Abstract
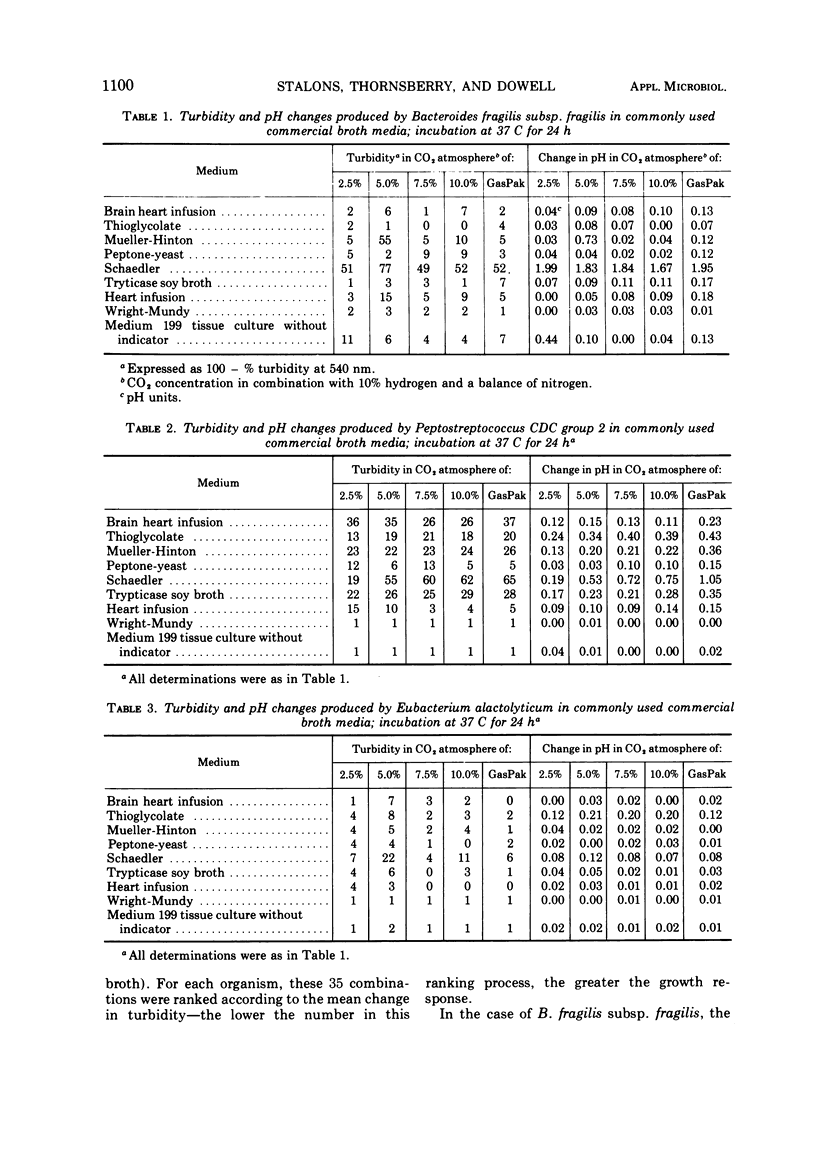
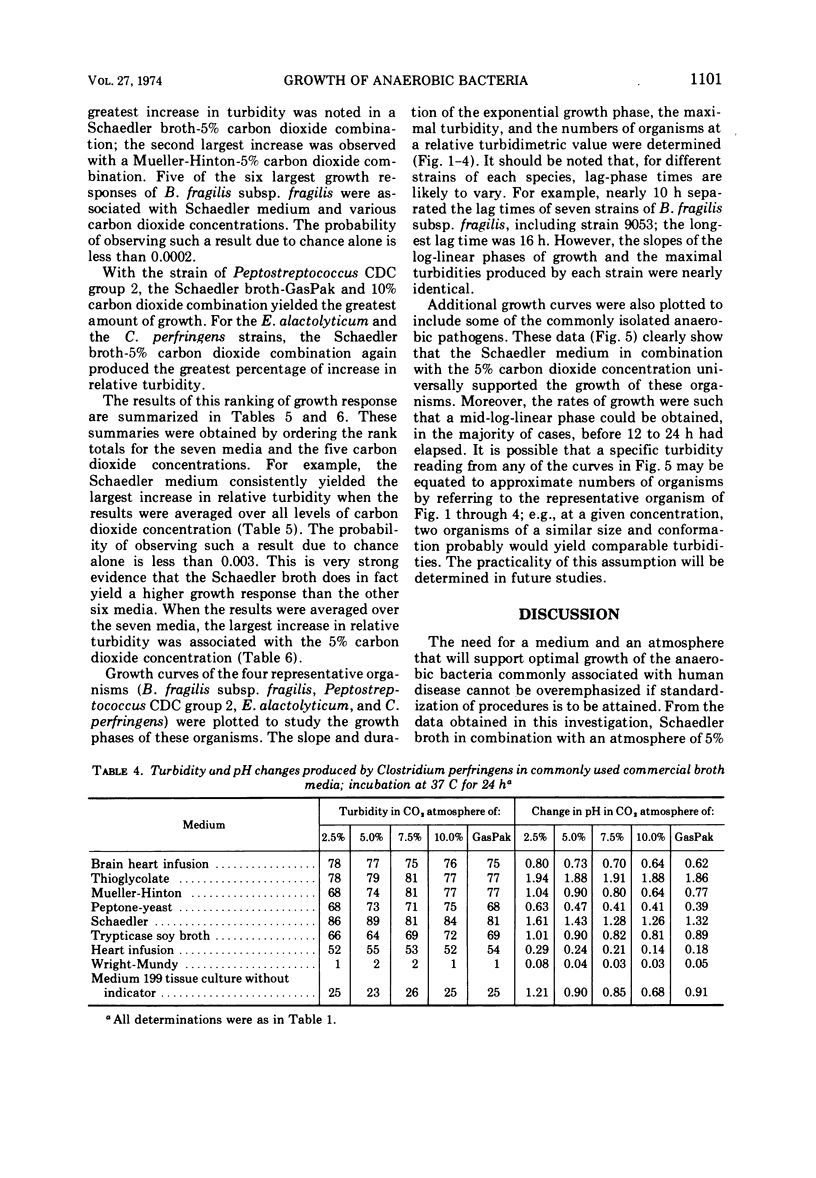
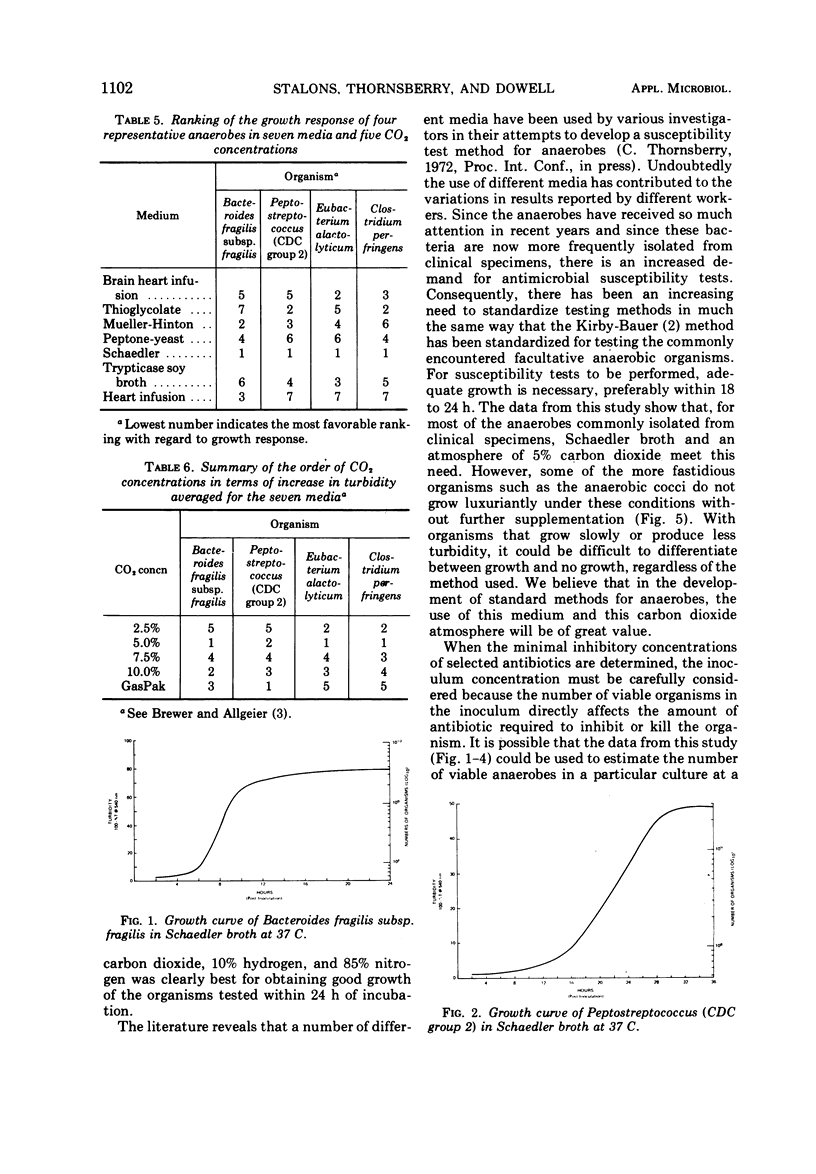
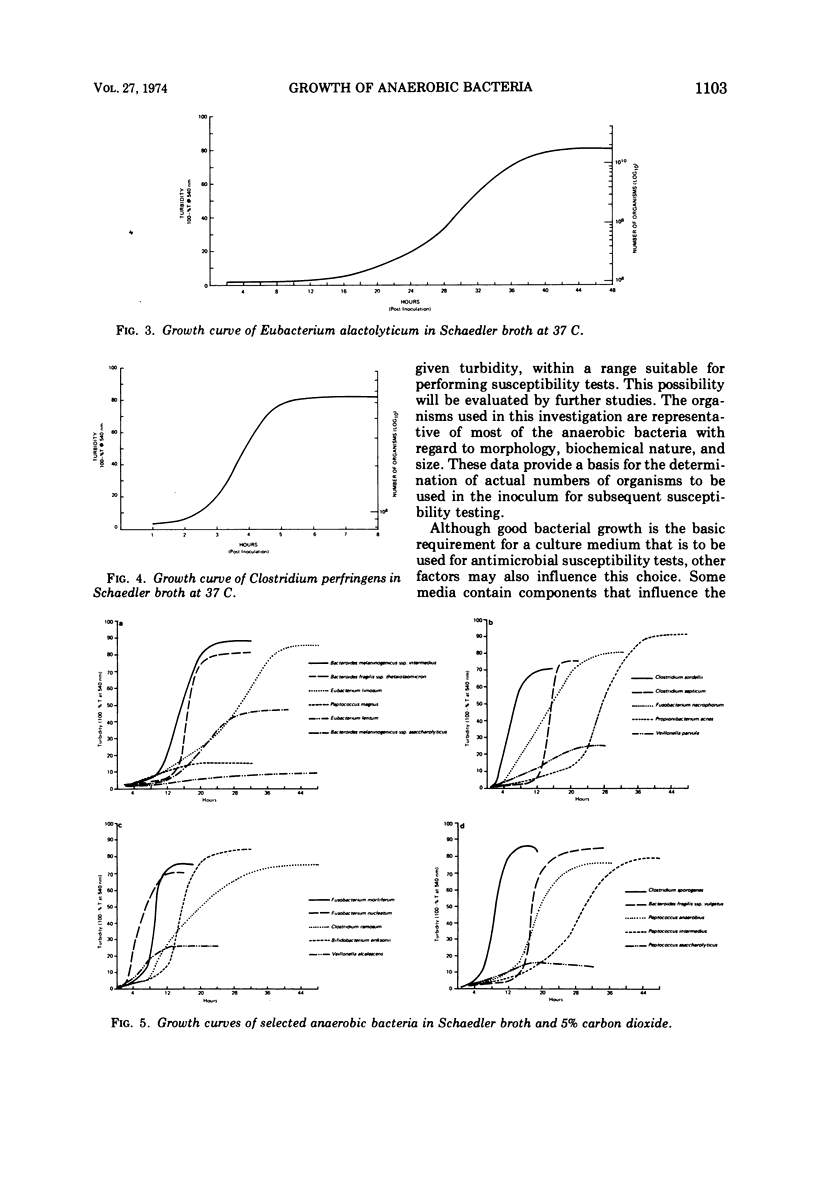
Representative strains of anaerobic bacteria from human infections were used to evaluate broth media, gas mixtures, and inocula for use in developing a procedure for performing minimal inhibitory concentration antimicrobic susceptibility tests. Nine commercially available media, including two that were chemically defined, were tested. Tests were performed in atmospheres with carbon dioxide concentrations between 2.5 and 10% and also in the GasPak system (BBL) that had a disposable hydrogen-carbon dioxide generator. Growth curves on each organism grown in schaedler broth and a 