Abstract
Objectives
To evaluate the outcome of TNF-alpha inhibition (anti-TNFα) for pediatric uveitis.
Methods
We retrospectively assessed children (≤18 years) with non-infectious uveitis receiving anti-TNFα at five uveitis centers and one pediatric-rheumatology center. Incident treatment success was defined as minimal or no uveitis activity at ≥2 consecutive ophthalmological exams ≥28 days apart while taking no oral and ≤2 eyedrops/day of corticosteroids. Eligible children had active uveitis and/or were taking higher corticosteroid doses.
Results
Among 56 eligible children followed over 33.73 person-years, 52% had juvenile idiopathic arthritis (JIA) and 75% had anterior uveitis (AU). The Kaplan-Meier estimated proportion achieving treatment success within 12 months was 75% (95% confidence interval [95% CI]: 62–87%). Complete absence of inflammatory signs with discontinuation of all corticosteroids was observed in an estimated 64% by 12 months (95% CI: 51–76%). Diagnoses of JIA or AU were associated with greater likelihood of success, as was the oligoarticular subtype amongst JIA cases. In a multivariable model, compared to those with JIA-associated AU, those with neither or with JIA or AU alone had a 75–80% lower rate of achieving quiescence under anti-TNFα - independent of the number of immunomodulators previously or concomitantly prescribed. Uveitis re-activated within 12 months of achieving quiescence in 14% of those continuing anti-TNFα (95% CI: 6–31%). The incidence of discontinuation for adverse effects was 8%/year (95% CI: 1–43%).
Conclusion
Treatment with anti-TNFα was successful and sustained in a majority of children with non-infectious uveitis and treatment-limiting toxicity was infrequent. JIA-associated AU may be especially responsive to anti-TNFα.
Keywords: uveitis, tumor necrosis factor-alpha antagonist, juvenile idiopathic arthritis
INTRODUCTION
Uveitis is an important contributor to visual morbidity in children. In Northern California, the annual incidence of uveitis was 7/100,000 among children ≤14 years and 27/100,000 among adolescents (1). In the developed world, most pediatric uveitis cases are undifferentiated or associated with Juvenile Idiopathic Arthritis (JIA) (13–47%) (2–6). Across JIA subtypes, 12–13% of children develop uveitis, although this percentage rises much higher amongst certain subgroups, particularly those who are anti-nuclear antibody (ANA) positive (7). The uveitis leads to complications in over half of children, including iris synechiae (iris scarring), corneal calcium deposition, glaucoma, cataracts, macular edema, and/or visual loss (8–11). Many complications necessitate surgery. Early and aggressive treatment is needed to prevent the most devastating long-term outcomes (12). Even with the broad array of treatments available, the incidence of vision loss (≤20/50) is 0.17/person-year (13).
Topical corticosteroids are the first line of treatment, but are poorly tolerated in children, leading to early use of second-line immunomodulatory therapies such as methotrexate (12). More recently, biologic immunomodulatory agents, especially TNFα inhibitors (anti-TNFα), also have been used. Particularly for JIA-associated uveitis, anti-TNFα have shown impressive effects regarding control of inflammation and improvement of visual acuity in small, single center observational studies (14–24). Definitions of success varied by study, making comparisons across these small studies impractical.
Whether certain patients are better suited to treatment with anti-TNFα is unknown. Because anti-TNFα treatment is costly, it would be important to identify whether particular factors are associated with response to these agents, yet this has not been explored. As some studies have suggested that children with JIA-associated uveitis have worse visual outcomes than those with non-JIA-associated uveitis (25), we hypothesized that children with JIA might respond less well to anti-TNFα. Further, in JIA-associated uveitis, certain factors have been associated with an increased likelihood of developing severe uveitis or poor visual outcomes. These included: male sex (4); non-white race (13); younger age at onset (4,7); oligoarticular JIA (11); ANA positivity (7,13); complications at presentation (4,9,13,26); and intermediate, or posterior, rather than anterior, uveitis (AU) (3,4,6). We hypothesized that these factors also might be associated with worse response to anti-TNFα. To address these issues, we assembled a relatively large multicenter cohort of children with uveitis receiving anti-TNFα treatment begun while the uveitis was active or else required an unsustainable dose of corticosteroids to maintain quiescence. We wished to examine the outcomes of anti-TNFα therapy and to identify variables positively or negatively associated with achieving control of uveitis with minimal or no use of corticosteroids.
PATIENTS AND METHODS
Study setting and study population
This was a retrospective cohort study of patients from five uveitis subspecialty centers participating in the retrospective Systemic Immunosuppressive Therapy for Eye Disease (SITE) Cohort Study combined with patients managed in the rheumatology and ophthalmology practices of The Children’s Hospital of Philadelphia (CHOP) during the anti-TNFα era (1999–2010) (SITE: 1999–2007; CHOP 2004–2010). At SITE centers, data had been collected from all available medical records of every patient with non-infectious uveitis (27,28). Additional subjects were identified by searching the CHOP electronic medical record system for ICD-9 codes possibly indicating non-infectious uveitis (ICD-9 363.x, 364.x) (1). The charts of patients thus identified, who presented for care between 2000–2010, were reviewed to determine if they had uveitis and if ophthalmologic records were available
Inclusion criteria
Subjects selected for this analysis were ≤18 years old at the time of initiation of anti-TNFα treatment and either had active uveitis or slightly active/inactive uveitis controlled by systemic and/or topical corticosteroids >2 drops/day. Anti-TNFα therapies included infliximab, etanercept, and adalimumab. Concomitant treatment with corticosteroids and/or methotrexate, azathioprine, mycophenolate mofetil, or cyclosporine was recorded. A subject was included only if uveitis activity status was available within 30 days prior to anti-TNFα initiation and for ≥2 visits after treatment initiation (the minimum follow-up required to meet the success definition). If a subject had more than one treatment course with anti-TNFα that met inclusion criteria (drug episode) and success never was achieved with the first anti-TNFα treatment course, that subject could be included in the cohort more than once. As per survival analysis, which measures time-to-event, person-time after an event could not be included. Drug episodes discontinued during the first infusion were not included.
