Abstract
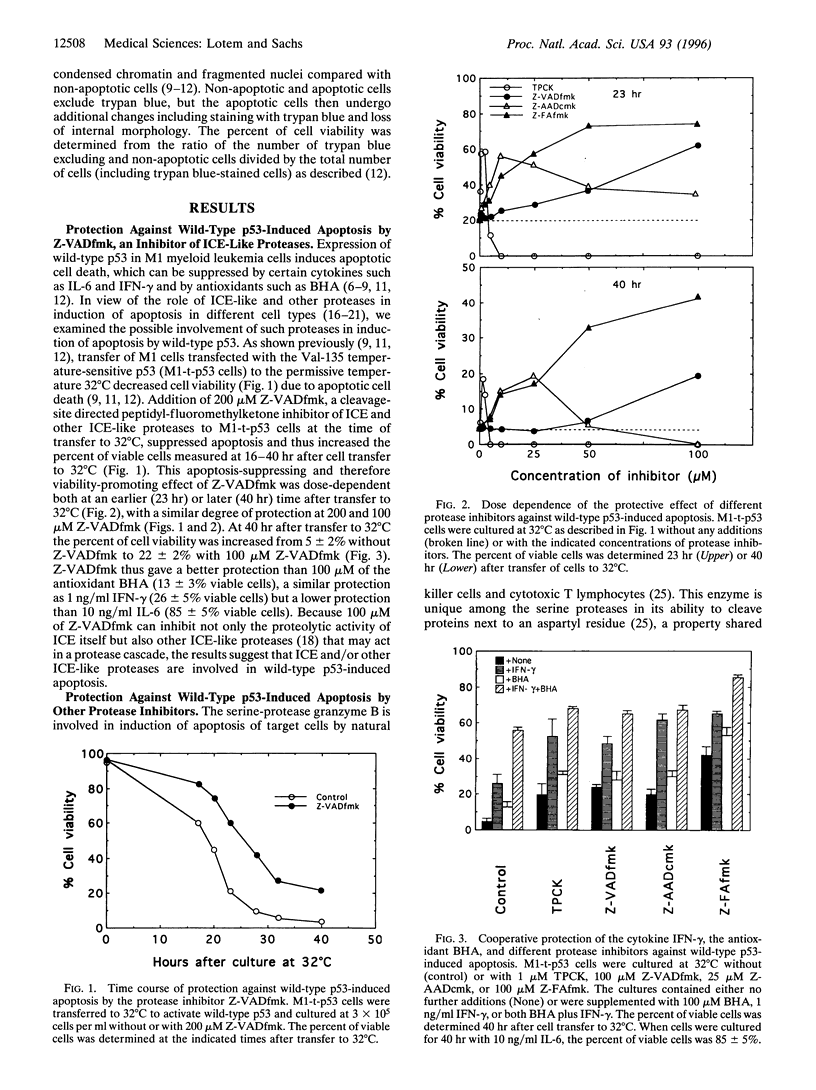
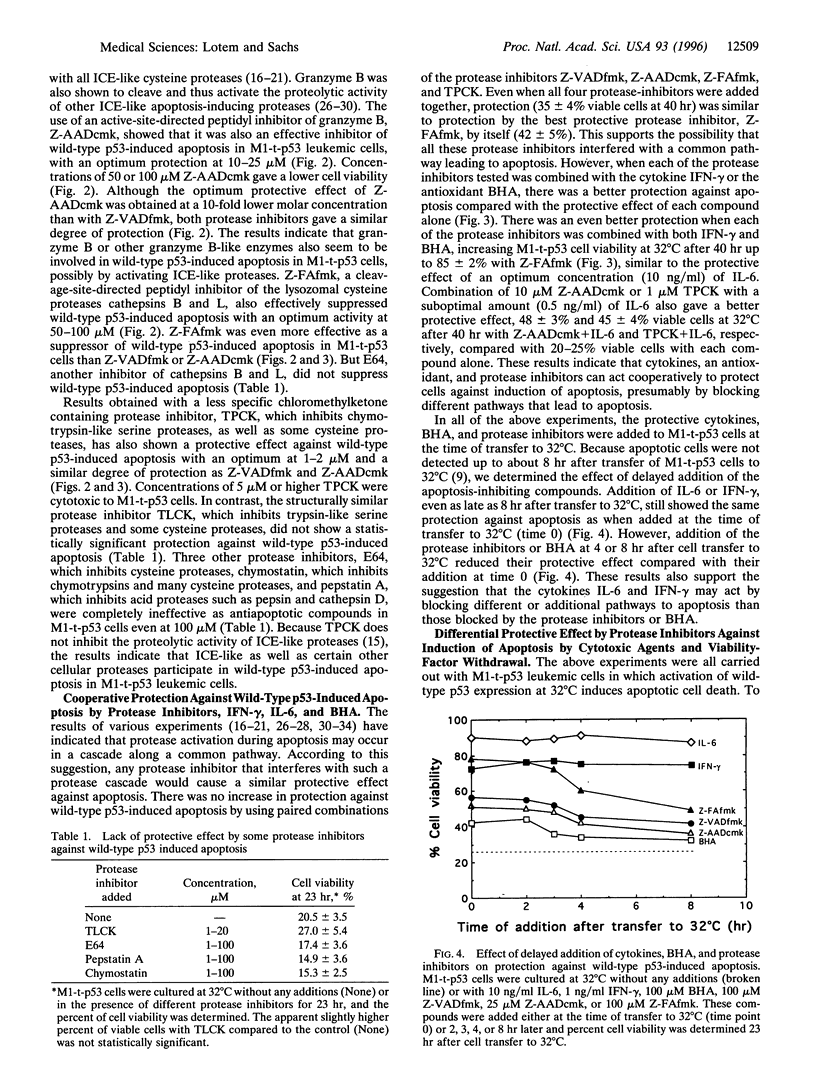
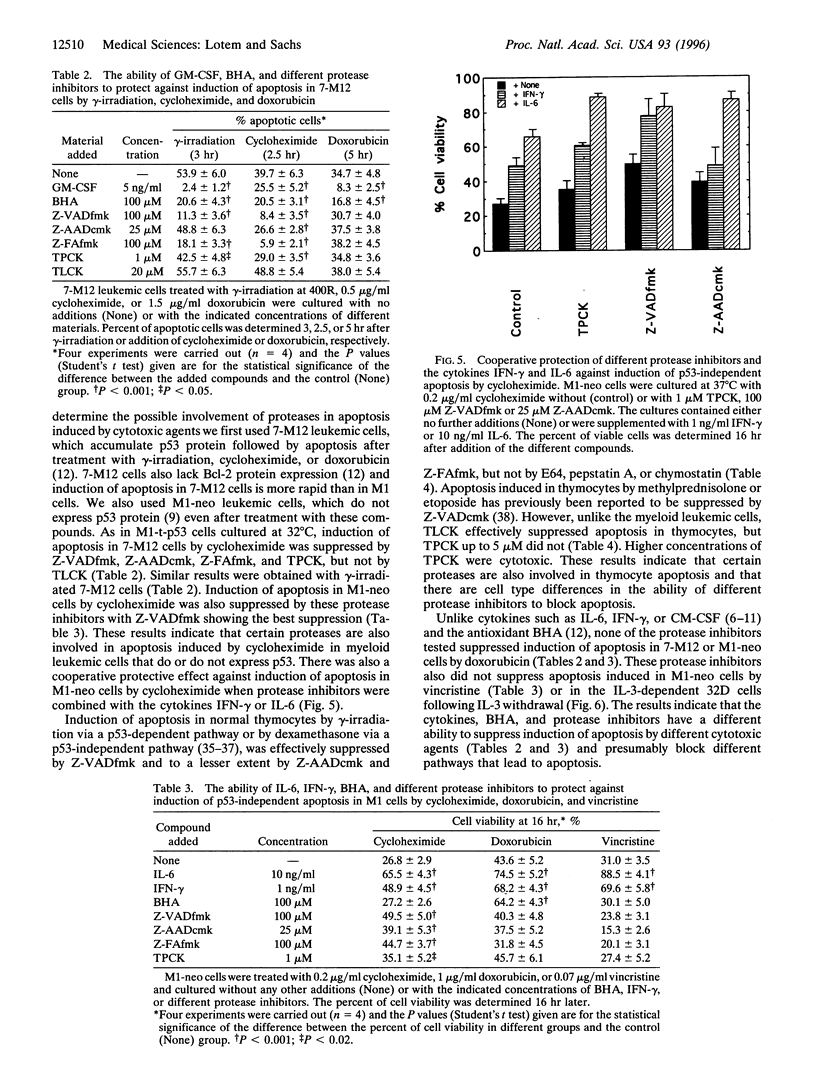
Apoptosis induced in myeloid leukemic cells by wild-type p53 was suppressed by different cleavage-site directed protease inhibitors, which inhibit interleukin-1 beta-converting enzyme-like, granzyme B and cathepsins B and L proteases. Apoptosis was also suppressed by the serine and cysteine protease inhibitor N-tosyl-L-phenylalanine chloromethylketone (TPCK) [corrected], but not by other serine or cysteine protease inhibitors including N alpha-p-tosyl-L-lysine chloromethylketone (TLCK), E64, pepstatin A, or chymostatin. Protease inhibitors suppressed induction of apoptosis by gamma-irradiation and cycloheximide but not by doxorubicin, vincristine, or withdrawal of interleukin 3 from interleukin 3-dependent 32D non-malignant myeloid cells. Induction of apoptosis in normal thymocytes by gamma-irradiation or dexamethasone was also suppressed by the cleavage-site directed protease inhibitors, but in contrast to the myeloid leukemic cells apoptosis in thymocytes was suppressed by TLCK but not by TPCK. The results indicate that (i) inhibitors of interleukin-1 beta-converting enzyme-like proteases and some other protease inhibitors suppressed induction of apoptosis by wild-type p53 and certain p53-independent pathways of apoptosis; (ii) the protease inhibitors together with the cytokines interleukin 6 and interferon-gamma or the antioxidant butylated hydroxyanisole gave a cooperative protection against apoptosis; (iii) these protease inhibitors did not suppress induction of apoptosis by some cytotoxic agents or by viability-factor withdrawal from 32D cells, whereas these pathways of apoptosis were suppressed by cytokines; (iv) there are cell type differences in the proteases involved in apoptosis; and (v) there are multiple pathways leading to apoptosis that can be selectively induced and suppressed by different agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenas J., Werb Z. Proteolysis and the biochemistry of life-or-death decisions. J Exp Med. 1996 May 1;183(5):1947–1951. doi: 10.1084/jem.183.