Li Y., Birren S. J., Yasukawa K., Kishimoto T., Anderson D. J. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992 Jun 26;69(7):1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Muglia L., Jacobson L., Dikkes P., Majzoub J. A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995 Feb 2;373(6513):427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Okajima T., Nakamura K., Zhang H., Ling N., Tanabe T., Yasuda T., Rosenfeld R. G. Sensitive colorimetric bioassays for insulin-like growth factor (IGF) stimulation of cell proliferation and glucose consumption: use in studies of IGF analogs. Endocrinology. 1992 Apr;130(4):2201–2212. doi: 10.1210/endo.130.4.1372238. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. Leukemia inhibitory factor, a cytokine at the interface between neurobiology and immunology. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7833–7835. doi: 10.1073/pnas.91.17.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. W., Ren S. G., Melmed S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest. 1996 Apr 15;97(8):1852–1859. doi: 10.1172/JCI118615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte B., Reynders M. M., Bosman F. T., Blijham G. H. Effect of tissue fixation on anti-bromodeoxyuridine immunohistochemistry. J Histochem Cytochem. 1987 Nov;35(11):1343–1345. doi: 10.1177/35.11.3116075. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Cherny R. Intercellular communication within the anterior pituitary influencing the secretion of hypophysial hormones. Endocr Rev. 1992 Aug;13(3):453–475. doi: 10.1210/edrv-13-3-453. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984 Sep 10;259(17):10978–10982. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ren S. G., Melmed S. Hypothalamic and pituitary leukemia inhibitory factor gene expression in vivo: a novel endotoxin-inducible neuro-endocrine interface. Endocrinology. 1996 Jul;137(7):2947–2953. doi: 10.1210/endo.137.7.8770918. [DOI] [PubMed] [Google Scholar]
- Waring P. M., Waring L. J., Billington T., Metcalf D. Leukemia inhibitory factor protects against experimental lethal Escherichia coli septic shock in mice. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1337–1341. doi: 10.1073/pnas.92.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P., Wycherley K., Cary D., Nicola N., Metcalf D. Leukemia inhibitory factor levels are elevated in septic shock and various inflammatory body fluids. J Clin Invest. 1992 Nov;90(5):2031–2037. doi: 10.1172/JCI116083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kobayashi R., Galaktionov K., Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995 Sep 22;82(6):915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]