Data collection
Protocol-driven retrospective paper chart reviews had been performed at the 5 SITE centers, and data were entered into an electronic database created for the SITE Cohort Study. At CHOP, data were obtained from electronic medical or paper records through the Divisions of Rheumatology and/or Ophthalmology and were entered into a simplified version of the SITE database addressing the outcomes of interest for this study. Paper charts included clinic notes from both CHOP-affiliated and community ophthalmologists. CHOP subcohort JIA patients were characterized further into subtypes according to the International League of Associations for Rheumatology (ILAR) criteria (29). Age at uveitis was dichotomized (≤6 vs. > 6 years), following the American Academy of Pediatrics ophthalmologic screening guidelines for children with JIA (30). Anti-nuclear antibody (ANA) status and whether uveitis involved pain/redness/photosensitivity at the onset were only available from the CHOP subcohort.
Definition of disease activity/location
Disease activity at each visit was characterized as an ordered categorical outcome, using the approach the SITE Cohort Study has used previously: inactive, slightly active, or active, incorporating information from sites of inflammation other than the Anterior chamber (AC) into a single activity variable (31–35). Categories closely parallel the uveitis definitions of the Standardization of Uveitis Nomenclature (SUN) Working Group (36,37). Chart reviewers ascertained disease status from a combination of the physician’s overall assessment at the visit through the use of descriptors such as quiet or quiescent, as well as quantitative descriptors of cell grade and vitreous haze. “Inactive” reflected designations such as no cells, rare cells, no vitreous haze and no corticosteroids; “slightly active” reflected gradings such as trace or fewer AC cells (≤0.5+), and minimal vitreous haze or cells, and “active” reflected higher levels of inflammation. Disease state was recorded by eye; a patient’s disease state was considered as the worse level of the two eyes if both had uveitis or else the level of the eye with uveitis for unilateral cases. Uveitis location was categorized as anterior, intermediate (+/− anterior), posterior, or panuveitis (36).
Outcome definitions
The primary outcome (success) was defined as achieving either “slightly active” or “inactive” uveitis status as assessed by the evaluating ophthalmologist while on ≤2 drops/day topical corticosteroids and no oral corticosteroids. Success was evaluated secondarily in the most restrictive fashion, as the complete absence of inflammatory signs (“inactive”) while taking no topical or oral corticosteroids. Only “successes” documented over ≥2 visits spanning ≥28 days were counted, so as to avoid counting transient control of inflammation as a success (31–35).
Discontinuation of therapy
The reason for discontinuation of anti-TNFα was coded as: ineffective, remission, missing, or adverse effects.
Follow-up time
Follow-up time began with the first visit at which an anti-TNFα was prescribed (subcutaneous etanercept or adalimumab) or from the first day of intravenous administration (infliximab). Only treatment courses initiated under observation during the study period were included. Visit frequency varied, and, for survival analyses, data were used from clinical visits while a subject was on therapy until uveitis quiescence. Uveitis information was collected from all available visits to an ophthalmologist. Time-to-success was measured to the first of the two sequential visits necessary for success. Censoring occurred when the anti-TNFα was discontinued (in order not to attribute success to a subsequent drug) or the subject was lost to follow-up without achieving quiescence before the end of observation. In a secondary analysis of time to re-activation while on anti-TNFα, analysis time began when a subject achieved quiescence; each subject was followed through uveitis re-activation, drug discontinuation or loss to follow-up.
Covariates
Variables evaluated for their association with treatment success (achievement of quiescence) are listed in Table 1.
Table 1.
Characteristics of Children with Inadequately Controlled Uveitis Starting Tumor Necrosis Factor-α Inhibitor Therapy *
| Variable | CHOP | SITE | p value | Total cohort |
|---|---|---|---|---|
| n=40 | n=16 | n=56 | ||
| DEMOGRAPHIC | ||||
| Sex (% Female) | 50 | 56 | 0.67 | 52 |
| Race (% Caucasian) † | 69 | 81 | 0.35 | 73 |
| Median Age Diagnosis, yrs (range)† | 6.88 (1.42, 16.33) | 4.84 (2.61,8.11) | 0.06‡ | 6.04 (1.42, 16.33) |
| Age at Diagnosis (%≤6 yrs)† | 40 | 89 | 0.01§ | 50 |
| CLINICAL | ||||
| Prior Ocular Surgeries (%): | ||||
| 0 | 90 | 76 | 86 | |
| 1 | 7 | 18 | 11 | |
| ≥2 | 2 | 6 | 0.36 | 4 |
| MTX prior to anti-TNFα (%) | 98 | 29 | <0.01§ | 78 |
| Prior IMT (%): | ||||
| 0 | 2 | 12 | 5 | |
| 1 | 90 | 41 | 76 | |
| ≥2 | 7 | 47 | <0.01§ | 19 |
| Pain/Redness/Photosensitivity (%)† | 54 | -- | -- | -- |
| Anterior Uveitis (%) | 75 | 59 | 0.35 | 70 |
| Activity >0.5+ (%) | 57 | 50 | 0.63 | 55 |
| Active uveitis at start (%)¶ | 57 | 47 | 0.48 | 54 |
| Ever Synechiae (%) | 78 | 69 | 0.50 | 75 |
| Systemic Diagnosis (%): | ||||
| JIA | 43 | 75 | 52 | |
| Sarcoidosis** | 15 | 0 | 11 | |
| None/Unknown | 43 | 25 | 0.06 | 38 |
| JIA Characteristics (%):††† | ||||
| Oligoarticular | 47 | -- | -- | -- |
| ANA (% >1:40) | 71 | -- | -- | -- |
| TREATMENT‡‡ | ||||
| >1 year from diagnosis (%)† | 31 | 100 | <0.01§ | 46 |
| Anti-TNFα Type (%): | ||||
| Etanercept | 10 | 47 | 20 | |
| Infliximab | 83 | 53 | 75 | |
| Adalimumab | 7 | 0 | <0.01§|| | 5 |
| MTX at start anti-TNFα (%) | 98 | 59 | <0.01§ | 86 |
| IMT other than MTX at start (%) | 5 | 100 | <0.01§ | 32 |
SITE, Systemic Immunosuppressive Therapy for Eye Disease Cohort Study; CHOP, The Children’s Hospital of Philadelphia; anti-TNFα, Tumor Necrosis Factor-α inhibitor; IMT, immunomodulatory therapies; JIA, juvenile idiopathic arthritis/juvenile rheumatoid arthritis; ANA, anti-nuclear antibodies; MTX, methotrexate. Synechiae=adhesions between the iris and the lens (posterior) or cornea (anterior).