5% carbon dioxide atmosphere were used to determine growth characteristics, equate time of the particular growth phases to turbidity readings, and determine the numbers of viable organisms present in the culture. Schaedler broth proved to be most advantageous in combination with an atmosphere of 5% carbon dioxide, 10% hydrogen, and 85% nitrogen. The growth curve studies yielded valuable data on the rapidity and quantity of growth under these conditions. We believe these data have provided information which can be used as the basis for developing a standardized procedure for antimicrobic susceptibility testing for anaerobic bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Brewer J. H., Allgeier D. L. A disposable anaerobic system designed for field and laboratory use. Appl Microbiol. 1968 Jun;16(6):848–850. doi: 10.1128/am.16.6.848-850.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felner J. M., Dowell V. R., Jr "Bacteroides" bacteremia. Am J Med. 1971 Jun;50(6):787–796. doi: 10.1016/0002-9343(71)90187-2. [DOI] [PubMed] [Google Scholar]
- Felner J. M., Dowell V. R., Jr Anaerobic bacterial endocarditis. N Engl J Med. 1970 Nov 26;283(22):1188–1192. doi: 10.1056/NEJM197011262832203. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dowell V. R., Jr, Farshtchi D., Raines L. J., Cherry W. B. Cultural characteristics and fatty acid composition of propionibacteria. J Bacteriol. 1969 Feb;97(2):561–570. doi: 10.1128/jb.97.2.561-570.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