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M. P., Goncharov T. M., Goltsev Y. V., Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996 Jun 14;85(6):803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Darmon A. J., Nicholson D. W., Bleackley R. C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995 Oct 5;377(6548):446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Enari M., Talanian R. V., Wong W. W., Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996 Apr 25;380(6576):723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Fraser A., Evan G. A license to kill. Cell. 1996 Jun 14;85(6):781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- Gu Y., Sarnecki C., Fleming M. A., Lippke J. A., Bleackley R. C., Su M. S. Processing and activation of CMH-1 by granzyme B. J Biol Chem. 1996 May 3;271(18):10816–10820. doi: 10.1074/jbc.271.18.10816. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. ICE family proteases: mediators of all apoptotic cell death? Immunity. 1996 Mar;4(3):195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Askew D. Origins and properties of hematopoietic growth factor-dependent cell lines. Int J Cell Cloning. 1989 Mar;7(2):68–91. doi: 10.1002/stem.5530070202. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995 Mar 31;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1995 May;20(5):198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- Lotem J., Peled-Kamar M., Groner Y., Sachs L. Cellular oxidative stress and the control of apoptosis by wild-type p53, cytotoxic compounds, and cytokines. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9166–9171. doi: 10.1073/pnas.93.17.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of apoptosis in hematopoiesis and leukemia by cytokines, tumor suppressor and oncogenes. Leukemia. 1996 Jun;10(6):925–931. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Genetic dissection of the control of normal differentiation in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5554–5558. doi: 10.1073/pnas.74.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993 Aug 15;82(4):1092–1096. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cytokines inhibit apoptosis induced by transforming growth factor beta 1 and cancer chemotherapy compounds in myeloid leukemic cells. Blood. 1992 Oct 1;80(7):1750–1757. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Interferon-gamma inhibits apoptosis induced by wild-type p53, cytotoxic anti-cancer agents and viability factor deprivation in myeloid cells. Leukemia. 1995 Apr;9(4):685–692. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Amarante-Mendes G. P., Shi L., Chuang T. H., Casiano C. A., O'Brien G. A., Fitzgerald P., Tan E. M., Bokoch G. M., Greenberg A. H. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J. 1996 May 15;15(10):2407–2416. [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Muzio M., Chinnaiyan A. M., Kischkel F. C., O'Rourke K., Shevchenko A., Ni J., Scaffidi C., Bretz J. D., Zhang M., Gentz R. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996 Jun 14;85(6):817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W. ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nat Biotechnol. 1996 Mar;14(3):297–301. doi: 10.1038/nbt0396-297. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Quan L. T., Tewari M., O'Rourke K., Dixit V., Snipas S. J., Poirier G. G., Ray C., Pickup D. J., Salvesen G. S. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Sachs L. The control of hematopoiesis and leukemia: from basic biology to the clinic. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4742–4749. doi: 10.1073/pnas.93.10.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Earnshaw W. C. ICE-related proteases in apoptosis. Curr Opin Genet Dev. 1996 Feb;6(1):50–55. doi: 10.1016/s0959-437x(96)90010-6. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Wang X., Zelenski N. G., Yang J., Sakai J., Brown M. S., Goldstein J. L. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 1996 Mar 1;15(5):1012–1020. [PMC free article] [PubMed] [Google Scholar]
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996 Jan 1;10(1):1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- Xue D., Shaham S., Horvitz H. R. The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 1996 May 1;10(9):1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Gahm A., Ankarcrona M., Nicotera P., Orrenius S. Multiple proteases are involved in thymocyte apoptosis. Exp Cell Res. 1995 Dec;221(2):404–412. doi: 10.1006/excr.1995.1391. [DOI] [PubMed] [Google Scholar]