Data are not available from all patients.
Rank sum.
Significant two-sided p value, <0.05 (for difference between CHOP and SITE).
p value for the distribution of categorical variables.
Active uveitis, vs. uveitis controlled, but on corticosteroids, at anti-TNFα initiation.
One child with onset suggestive of early-onset Sarcoid (3 years); none with familial Sarcoid.
Of those with JIA in the CHOP subcohort.
Represent the percentage of anti-TNFα treatment courses, rather than patients: CHOP n=42; SITE n=17.
Data analysis
Data were analyzed using Stata 11.0 (College Station, TX). Differences in categorical demographic and clinical characteristics between subcohorts were assessed using the χ2 test. Nominal statistical significance was defined as a two-tailed p value ≤0.05. Kaplan-Meier methods evaluated time-to-success. As a subject could have more than one drug episode, analyses were performed using a robust variance estimator to account for clustering by subject. Cox proportional hazards model-derived hazard ratios (HR) estimated the association of each independent patient-level or treatment-level variable with each outcome variable. When subjects discontinued treatment because of an adverse event or failure they were no longer considered “at-risk” for succeeding under that drug. Consequently a competing risks analysis was performed using proportional subhazard regression (Stata “stcrreg” command) (38). For variable selection in multivariable regression, a variable was included if it was significant at the 0.1 level. Two-way interactions were assessed for variables that were statistically significant in the unadjusted analysis; an interaction was said to exist if it was significant at the 0.05 level. To account for possible differences between CHOP vs. SITE, subcohort was included as a covariate.
Ethics Boards Approval
The institutional review boards of the participating centers approved the SITE Cohort Study. The institutional review boards of CHOP and The University of Pennsylvania approved this study.
RESULTS
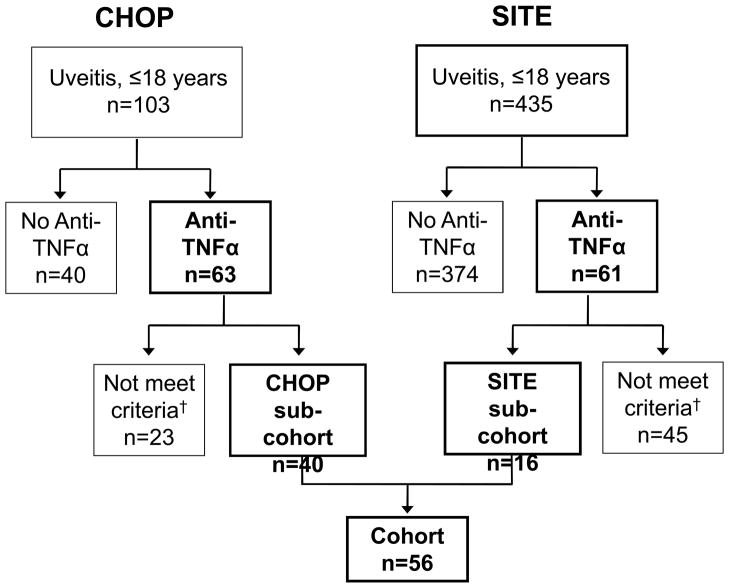
The records of 538 children (ages 18 years or younger) with uveitis were evaluated for inclusion in this cohort (Figure 1). Of these, 103 children at CHOP and 435 children in the SITE Cohort were treated for non-infectious uveitis. Overall, 23% of the children diagnosed with uveitis in childhood were treated with anti-TNFα. At CHOP, 63 children were treated with anti-TNFα; only 40 met inclusion criteria. In the SITE Cohort, 61 children received anti-TNFα; only 16 of these met inclusion criteria. The age, race, and sex distributions were similar between those included and those excluded from the cohort because of missing data (data not shown). The 56 children, who met inclusion criteria and had adequate follow-up information to be “at risk” of quiescence, were followed over 33.73 person-years (median 0.26, range 0.04–4.12 person-years/patient).
Figure 1.
Study cohort. Of 538 patients treated for non-infectious uveitis, 124 were treated with anti-TNFα. 56 of these met inclusion criteria for anti-TNFα/Corticosteroid/Immunomodulatory therapy use. †68 were excluded (23 potential subjects from CHOP and 45 from SITE) due to: inadequate records (did not have a visit within 30 days prior to initiation of anti-TNFα; no documentation of uveitis status at onset of anti-TNFα therapy; uveitis that developed after starting anti-TNFα; or initiation of anti-TNFα after the cut-off for the study). SITE=Systemic Immunosuppressive Therapy for Eye Disease Cohort Study; CHOP=The Children’s Hospital of Philadelphia; anti-TNFα, Tumor Necrosis Factor-α inhibitor.
Baseline Characteristics of the Cohort
Subject characteristics are described in Table 1. The ILAR subtype of JIA and ANA status was available only from CHOP. Subjects from CHOP and SITE were balanced in most baseline characteristics, except that at CHOP: a lower proportion of children were diagnosed at ≤6 years of age (40% vs. 89%); more were treated with methotrexate (98% vs. 29%), but fewer were treated with ≥2 conventional immunomodulators (7% vs. 47%) before anti-TNFα; and a lower proportion had an underlying diagnosis of JIA (43% vs. 75%). Only 5% of children in the overall cohort began anti-TNFα prior to failing ≥1 other immunomodulatory agent. Besides corticosteroids and methotrexate (n=44), children had been treated previously with cyclosporine (n=13), mycophenolate mofetil (n=12), chlorambucil (n=2), and cyclophosphamide (n=1).
Treatment Characteristics
Subjects from CHOP had a shorter time between diagnosis of uveitis and anti-TNFα treatment relative to subjects from SITE (median=0.32 vs. 4.25 years) and fewer of them were treated with the soluble TNF receptor (etanercept) than with monoclonal anti-TNF antibodies (infliximab or adalimumab) (10% vs. 47%) (Table 1). Children with sarcoidosis-associated uveitis had been treated with infliximab (n=5) or adalimumab (n=1).
Of the subjects with known anti-TNFα dose information (n=40, from CHOP), 35 were treated with infliximab; all but one began treatment on an every 4-week schedule after drug loading. Subjects had a median starting dose of 9.3 mg/kg (range 4.5 to 13.3 mg/kg); only 5 subjects were treated with doses <7mg/kg. All subjects treated with adalimumab received 40 mg every other week. Subjects on etanercept received between 12.5–25 mg twice weekly, except for one young child who received 0.8 mg/kg weekly. Three subjects initiated treatment with anti-TNFα on two occasions that met inclusion criteria for the final analysis. In each case, the initial drug was etanercept, which was discontinued for failure (n=2, CHOP) or unknown reasons (n=1, SITE); none were successful on their second drug, infliximab.
Response to anti-TNFα Treatment
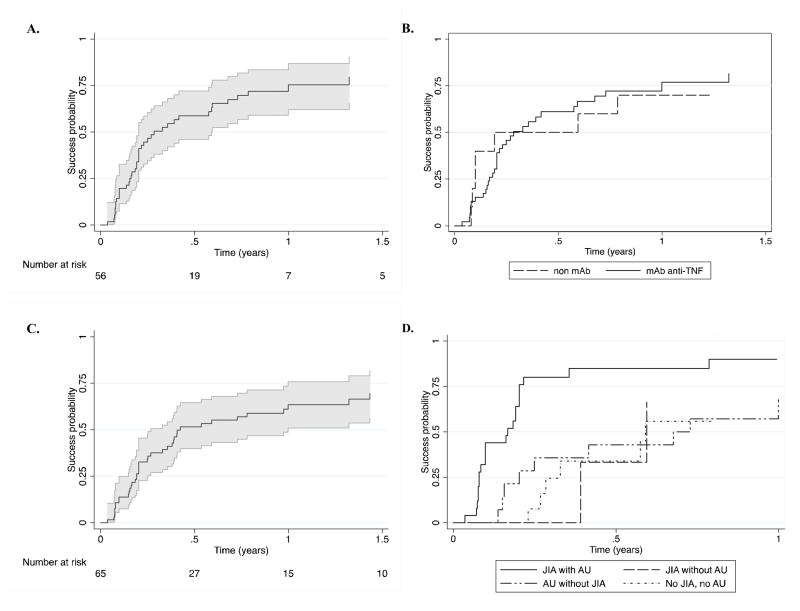
The estimated proportion who achieved treatment success that was then sustained for at least 28 days within 12 months was 75% (95% CI: 62–87%) (Figure 2 (2A)). The estimated probability of a child achieving quiescence was 47% by three months (95% CI: 35%–60%) and 59% by 6 months (95% CI: 46–72%); the median time to success was 3.4 months. The cumulative incidence of success did not differ significantly between subcohorts (p=0.37); treatment success at 12 months was 79% (95% CI: 63–91%) and 68% (95% CI 44–89%) in the CHOP and SITE subcohorts respectively. The cumulative incidence of success did not differ significantly between those treated with different classes of anti-TNFα agents (monoclonal anti-TNFα antibodies vs. non-monoclonal antibody) (p=0.55); treatment success at 12 months was 0.77 (95%CI: 0.62, 0.89) vs. 0.70 (95% CI, 0.32, 0.93) (Figure 2 (2B)). Most of these also met the higher standard of complete uveitis inactivity without concomitant topical or oral corticosteroids, 34% by 3 months (95% CI: 24–47%), 52% by 6 months (95% CI: 40–65%), and 64% by 12 months (95% CI: 51–76%) (Figure 2 (2C)). Sensitivity analysis including only one drug episode per subject did not alter results qualitatively.
Figure 2.
Time-to-treatment success following initiation of Tumor Necrosis Factor-α inhibitors in children with uveitis. The 95% CI is represented by the areas shaded in grey. A. “Slightly active” (trace or fewer AC cells (≤0.5+) or better control of inflammation in all eyes with uveitis absent use of oral corticosteroids and with no more than two drops/day of topical corticosteroids. B. “Slightly active” in children treated with monoclonal antibody anti-TNFα (infliximab or adalimumab) (mAb) vs. those treated with etanercept (non-mAb). C. Complete uveitis inactivity without concomitant topical or oral corticosteroids. D. “Slightly active” or better control of inflammation in different subgroups of children with uveitis; treatment success over time is demonstrated in subgroups determined by their juvenile idiopathic arthritis (JIA) and anterior uveitis (AU) statuses.
Factors Predictive of Treatment Success
Diagnoses of JIA (HR 2.41, 95% CI 1.29–4.56) and AU (vs. intermediate, posterior or panuveitis, HR 2.52, 95% CI: 1.35, 4.69) each were associated with faster development of quiescence (see Table 2). Within the CHOP subcohort, children with oligoarticular JIA (vs. other forms of JIA) had a higher rate of treatment success (HR 5.93, 95% CI: 2.15–16.36). No other variables were significantly associated with treatment success. Results from the competing risks analysis were similar to those from standard proportional hazards analysis.
Table 2.
Factors Associated with Tumor Necrosis Factor-α Inhibitor Treatment Success*
| Variable | Competing Risk HR† ** |
|---|---|
| Female Sex | 1.36 (0.75, 2.47) |
| Caucasian | 1.60 (0.84, 3.05) |
| Age at Diagnosis ≤6 | 1.30 (0.68, 2.49) |
| JIA | 2.41 (1.28, 4.56) |
| Oligo-JIA‡ | 5.93 (2.15, 16.36) |
| ANA Positive‡ | 1.67 (0.71, 3.98) |
| Anterior Uveitis | 2.52 (1.35, 4.69) |
| Active Uveitis at start§ | 1.00 (0.54, 1.85) |
| Iris synechiae|| | 1.00 (0.46, 2.21) |
| Pain/Redness/Photosensitivity‡ | 0.65 (0.31, 1.39) |
| ≥ 2 Prior surgeries | 2.29 (0.70, 7.54) |
| ≥ 2 Prior IMT | 0.46 (0.18, 1.22) |
| On MTX at start anti-TNFα | 1.47 (0.56, 3.89) |
| On IMT other than MTX at start | 0.62 (0.30, 1.30) |
| Diagnosis to anti-TNFα >1 yr | 1.41 (0.73, 2.71) |
| Anti-TNFα: | |
| Etanercept | 1.00 (reference) |
| Infliximab | 1.34 (0.53, 3.43) |
| Adalimumab | 1.07 (0.22, 5.12) |
| Monoclonal antibody anti-TNFᶠ| 1.33 (0.52, 3.38) |
| CHOP Subcohort | 0.74 (0.35, 1.58) |
HR, hazard ratio; CI, confidence interval; JIA, juvenile idiopathic arthritis; Oligo-JIA, oligoarticular JIA; ANA, anti-nuclear antibodies; IMT, Immunomodulatory therapy; MTX, methotrexate; Anti-TNFα, Tumor Necrosis Factor-α inhibitor; CHOP, The Children’s Hospital of Philadelphia; SITE, the Systemic Immunosuppressive Therapy for Eye Disease Cohort Study.
A HR greater than 1 is favorable, representing a relatively higher incidence of treatment success. HR’s whose 95% confidence intervals do not cross 1 are in bold text.
Proportional subhazards analysis, with drug discontinuation for failure or adverse reaction as a competing risk.
Data are only available on subjects from CHOP.
Active uveitis, vs. uveitis controlled but on corticosteroids, at the onset of anti-TNFα.
Synechiae=adhesions between the iris and the lens (posterior) or cornea (anterior).
Monoclonal anti-TNFα (Infliximab or Adalimumab) vs. Etanercept.
Conjoint analysis of the effects of JIA and AU on treatment success, adjusted for subcohort, revealed that compared to patients with JIA-associated AU, the rate of success was 75–80% lower in patients with neither or with JIA or AU alone (see Table 3). The Kaplan-Meier estimate of the proportion with treatment success by 6 months in those with JIA-associated AU was 0.85 (95% CI: 0.68–0.96) versus 0.37 (95% CI 0.23–0.58) for the other three groups combined (Figure 2D).
Table 3.
Multivariable Hazard Ratios for Treatment Success Variables under Tumor Necrosis Factor-α Inhibitors * † ** ††
| Uveitis Characteristics | ||
|---|---|---|
| Systemic Disease | Anterior Uveitis | Intermediate/Posterior/Pan-Uveitis |
| JIA | 1.00 (reference)** | 0.19 (0.04, 0.85) |
| No JIA | 0.25(0.10, 0.61) | 0.24 (0.11, 0.50) |
Covariates included in the multiple regression model: diagnoses of JIA and anterior uveitis (with interaction between the two) and source of data (The Children’s Hospital of Philadelphia subcohort vs. Systemic Immunosuppressive Therapy for Eye Disease Cohort Study).
Referent different than in table 2; it is JIA and AU (rather than no JIA no AU).
JIA, Juvenile Idiopathic Arthritis.
Hazard ratio (95% CI).
Treatment-limiting Adverse Effects
Eight percent of anti-TNFα treatment episodes were discontinued within 12 months because of adverse effects (n=1) (95% CI: 1–43%). Only one subject stopped infliximab before control was achieved (allergic reaction). Four subjects discontinued medications because of side effects after they had already achieved success: allergic reaction to infliximab (n=2); side effects to infliximab (n=1); side effects to etanercept (n=1. None of the subjects treated with adalimumab in the cohort stopped therapy because of adverse effects.
Relapse of Uveitis during anti-TNFα Treatment
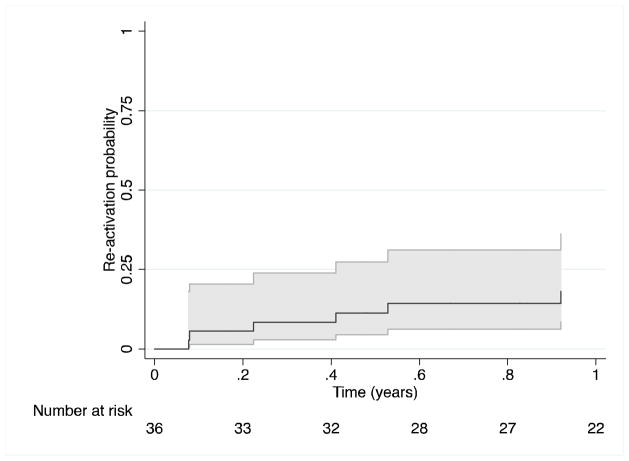
Among 45 subjects on anti-TNFα agents who initially achieved treatment success, 36 had sufficient follow-up while remaining on anti-TNFα to observe a relapse of uveitis. The Kaplan-Meier estimates of the risk of relapse while on anti-TNFα by 3,6, and 12 months were 8% (95% CI: 3 to 24%), 11% (95% CI: 04–27%), and 14% (95% CI: 6–31%) respectively (see Figure 3).
Figure 3.
Time-to-reactivation of uveitis after achieving treatment success (“slightly active” or better control of inflammation in all eyes with uveitis, absent use of oral corticosteroids, and with no more than two drops/day of topical corticosteroids), while receiving ongoing Tumor Necrosis Factor-α inhibitor therapy. The 95% CI is represented by the areas shaded in grey.
DISCUSSION
Our results suggest that anti-TNFα is a useful treatment for pediatric non-infectious uveitis, particularly for patients with JIA-associated AU. Even though 95% of children in our cohort had failed one or more prior immunosuppressive drugs, the substantial majority had a favorable or very favorable response to treatment. Although JIA-associated uveitis has been thought to be more treatment-resistant than other forms of pediatric uveitis (39), in our study JIA-associated uveitis responded better under TNFα inhibition than did other forms of uveitis, such as sarcoidosis-associated or undifferentiated uveitis. As expected, AU responded better than did other types of uveitis (31,33), but the benefit seemed to be primarily among those with JIA-associated AU. Suppression was sustained for twelve months in a large majority of cases. Therapy was well tolerated with a low rate of discontinuation of anti-TNFα agents for complications of therapy.
While presumably there is overlap of some SITE Cohort patients with those described in previous reports (15,22,40–42), here we describe the outcomes in 56 patients, from six tertiary-care centers, not previously reported together. This is one of the largest pediatric cohorts of anti-TNFα therapy for pediatric uveitis to date. Comparisons of response rates with those from prior smaller studies are complicated in that each used different measures of improvement in inflammation, only some of which took into account the degree of corticosteroid usage. Once uveitis is quiet, many ophthalmologists maintain patients on low-dose topical corticosteroids and vary in their attempts to completely discontinue them. If success required complete corticosteroid discontinuation, time-to-success would be artificially lengthened by clinicians who did not attempt to completely wean corticosteroids. Given the heterogeneous group of ophthalmologists who saw cases in this cohort, we defined quiescence as uveitis inactivity while on ≤2 corticosteroid drops/day for our primary analysis, in addition to reporting complete quiescence off all corticosteroid therapy.
Overall, our results confirm the generally favorable results reported in small series. The 3.4-month median time-to-response is identical to that observed previously (15,21,43). It has been hypothesized that the effectiveness of anti-TNFα agents may be affected by their mechanism of action, and that monoclonal anti-TNFα antibodies (infliximab and adalimumab) may be more effective than the soluble TNF receptor (etanercept). In fact, there was no benefit of etanercept over placebo for uveitis in a small randomized controlled trial (40). Later studies suggested that infliximab was more effective than etanercept (15,43) and that adalimumab was more effective than infliximab for uveitis (44). Although we did not demonstrate a decreased effectiveness of etanercept relative to the other agents, we had limited power to discriminate between the effects of different anti-TNFα agents or between monoclonal antibody and soluble TNF receptor anti-TNFα agents. In our combined evaluation of anti-TNFα agents, quiescence was achieved in 64–75% by one year, depending on the stringency of the definition of quiescence used, which is on the same order as in two other studies describing the one-year outcome of infliximab treatment (14,21). Our observation that the cumulative incidence of complete control of inflammation absent all corticosteroids was only slightly lower than a less stringent definition of success permitting low dose topical corticosteroids suggests that most patients can be tapered off all corticosteroids while under anti-TNFα therapy. Had all ophthalmologists pressed tapering more aggressively, perhaps an even higher incidence of success would have been achieved. The percentage of patients who maintained quiescence through 12 months of continued anti-TNFα therapy was higher than that in another study (86% [95% CI 69–94%] vs. 58% [95% CI 32–82%]) (14), possibly because of higher initial infliximab doses (9 vs. 5 mg/kg).
Because physicians may choose anti-TNFα over other conventional immunomodulators when uveitis is more severe or recalcitrant, success under different treatment types cannot be contrasted directly. However, review of our results alongside historical controls suggests that anti-TNFα may be more effective for inflammatory uveitis. Most pediatric studies did not describe the anti-inflammatory and topical corticosteroid-sparing effect of immunomodulators in a single statistic (45–47). Yet, in one study of azathioprine for methotrexate-resistant uveitis, only 29% of children (95% CI 4–71%) achieved uveitis control on fewer than 2 drops/day of topical corticosteroids (48). In the large SITE Cohort studies, including patients of all ages with a variety of sites of inflammation, by one year, fewer than 20% of individuals achieved control of inflammation off of systemic corticosteroids with mycophenolate mofetil, methotrexate, cyclosporine A, or azathioprine (31–35). Thus, the more favorable incidence of success in this study, even in a population that had largely failed conventional immunomodulators and using more stringent success criteria, suggests that anti-TNFα treatment may be more effective than alternative immunomodulatory drugs.
Little information previously has been available regarding factors predictive of favorable response to anti-TNFα. One study of 20 children with JIA demonstrated a positive correlation between response to adalimumab and a younger age at diagnosis and/or a shorter time between uveitis diagnosis and adalimumab treatment (49). Our analysis did not confirm these associations. However, we found that children with JIA-associated AU have a particularly favorable prognosis with anti-TNFα therapy. Among patients with JIA in the CHOP subcohort, we observed a particularly favorable prognosis for children with oligoarticular JIA. Many animal models of autoimmune uveitis are primarily driven by antigen specific Th1 cells, yet innate immune cells are also involved (50). JIA-associated AU may be more Th1, or specifically TNFα-driven, than uveitis of other etiologies. Further work is needed to ascertain if specific blockade of other cytokines (e.g. interferon-γ) or cellular pathways (e.g. Th17) also might be especially effective in non-JIA-associated uveitis than conventional immunomodulators.
In previous SITE Cohort reports, the one-year incidence of discontinuation for toxicity, defined in the same manner as this study, for cyclosporine, mycophenolate mofetil, methotrexate, azathioprine, and cyclophosphamide respectively were 10.7%, 14.5%, 17.5%, 24.1%, 33.5% (31–35). Thus the tolerability profile of anti-TNFα was no worse than, and may be favorable to, alternative conventional immunosuppressive agents. The greatest limitation of this study is its observational nature. Due the absence of a single prospective study protocol, follow-up schedule, methotrexate usage patterns, topical corticosteroid weaning approaches, and anti-TNFα doses varied. A number of potential subjects had to be excluded because of missing information. As in any retrospective cohort study, we are unable to assess whether the outcomes of excluded patients were different than those of observed patients. That their demographic and clinical characteristics were similar suggests that the patients studied were likely to be representative of the larger population. Also, because of the variability in follow-up, improvement may have been mischaracterized as occurring later or initial treatment success may have been missed altogether, thus underestimating the proportion achieving quiescence at each time point. Furthermore, uveitis treated at these tertiary care centers, and the uveitis treated therein with anti-TNFα, may have represented a subset of more severe or treatment-refractory disease. If so, our analysis may underestimate the benefits of anti-TNFα therapy. However, that patients who had failed a larger number of prior immunomodulatory therapies did not have a lower rate of success suggests that anti-TNFα therapy often succeeds even in that context. Because standard survival analysis may overestimate the incidence of a favorable event in the face of informative censoring, as might occur when a drug was switched because an individual failed to respond to the initial anti-TNFα, we used a competing risk analysis. While a competing risk analysis is designed to overcome this problem, it is possible that violations of its assumptions may have led to over or underestimation of the time-to-treatment success.
Our study’s statistical power also was limited to identify associations with variables that were not collected in the SITE Cohort (anti-TNFα dose, JIA subtype, ANA status, symptomatic uveitis, or joint activity). Because at CHOP few patients had active musculoskeletal disease at cohort entry, we were unable to evaluate the concordance of joint and eye activity in response to treatment. In our study, concomitant anterior and intermediate uveitis was classified as intermediate. Our analysis may underestimate those with AU, thus underestimating the association between AU and response to anti-TNFα. While a difference between subcohorts, CHOP vs. SITE, is a potential limitation, outcomes did not differ between subcohorts in a sensitivity analysis. Strengths of the study include a sample size large enough to allow substantially more precise estimates of treatment success, time to success and risk factor associations than previously were available; and the use of quality control measures in the data collection to maximize retrospective data quality.
In summary, our results suggest that anti-TNFα medications are often useful for children with uveitis, even in children who have failed other immunomodulatory therapies, and are generally well tolerated. These agents result in a corticosteroid sparing benefit, likely limiting the ocular toxicities of long-term topical corticosteroid use. Patients with JIA-related AU or patients with oligoarticular JIA-associated uveitis may be especially likely to respond under such therapy, as the large majority of these patients achieve treatment success relatively quickly. Clinical trials evaluating the relative merits of TNF inhibitor therapy vs. conventional immunosuppression would be valuable to more thoroughly characterize the extent of any such advantage, but will be difficult to implement. In the meantime, it seems reasonable to recommend the use of anti-TNFα therapy relatively early on in the management of pediatric uveitis cases that fail an initial conventional immunomodulatory drug, before uveitis or corticosteroid therapy induces severe damage to the eye(s).
Acknowledgments
Supported by: American College of Rheumatology Research Education Foundation Scientist Development Award (Atlanta, GA) (MAL); Autoimmunity Centers of Excellence (National Institute of Allergy and Infectious Diseases) (Bethesda, MD) (JMB); R01 EY014943 National Eye Institute (NEI), National Institutes of Health (Bethesda, MD) (ED, JTR, EBS, JET, JHK); grants AG025152 and AG035751 from the National Institute on Aging (SH); cooperative agreements from the NEI, the National Institutes of Health, Bethesda, MD to the Mount Sinai School of Medicine, New York, NY (U10 EY08052), the Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (U10 EY08057), and the University of Wisconsin, Madison, Madison, WI (U10 EY08067) (DAJ); intramural funding from the National Eye Institute (Bethesda, MD) (GAL (previously), RBN); The Department of Veterans Affairs (EBS); research grants from Abbott, LuxBio Genentech, Bristol Myers Squibb, Novartis, and EyeGate (EBS); RPB Sybil B. Harrington Special Scholars Award (JET); Research to Prevent Blindness (New York, NY) (JHK); Paul and Evanina Mackall Foundation (New York, NY) (JHK).
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern california; the northern california epidemiology of uveitis study. Ophthalmology. 2004 Mar;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 2.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005 Apr;89(4):444–8. doi: 10.1136/bjo.2004.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003 May;135(5):676–80. doi: 10.1016/s0002-9394(02)02148-7. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: Clinical characteristics and complications. Am J Ophthalmol. 2009 Apr;147(4):667–678.e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005 Jul;112(7):1287–92. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009 Aug;116(8):1544–51. 1551.e1. doi: 10.1016/j.ophtha.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis Rheum. 2010 Feb 22;62(6):1824–8. doi: 10.1002/art.27416. [DOI] [PubMed] [Google Scholar]
- 8.Foster CS. Diagnosis and treatment of juvenile idiopathic arthritis-associated uveitis. Current Opinion in Ophthalmology. 2003;14(6):395. doi: 10.1097/00055735-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K German Uveitis in Childhood Study Group . Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in germany: Suggested modification of the current screening guidelines. Rheumatology (Oxford) 2007 Jun;46(6):1015–9. doi: 10.1093/rheumatology/kem053. [DOI] [PubMed] [Google Scholar]
- 10.Kump LI, Castañeda RA, Androudi SN, Reed GF, Foster CS. Visual outcomes in children with juvenile idiopathic arthritis-associated uveitis. Ophthalmology. 2006 Oct;113(10):1874–7. doi: 10.1016/j.ophtha.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, Silverman ED. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: A long-term followup study. Arthritis Rheum. 2007 Feb;56(2):647–57. doi: 10.1002/art.22381. [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. American Journal of Ophthalmology. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 13.Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: Incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007 May;143(5):840–6. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: Retrospective case series with long-term follow-up. Am J Ophthalmol. 2007 Dec;144(6):844–9. doi: 10.1016/j.ajo.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher M, Quinones K, Cervantes-Castaneda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthlamol. 2007;91(10):1341. doi: 10.1136/bjo.2007.124081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006 May;113(5):860–4.e2. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006 Feb;113(2):308–14. doi: 10.1016/j.ophtha.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Reiff A, Takei S, Sadeghi S, Stout A, Shaham B, Bernstein B, et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum. 2001 Jun;44(6):1411–5. doi: 10.1002/1529-0131(200106)44:6<1411::AID-ART235>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk of new-onset uveitis in patients with juvenile idiopathic arthritis treated with anti-tnfalpha agents. J Pediatr. 2006 Dec;149(6):833–6. doi: 10.1016/j.jpeds.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SM, Ramanan AV, Riley P, Dick AD. Use of infliximab in juvenile onset rheumatological disease associated refractory uveitis: Efficacy in joint and ocular disease. British Medical Journal. 2006 doi: 10.1136/ard.2006.065441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonini G, Zannin ME, Caputo R, Falcini F, de Martino M, Zulian F, Cimaz R. Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis. Rheumatology (Oxford) 2008 Oct;47(10):1510–4. doi: 10.1093/rheumatology/ken298. [DOI] [PubMed] [Google Scholar]
- 22.Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125(7):895. doi: 10.1001/archopht.125.7.895. [DOI] [PubMed] [Google Scholar]
- 23.Tugal-Tutkun I, Ayranci O, Kasapcopur O, Kir N. Retrospective analysis of children with uveitis treated with infliximab. J AAPOS. 2008 Dec;12(6):611–3. doi: 10.1016/j.jaapos.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Cobian LB, Flynn T, Lehman TJA. Adalimumab therapy for childhood uveitis. The Journal of Pediatrics. 2006;149(4):572–5. doi: 10.1016/j.jpeds.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 25.Heinz C, Mingels A, Goebel C, Fuchsluger T, Heiligenhaus A. Chronic uveitis in children with and without juvenile idiopathic arthritis: Differences in patient characteristics and clinical course. Journal of Rheumatology. 2008;35(7):1403–7. [PubMed] [Google Scholar]
- 26.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010 Jul;117(7):1436–41. doi: 10.1016/j.ophtha.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempen JH, Daniel E, Gangaputra S, Dreger K, Jabs DA, Kaçmaz RO, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: The systemic immunosuppressive therapy for eye diseases (SITE) cohort study. Ophthalmic Epidemiol. 2008;15(1):47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 28.Kempen JH, Daniel E, Dunn JP, Foster CS, Gangaputra S, Hanish A, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: Retrospective cohort study. BMJ. 2009;339:b2480. doi: 10.1136/bmj.b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: Second revision, edmonton, 2001. J Rheumatol. 2004 Feb;31(2):390–2. [PubMed] [Google Scholar]
- 30.Cassidy J, Kivlin J, Lindsley C, Nocton J. Section on Rheumatology, Section on Ophthalmology Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006 May;117(5):1843–5. doi: 10.1542/peds.2006-0421. [DOI] [PubMed] [Google Scholar]
- 31.Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kaçmaz RO, Levy-Clarke GA, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010 Mar;149(3):423–32. e1–2. doi: 10.1016/j.ajo.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009 Nov;116(11):2188–98.e1. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010 Mar;117(3):576–84. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasadhika S, Kempen JH, Newcomb CW, Liesegang TL, Pujari SS, Rosenbaum JT, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009 Oct;148(4):500–509.e2. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pujari SS, Kempen JH, Newcomb CW, Gangaputra S, Daniel E, Suhler EB, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010 Feb;117(2):356–65. doi: 10.1016/j.ophtha.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group . Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005 Sep;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008 Dec;146(6):813–8.e1. doi: 10.1016/j.ajo.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54. [PubMed] [Google Scholar]
- 39.Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: Analysis of a case series of 160 patients. Ophthalmology. 2001 Jun;108(6):1134–9. doi: 10.1016/s0161-6420(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 40.Smith JR, Levinson RD, Holland GN, Jabs DA, Robinson MR, Whitcup SM, Rosenbaum JT. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001 Jun;45(3):252–7. doi: 10.1002/1529-0131(200106)45:3<252::AID-ART257>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005 Feb 15;53(1):18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 42.Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S, Vredeveld CM. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol. 2003 Apr;121(4):437–40. doi: 10.1001/archopht.121.4.437. [DOI] [PubMed] [Google Scholar]
- 43.Saurenmann RK, Levin AV, Rose JB, Parker S, Rabinovitch T, Tyrrell PN, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006 Aug;45(8):982–9. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 44.Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the italian registry. J Rheumatol. 2012 Nov 1; doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]
- 45.Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. 2007 Feb;91(2):180–4. doi: 10.1136/bjo.2006.094698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. Br J Ophthalmol. 1998;82(7):737. doi: 10.1136/bjo.82.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss AW, Wallace CW, Sherry DS. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr. 1998 Aug;133(133):266–8. doi: 10.1016/s0022-3476(98)70232-x. [DOI] [PubMed] [Google Scholar]
- 48.Goebel JC, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol. 2011 Feb;95(2):209–13. doi: 10.1136/bjo.2009.173542. [DOI] [PubMed] [Google Scholar]
- 49.Tynjälä P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, Lahdenne P. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford) 2008 Mar;47(3):339–44. doi: 10.1093/rheumatology/kem356. [DOI] [PubMed] [Google Scholar]
- 50.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010 Sep 1;120(9):3073–83. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]